Journal of Sensor Technology and Application
Vol.03 No.03(2015), Article ID:15657,7
pages
10.12677/JSTA.2015.33004
Construction and Evaluation of NADH Biosensor Based on Ion-Exchange Behavior between Nafion and Thionine
Yue Shui1, Yan Ji1, Yujia Liang1, Qinghe Wei1, Yue Tang1, Xiang Gao2, Bin Qi1*
1Faculty of Chemistry, Northeast Normal University, Changchun Jilin
2Institute of Genetics and Cytology, Northeast Normal University, Changchun Jilin
*通讯作者。
Email: *qib219@nenu.edu.cn
Received: Jun. 18th, 2015; accepted: Jul. 8th, 2015; published: Jul. 14th, 2015
Copyright © 2015 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



ABSTRACT
Thionine/OMC/GCE was prepared by ion-exchange procedure. Electrochemical behaviors of the resulting electrode were investigated thoroughly with cyclic voltammetry, and a well-defined redox couple was clearly visualized. Under a lower operation potential of 0 V in 0.1 mol∙L−1 PBS (pH 7.0), NADH could be detected linearly up to a concentration of 1 × 10−6 mol∙L−1 to 6 × 10−4 mol∙L−1 with an extremely lower detection limit of 1 × 10−7 mol∙L−1 estimated (S/N = 3). The feasibility for simultaneous determination of uric acid, dopamine, AP and NADH was discussed. They did not interfere with each other in certain concentration. Based on the results, a new NADH sensor was successfully established using the PTH/OMC/GCE.
Keywords:Ordered Mesoporous Carbon, Electrocatalysis, Thionine, NADH

