Advances in Analytical Chemistry
Vol.1 No.2(2011), Article ID:359,5 pages DOI:10.4236/aac.2011.12002
Progress on the Electrochemical Co-Precipitation Determination for Trace Amounts of Heavy Metal Ions
Anhui Key Laboratory of Chemo-Biosensing, College of Chemistry and Materials Science, Anhui Normal University, Wuhu
Email: binfang_47@yahoo.com.cn
Received: Jul. 16th, 2011; revised: Sep. 20th, 2011; accepted: Oct. 8th, 2011.
ABSTRACT:
Description on the electrochemical determination of heavy metal ions in recent years was presented in this paper. Research of electrochemical co-precipitation detection of trace amounts of heavy metal ions was high lightly introduced. The method was that separation, enrichment and electrochemical detection of trace heavy metal ions were simultaneously and synchronously carried through and it shows high selectivity, sensitivity and stability. And thirty-six literatures were cited.
Keywords: Heavy Metal Ions; Trace Amount; Modified Electrode; Electrochemical Co-Precipitation
电化学共沉淀分离测定痕量重金属离子的研究进展
刘 敏,王广凤,方 宾*
安徽师范大学化学与材料科学学院,安徽省化学生物传感器重点实验室,芜湖
Email: binfang_47@yahoo.com.cn
摘 要:
本文简述了近年来测定重金属离子的电分析方法,着重介绍了电化学共沉淀分离测定痕量重金属离子的研究进展。该方法集分离、富集和测定于一体的优势,有着良好的选择性、灵敏度和稳定性。引用文献36篇。
收稿日期:2011年7月16日;修回日期:2011年9月20日;录用日期:2011年10月8日
关键词:重金属离子;痕量;修饰电极;电化学共沉淀
1. 引言
随着工农业以及经济的迅猛发展,环境中重金属污染日趋加剧已成为不争的事实[1-3]。Leung[4]报道,中国东南部的重金属污染严重,尤其是Co,Hg,Cd,Cr,Pb,Cu,As,在靠近城市、工业区及主要支流的重金属离子浓度尤其高。在环境污染方面的重金属主要是指汞、镉、铅、铬以及类金属砷等生物毒性显著的重金属元素。重金属污染物在环境中难以降解,能在动物和植物体内积累,通过食物链逐步富集,浓度能成千成万甚至成百万倍地增加[5],最后进入人体造成危害,是危害人类最大的污染物之一[6]。重金属的定量分析在环境监测方面一直受到人们的重视[7,8],因此检测环境中的重金属具有现实的意义。
目前,提高检测的灵敏度、选择性和有效分离痕量元素仍是环境中重金属离子分析的两个关键点[9,10]。前些年以络合吸附波测定痕量物质的方法是重要的电化学分析方法之一。李启隆[11]曾对1991年前的有机试剂在极谱吸附波中的应用进行过评述,刘道杰等[12]2003年又对有机试剂在络合吸附波中的应用进行简要综述,这里不再赘述。近年来,利用修饰电极的电化学方法已经广泛地应用于重金属离子的分析测定。Leech等[13]通过金属硫蛋白修饰电极对痕量汞离子的富集,然后采用阴极脉冲伏安法对其进行测定;Oztekin等[14]则采用电化学聚合法制备了聚多酚修饰电极,同时采用脉冲伏安法考察了该修饰电极对铜离子(Cu2+)响应的灵敏度;姜林等[15]则采用自组装膜法制备离子载体–萘酚修饰电极,实现对痕量镉离子(Cd2+)的测定。任鑫胜等[16]则通过低聚核苷酸和DNA大分子之间的共轭成对作用,制备高灵敏高选择性的共聚物修饰电极,实现了对汞离子的测定。随着纳米技术的发展结合光学分析和电化学分析在重金属分析测定方面有着广泛的应用。刘燕等[17]则以钛酸丁酯为前驱物、无水乙醇为溶剂,采用溶胶–凝胶法将纳米二氧化钛(TiO2)固定在硅胶表面,通过纳米TiO2对Cd,Cr,Mn和Cu的吸附,并采用电感耦和等离子体–原子发射光谱法(ICP-AES)对其进行测定。Toh等[18]利用金纳米巨大的表面积对DNA的吸附能力,将DNA组装到金纳米表面,采用紫外可见光谱和荧光检测法探讨了汞离子测定的机理。杨斌武等[19]通过电化学沉积的方法将二氧化锰纳米粒子成功地吸附到多壁碳纳米管(MWCNTs)上面,得到γ-MnO2/MWCNTs纳米复合物,基于该纳米复合物对痕量铅离子(Pb2+)和镉离子(Cd2+)分离富集的原理,通过电热原子吸收光谱法实现了对痕量铅离子和镉离子的测定。以上是利用无机载体或有机载体的吸附性,首先对痕量元素进行富集,然后对其进行测定,但未集分离、富集、检测为一体。金利通等[20]则以乙二醇作氯铂酸的还原剂,采用微波法在浓硝酸回流纯化和羟基化的碳纳米管(CNTs)上负载铂纳米粒子,制备了铂纳米粒子/碳纳米管修饰电极,实现了对三价砷离子的电化学测定,该修饰电极对痕量砷的测定有较高的灵敏度、选择性和重现性。
Ganjali等[21]则采用纳米自组装术制备了MWCNTs和纳米硅的复合物修饰碳糊电极,实现了对痕量铅离子的测定。赵红等[22]则采用纳米掺杂技术通过电化学聚合的方法,将2-氨基-4-噻唑酸聚合到MWCNTs饰电极上,制得聚合物/MWCNTs修饰玻碳电极,并用脉冲伏安法考察了痕量铜离子在该修饰电极上的电化学响应,检测线达到5.0 × 10−10 M。邢苏杰等[23]通过自组装膜法利用Nafion膜将银纳米粒子固定到电极表面上,制备Nafion/银纳米粒子修饰玻碳电极,用来测定环境中的重金属铬离子(Cr6+)。以上几种方法均利用载体对痕量元素功能性吸附,实现了集分离、富集、测定为一体的优势。
2. 研究进展
2.1 电化学共沉淀