基于Nafion与硫堇离子交换行为的NADH生物传感器的构建与性能评价
税月1,吉艳1,梁羽佳1,魏倾鹤1,唐悦1,高翔2,齐斌1*
1东北师范大学化学学院,吉林 长春
2东北师范大学生命科学学院,吉林 长春
Email: *qib219@nenu.edu.cn
收稿日期:2015年6月18日;录用日期:2015年7月8日;发布日期:2015年7月14日

摘 要
以Nafion为离子交换材料,通过离子交换的方法制备了硫堇(Thionine, TH)/有序介孔碳(Ordered mesoporous carbon, OMC)复合材料修饰玻碳电极(Glassy carbon electrode, GCE),用电化学方法对该修饰电极进行了详细表征,在循环伏安曲线上可以观察到硫堇的一对可逆的氧化还原峰,证明硫堇已经被成功的固定到电极表面;对修饰电极的动力学研究表明电极过程是受表面控制。利用此修饰电极,0 V电位下即可以实现对烟酰胺腺嘌呤二核苷酸(Nicotinamide adenine dinucleotide, NADH)的安培检测,线性范围可达1 × 10−6 mol∙L−1~6 × 10−4 mol∙L−1,检出限达到1 × 10−7 mol∙L−1 (信噪比为3),并且能很好的消除尿酸、多巴胺以及对乙酰氨基酚(p-acetamidophenol, AP)的干扰,有希望成为有应用价值的NADH传感器。
关键词 :有序介孔碳,电催化,硫堇,NADH

1. 引言
烟酰胺腺嘌呤二核苷酸广泛存在于一切动物、植物和微生物的活细胞中,是目前已知的300多种脱氢酶的辅酶,也是许多生物氧化还原电子传递中的重要物质,但是通常它在电极上的氧化具有较大的过电位[1] [2] ,而且其氧化产物易在电极表面吸附而引起电极钝化,这使NADH的直接电化学测量十分困难。为了克服这一难题,人们提出了以醌类、氧化还原染料、金属配合物等作为电子传递媒介体修饰电极的方法来加速NADH的电化学氧化过程,从而降低其过电位[3] -[7] 。
吩噻嗪类染料可以介导一些生物大分子和一些简单无机离子在电极上的电化学氧化、还原过程的进行,其中以硫堇作为电子媒介体可以用包埋、交联剂的方式修饰到电极表面,同时也可采用电化学的方法将硫堇聚合到电极表面形成聚合物膜修饰电极,已经报道的聚硫堇修饰电极一般都直接在电极表面进行[8] -[13] 。对于以硫堇作为电子媒介体催化NADH已经有了一些报道,蔡称心等人[14] 以含硫堇的乙腈溶液为电解质在微带金电极上聚合硫堇,制备成的聚硫堇微带金电极可有效的催化NADH;Gao Qiang等人[15] 将硫堇聚合到screen-printed电极表面,用来检测NADH最低检测线达到3 μM;Huang Minghua等人[16] 将CNTs/Nafion修饰电极浸泡在硫堇溶液中,得到了硫堇修饰的CNTs/Nafion电极,此电极检测NADH的线性范围为2~400 μM,最低检出限达1 μM。