电化学共沉淀是指共沉淀分离法与电化学分析法有机结合。它集共沉淀分离、富集和分析测定于一体,同步进行。共沉淀按机理包括吸附,混晶,吸留等。共沉淀载体可分无机共沉淀载体和有机共沉淀载体。有机共沉淀载体又分为离子缔合物、金属螯合物和惰性共沉淀剂等。若将有机共沉淀载体修饰于电极,让痕量元素在电极–溶液界面进行分离富集,然后在一定电位窗口,让分离富集的痕量元素于电极上发生电化学反应,产生一定电信号响应,用于检测分析。在修饰有有机共沉淀载体时,为加强稳定性和防止薄层致密(薄层致密时不利于离子缔合和金属螯合),可运用纳米技术修饰和纳米材料掺杂,充分利用纳米材料大的比表面、良好的吸附性和提高电子传递速率等特性。我们采用自组装膜法、电化学沉积、滴涂等方法结合现代纳米技术将有机共沉淀剂或共沉淀载体修饰到玻碳电极表面,研讨了痕量重金属离子在该修饰电极上的电化学行为,成功实现了集分离、富集、检测为一体的电化学共沉淀法。
2.2. 离子缔合物共沉淀载体修饰
由于结晶紫(CV+)独特的结构和它的正电性,可与阴离子形成离子缔合物。在我们的实验“对痕量汞离子的分离富集和电化学测定同时同步进行的研究”[24]中,通过静电吸附作用,我们采用纳米自组装膜法制备了离子缔合物结晶紫碘化物(CV+I–)和MWCNTs的复合物修饰电极(CV+I–/MWCNTs/GCE)。首先是将10 µL羧基化的MWCNTs悬浮液滴涂到玻碳电极表面,待其干后将MWCNTs修饰电极浸泡到0.1 mM结晶紫溶液中60 min得到CV+/MWCNTs/GCE。最后将CV+/MWCNTs/GCE浸泡在1 mM碘离子溶液中30 min得到CV+I–/MWCNTs/GCE。其中离子缔合物(CV+I–)作为载体,表面吸附少量的I–,在电极–溶液界面,Hg2+先与I–形成 阴离子,再与CV+生成
阴离子,再与CV+生成 ·2CV+离子缔合物,在CV+I–载体诱导吸附下形成共沉淀,同时利用电化学方法对其进行测定。痕量汞离子在修饰电极上的作用机理如下:
·2CV+离子缔合物,在CV+I–载体诱导吸附下形成共沉淀,同时利用电化学方法对其进行测定。痕量汞离子在修饰电极上的作用机理如下:
电极表面:CV+ + I– → CV+I–(载体) I–(剩余力场吸附)
I–(剩余力场吸附)
界面:Hg2+ + 4I– → HgI42–
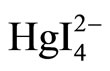 + 2CV+ →
+ 2CV+ →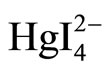 ·2CV+
·2CV+
诱导吸附:
 ·2CV+ + CV+ I–(载体) →
·2CV+ + CV+ I–(载体) → ·2CV+(CV+·I–)
·2CV+(CV+·I–)
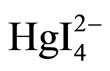 ·2CV+(CV+·I–)(被共沉淀化合物的组成)
·2CV+(CV+·I–)(被共沉淀化合物的组成)
在最优的实验条件下,汞离子的阳极氧化峰电流与其浓度在5.0 × 10−9~1.9 × 10−6 M范围内呈良好的线性关系,相关系数为0.9984,检出限为1.0 × 10−10 M (S/N = 3),而且避免了其他重金属和有机配体的干扰。因此,在CV+I−/MWCNTs修饰电极上,分离富集和电化学测定痕量的汞离子可以同时同步进行。试验结果表明CV+I−/MWCNTs修饰电极对汞离子有较高的选择性、灵敏度和稳定性。详见表1。
2.3. 金属螯合物共沉淀载体修饰
铜铁试剂是一种重要的螯合剂,具有吸附性和电活性[25,26],萘酚属于惰性共沉淀剂。我们在实验“聚铜铁试剂和萘酚/金纳米复合物修饰电极测定痕量铜”[27]中,基于纳米掺杂技术,采用循环伏安法制备了聚铜铁试剂和萘酚/金纳米复合物修饰电极(co-poly (cupferron and β-naphthol)/GNPs/GCE),研究了铜离子在该修饰电极上的电化学行为,并提出了一种新的用于检测铜离子的方法。基于修饰电极表面的有机配体铜铁试剂(CPf)对Cu2+螯合选择性,首先得到螯合物Cu(Ⅱ)-Cpf,然后通过有机共沉淀原理,电极表面的有机惰性载体萘酚(β-naphthol)将该螯合物诱导吸附到修饰电极表面,达到分离富集痕量Cu2+作用,在一定电位窗口测定电化学信号。铜离子在聚铜铁试剂和萘酚/金纳米复合物修饰电极上的作用机理如下:
界面:
Cu(Ⅱ)(溶液) + Cpf(电极表面) → Cu(Ⅱ)-Cpf (螯合)
诱导吸附:
Cu(Ⅱ)-Cpf + β-naphthol(载体) →
Cu(Ⅱ)-Cpf·β-naphthol(被共沉淀的化合物)
电化学反应:Cu(Ⅱ)-Cpf + e– → Cu(I) + Cpf(还原)
Cu(I) – e– → Cu(Ⅱ)(氧化)
在最优的实验条件,铜离子的阳极氧化峰电流与其浓度在9.0 × 10−10 M ~5.0 × 10−8 M和5.0 × 10−8 M~ 1.5 × 10−6 M范围内呈良好的线性关系,相关系数分别为0.9865和0.9948,检出限为5.0 M × 10−11 M (S/N = 3)。而且该修饰电极可以避免Cd2+(100),Ag+(500),Fe3+(200),Pb2+(100),Cr3+(100),Zn2+(500),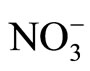 (500),Cl−(500),SO42−(500),EDTA(500)等物质的干扰。实验结果表明,该电极对铜离子有较高的选择性,灵敏度和稳定性。详见表2。
(500),Cl−(500),SO42−(500),EDTA(500)等物质的干扰。实验结果表明,该电极对铜离子有较高的选择性,灵敏度和稳定性。详见表2。
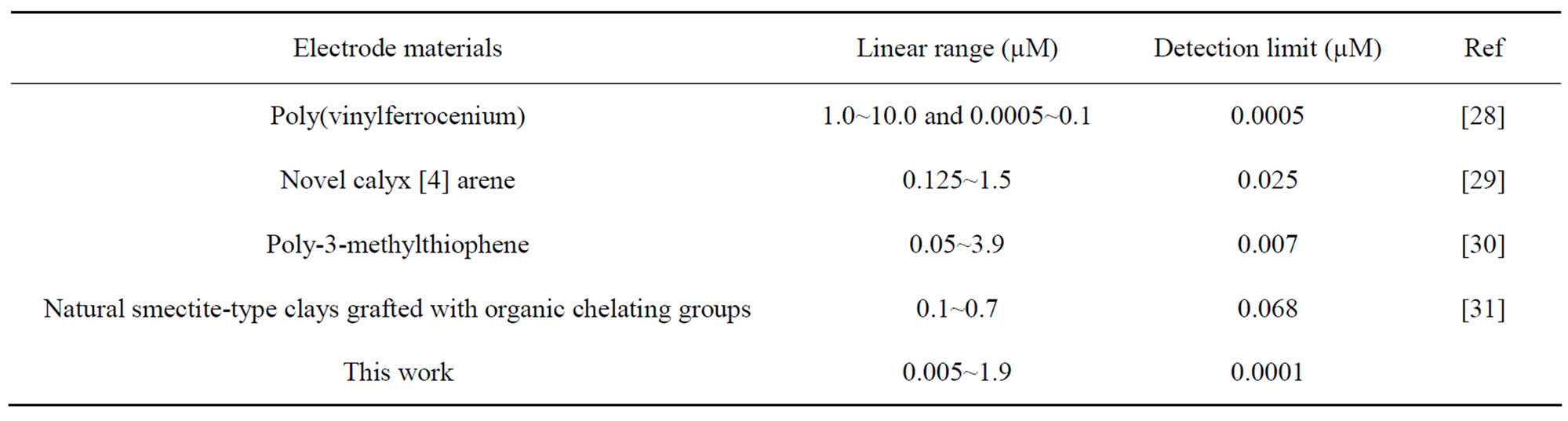
Table 1. Comparison of the responses for mercury based on different modified electrode
表1. 不同修饰电极对汞离子测定结果比较

Table 2. Comparison of the responses for copper based on different modified electrode
表2. 不同修饰电极对铜离子测定结果比较
3. 结论与展望
运用纳米技术修饰的电化学共沉淀法很有发展前景,实现了将有机共沉淀化学原理应用到电化学分析中,以及对痕量重金属离子的分离、富集和电化学分析测定同步进行的优势。对有机共沉淀剂的选择络合性、吸附性、电催化活性[35]的研究仍有发展空间。如基于铜铁试剂能与高价金属离子络合吸附形成催化循环[26]和纳米材料的独特的性质[36],利用化学修饰电极共沉淀法测定痕量钼(Mo6+)正在研究中,该研究的特点在于催化作用。
4. 致谢
感谢国家自然科学基金(20675001,20901003,21005001)、安徽省教育厅基金资助项目(No.2006kj145B)、安徽省自然科学基金资助项目(KJ2009B013Z)和安徽师范大学化学与材料科学学院。
参考文献 (References)
[1] X. X. Zhu, A. M. Kriegel, C. A. Boustany, et al. Single-Chain variable fragment (scFv) antibodies optimized for environmental analysis of uranium. Analytical Chemistry, 2011, 83(10): 3717- 3724.