有序介孔碳本身是很好的良导体,具有较好的导电性,这使其成为一种很新颖的电极材料[17] -[21] 。将有序介孔碳与电化学相结合,研究生物活性物质的电催化过程是一项非常有应用前景的研究工作[22] [23] 。介孔材料的结构和组成千变万化,不同的介孔材料可以适用于不同的客体的组装。同时,通过调变客体的种类以及组装的方法,在同一主体介孔材料中也可以组装不同的客体物质:这些客体物质可以表现出多种多样的化学物理性质。其中染料分子与介孔材料可形成的主客体复合物,有望为介孔材料开辟新的应用领域。有序介孔碳特有的组成与结构,加之高的比表面积、有序的孔径分布,不但有利于传质,更易于主客体组装,使每单位物质有非常高的表面积和高浓度的活性点[24] [25] 。现在对于有序介孔碳的研究多集中于基于有序介孔碳的电池或电容器[25] ,很少有基于有序介孔碳电极对物质电分析及电催化研究的报道,因此对有序介孔碳及其复合材料的电化学及电催化性质的研究是非常有意义的。
鉴于有序介孔碳的上述优点我们已经成功的将其应用于电化学催化研究中[26] -[29] ,并在此基础上将硫堇用离子交换的方法修饰到有序介孔碳修饰电极表面,制备了硫堇/有序介孔碳复合材料修饰电极,实现了有序介孔碳与染料分子的主客体组装。该电极不仅具有有序介孔碳的特性并且含有硫堇这种电子媒介体,应用于NADH的电化学催化显示出了良好的性能,将电位降低到0 V,线性范围为1 × 10−6 mol∙L−1~6 × 10−4 mol∙L−1,检出限达到1 × 10−7 mol∙L−1。这是首次将硫堇以离子交换的方式与有序介孔碳形成复合材料来构建NADH生物传感器,制备简单易行,性能优于传统的电极,有希望成为一种新型的NADH电化学传感器。
2. 实验部分
2.1. 试剂与仪器
烟酰胺腺嘌呤二核苷酸(NADH),硫堇(TH)均购于Aldrich-Sigma公司,有序介孔碳(OMC)由本实验室提供,其它试剂均为分析纯。实验用水为二次蒸馏水,由Milli-Q型石英亚沸高纯水蒸馏器蒸出(Millipore, Bedford, MA)。实验均在室温下进行。
0.1 mol∙L−1,pH = 7.0的磷酸缓冲液的配制:首先,用蒸馏水分别配制0.1 mol∙L−1的磷酸二氢钠和磷酸氢二钠溶液,再取两种溶液适量,用PH计调节混合溶液的pH为7.0,最后装入试剂瓶备用;实验均在PBS溶液中进行。
电化学实验采用CHI
2.2. 硫堇的结构
硫堇(Thionine),分子式为C12H10ClN3S,结构式如图1所示。
2.3. 传感器的制备
玻碳电极(GCE,直径
将OMC/GCE浸入含1 mM硫堇(TH)的DMF溶液中,40 min后取出,再在纯的DMF溶液中浸泡15 min,然后用二次蒸馏水洗净,自然晾干,得到硫堇/有序介孔碳复合材料修饰电极(TH/OMC/GCE),待用。
3. 结果与讨论
3.1. TH/OMC修饰电极电在缓冲溶液中的循环伏安行为
图2为TH/OMC修饰电极表面在0.1 mol∙L−1 PBS (pH 7.0)中的循环伏安图。如图所示,当扫描时出现了一对可逆的氧化还原峰,为硫堇发生电化学反应所引起的氧化还原峰,这对峰的出现证明了硫堇已成功的固定在电极表面上。当扫描16圈后此对峰的峰电流基本不再发生变化,且经过16圈循环伏安扫描后此TH/OMC修饰电极已经稳定,因此我们每次制备此修饰电极时,都在0.1 mol∙L−1 PBS中用循环伏安扫描16圈后再使用。
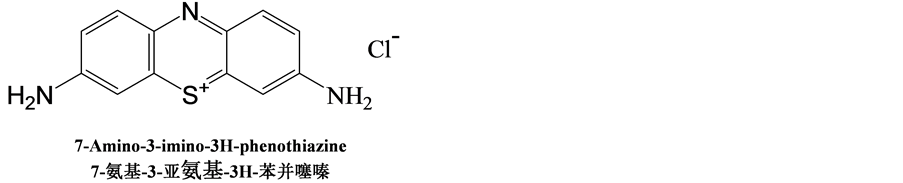
Figure 1. Structure of thionine
图1. 硫堇的结构