[2] G. Aragay, J. Pons and A. Merko. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy metal detection. Chemical Reviews, 2011, 111(5): 3433-3458.
[3] H. Tokuyama, T. Iwama. Temperature-swing solid-phase extraction of heavy metals on a poly(N-isopropylacrylamide) hydrogel. Langmuir, 2007, 23(26): 13104-13108.
[4] A. O. W. Leung, N. S. Duzgoren-Aydin, K. C. Cheung, et al. Heavy metals concentrations of surface dust from e-waste recycling and its human health implications in southeast China. Environmental Science and Technology, 2008, 42(7): 2674-2680.
[5] T. Chen, X. M. Liu, X. Li, et al. Heavy metal sources identification and sampling uncertainty analysis in a field-scale vegetable soil of Hangzhou, China. Environmental Pollution, 2009, 157(3): 1003-1010.
[6] M. B. Arain, T. G. Kazi, J. A. Baig, et al. Determination of arsenic levels in water, sediment and foodstuff from selected area of sindh, pakistan: Estimation of daily dietary intake. Food and Chemical Toxicology, 2009, 47(1): 242-248.
[7] S. Seoyoun, J. Jyongsik. Thiol containing polymer encapsulated magnetic nanoparticles as reusable and efficiently separable adsorbent for heavy metal ions. Chemical Communications, 2007, 17(6): 4230-4232.
[8] E. Margui, K. V. Meel, R. V. Grieken, et al. Method of the determination of Pd-catalyst residues in active pharmaceutical ingredients by means of high-energy polarized-beam energy dispersive X-Ray fluorescence. Analytical Chemistry, 2009, 81(4): 1404-1410.
[9] O. Chailapakul, S. Korsrisakul, W. Siangproh, et al. Fast and simultaneous detection of heavy metals using a simple and reliable microchip-electrochemistry route: An alternative approach to food analysis. Talanta, 2008, 74(4): 683-689.
[10] G. W. Li, L. H. Zhang, Z. W. Li, et al. PAR immobilized colorimetric fiber for heavy metal ion detection and adsorption. Journal of Hazardous Materials, 2010, 177(3): 983-989.
[11] 李启隆. 有机试剂在极谱吸附波中的应用[J]. 冶金分析, 1991, 11(2): 27-29.
[12] 张军红, 刘道杰. 有机试剂在络合吸附波中的应用[J]. 冶金分析, 2003, 23(6): 18-23.
[13] H. Ju, D. Leech. Electrochemical study of a metallothionein modified gold disk electrode and its action on Hg2+ cations. Journal of Electroanalytical Chemistry, 2000, 484(1): 150-152.
[14] Y. Oztekin, Z. Yazicigil, A. Ramanaviciene and A. Ramanavicius. Anodic stripping voltammetric determination of copper(II) usinga functionalized carbon nanotubes paste electrode modified with crosslinked chitosan. Sensors and Actuators B: Chemical, 2010, 151(1): 1-12.
[15] L. Jiang, Y. Wang, J. W. Ding, et al. An ionophore-Nafion modified bismuth electrode for the analysis of cadmium(II). Electrochemistry Communications, 2010, 12(3): 202-205.
[16] X. S. Ren, Q. H. Xu. Highly sensitive and selective detection of mercury ions by using oligonucleotides, DNA intercalators, and conjugated polymers. Langmuir, 2009, 25(1): 29-31.
[17] Y. Liu, P. Liang, Guo L. Nanometer titanium dioxide immobilized on silica gel as sorbent for preconcentration of metal ions prior to their determination by inductively coupled plasma atomic emission spectrometry. Talanta, 2005, 68(1): 25-30.
[18] J. Toh, X. B. Zuo, H. N. Wu, et al. Mechanism of mercury detection based on interaction of single-strand DNA and hybridized DNA with gold nanoparticles. Talanta, 2010, 82(6): 1642-1646.
[19] B. W. Yang, Q. J. Gong, L. P. Zhao, et al. Development of a threestage system for the treatment and reclamation of wastewater containing nano-scale particles. Desalination, 2011, 5(1): 1-5.
[20] L. T. Jin, H. Xu, L. P. Zeng, et al. Microwave-irradiated synthesized platinum nanoparticles/carbon nanotubes for oxidative determination of trace arsenic(III). Electrochemistry Communications, 2008, 10 (3): 551-554.
[21] M. R. Ganjali, N. Motaker-Kazami, F. Faridbod, et al. Separation and preconcentration of trace level of lead in one drop of blood sample by using graphite furnace atomic absorption spectrometry. Journal of Hazardous Materials, 2010, 173(3): 415-419.
[22] H. Zhao, Y. Jiang, Y. R. Ma, et al. Poly(2-amino-4-thiazoleacetic acid)/multiwalled carbon nanotubes modified glassy carbon electrodes for the electrochemical detection of copper(II). Electrochimica Acta, 2010, 55(6): 2518-2521.
[23] S. J. Xing, H. Xu, J. S. Chen, et al. Nafion stabilized silver nanoparticles modified electrode and its application to Cr(VI) detection. Journal of Electronalytical Chemistry, 2011, 652(1): 60-65.
[24] B. Fang, M. Liu, G. F. Wang, C. H. Zhang, et al. The study on Separating, enriching and determination of trace amount of Hg2+ synchronously. Analytical Methods, 2011, 3(5): 865-871.
[25] J. G. Lawless, M. D. Hawley. Electrochemical oxidation of cupferron. Analytical Chemistry, 1968, 40(6): 948-951.
[26] 李启隆, 胡劲波. 铜铁试剂在络合吸附波中的应用[J]. 冶金分析, 1994, 14(1): 39-43.
[27] M. Liu, Y. H. Feng, C. H. Zhang, et al. Electrochemical determination of copper (Ⅱ) using Co-poly (Cupferron and β-naphthol)/ gold nanoparticles modified glassy carbon electrodes. Analytical Methods, 2011, 3(6): 1440-1446.
[28] M. Sonmez, C. H. Ozyoruk, A. Yildiz, et al. Determination of Hg2+ on poly(vinylferrocenium) (PVF+)-modified platinum electrode. Talanta, 2009, 78(3): 405-409.
[29] J. Q. Lu, X. W. He, X. S. Zeng, et al. Voltammetric determination of mercury (II) in aqueous media using glassy carbon electrodes modified with novel calyx [4] arene. Talanta, 2003, 59(3): 553-560.
[30] H. Zejli, P. Sharrock, J. L. Cisneros, et al. Voltammetric determination of trace mercury at a sonogel-carbon electrode modified with poly-3-methylthiophene. Talanta, 2005, 68(1): 79-81.
[31] I. K. Tonle, E. Ngameni and A. Walcarius. Preconcentration and voltammetric analysis of mercury (II) at a carbon paste electrode modified with poly-3-methylthiophene. Talanta, 2005, 68(1): 79- 81.
[32] M. Etiene, J. Bessiere and A. Wlcarius. Voltammetric detection of copper at a carbon paste electrode containing an organically modified silica. Sensors and Actuators B: Chemical, 2001, 76(2): 531-538.
[33] B. C. Janegitz, L. H. Marcolino-Junior, S. P. Campana-Filh, et al. Anodic stripping voltammetric determination of copper (II) using a functionalized carbon nanotubes paste electrode modified with crosslinked chitosan Sensors and Actuators B: Chemical, 2009, 142(1): 260-266.
[34] M. Lin, M. Cho, W. S. Choe, et al. Electrochemical detection of copper ion using a modified copolythiophen electrode. Electrochimica Acta, 2009, 54(6): 7012-7017.
[35] S, Mustafa, D. Ayse. Determination of some heavy metals in food and environmental samples by flame atomic absorption spectrometry after coprecipitation. Food Chemical Toxicology, 2011, 49(6): 1242-1248.
[36] R. Sonia, G. Ana, G. Edgar, et al. Use of CeO2, TiO2 and Fe3O4 nanoparticles for the removal of lead from water: Toxicity of nanoparticles and derived compounds. Desalination, 2011, 277 (1): 213-220.