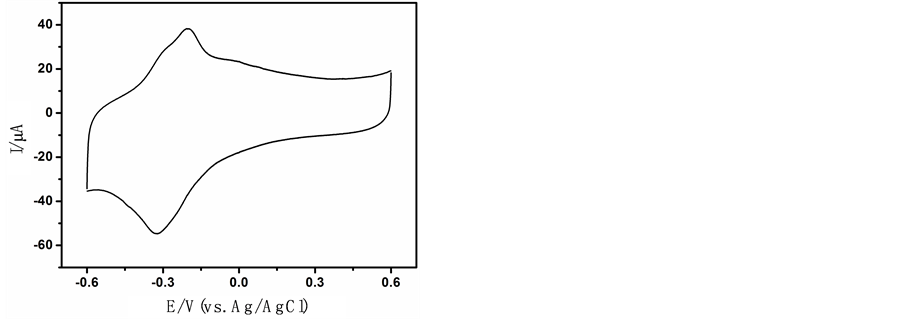
Figure 2. CV of TH/OMC/GCE in 0.1 mol/L PBS (pH = 7.0), scan rate: 100 mv/s
图2. TH/OMC修饰电极在0.1 mol/L PBS (pH = 7.0)中的循环伏安曲线,扫描速度为100 mv/s
3.2. TH/OMC修饰电极的动力学研究
我们通过CV法对TH/OMC/GCE进行了动力学研究,结果发现随着扫描速度v的增大,峰电流(Ip)和峰峰电位差(DEp)都增加(图3)。
由图4可知,Ip与v成线性关系,表明电极过程是受表面控制的。
3.3. TH/OMC修饰电极催化氧化NADH
图5实线/虚线为各种电极在有/没有1 mM NADH (0.1 mol∙L−1, pH 7.0 PBS)中的循环伏安曲线,扫描速度为50 mV/s。如图所示,在Nafion/GCE上,NADH在0.63 V处出现一个氧化峰。当用Nafion/OMC和TH/Nafion/GCE分别修饰电极后NADH的氧化峰峰电位负移到0.25 V和0.05 V处,这说明Nafion/OMC可以有效的降低NADH的过电位。当用硫堇修饰的OMC修饰电极在此溶液中进行循环伏安扫描时,我们可以观察到与此电极在空白PBS溶液中相比较,硫堇的氧化峰电流明显增大,这说明此修饰电极对NADH有较好的催化能力,将NADH的过电位降低到了0 V。由此可知,在OMC/GCE表面引入TH后,该电极将有序介孔碳和硫堇相结合,兼具了有序介孔碳的特点和TH的电子媒介作用,对NADH表现出良好的的催化作用。
3.4. TH/OMC修饰电极对NADH的计时电流响应
由图5(d)可知TH的氧化峰电流随NADH的加入而增加,所以我们选择0 V作为工作电位对NADH进行安培检测。图6为TH/OMC/GCE在0 V的工作电位下对NADH的计时电流曲线。随着NADH的加入,TH/OMC/GCE在较短的时间内可迅达到稳定。TH/OMC/GCE检测NADH的线性范围为1 × 10−6 mol∙L−1~6 × 10−4 mol∙L−1,检出限达到1 × 10−7 mol∙L−1 (信噪比为3)。
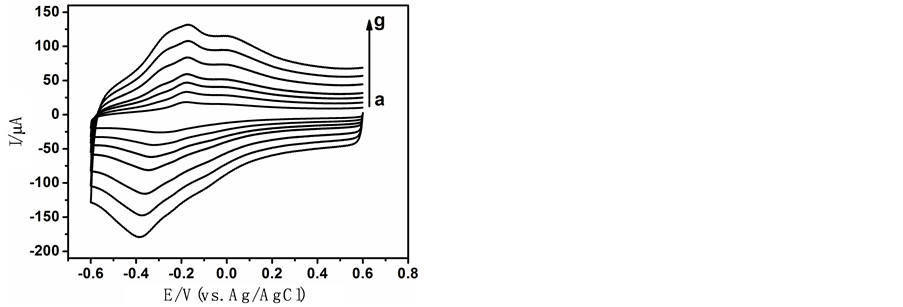
Figure 3. CVs of TH/OMC/GCE in 0.1 mol/L PBS (pH = 7.0) with different scan rates (a: 50 mv/s. b: 100 mv/s. c: 150 mv/s. d: 200 mv/s. e: 300 mv/s. f: 400 mv/s. g: 500 mv/s)
图3. TH/OMC修饰电极在不同扫描速度下的循环伏安图。扫描速度从a到g分别为a: 50 mv/s. b: 100 mv/s. c: 150 mv/s. d: 200 mv/s. e: 300 mv/s. f: 400 mv/s. g: 500 mv/s

Figure 4. The relationship of scan rate and peak current
图4. 扫描速度与电流关系图
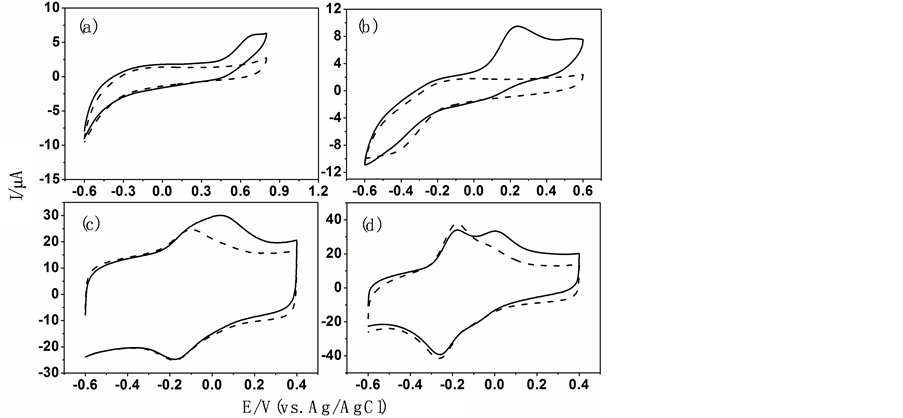
Figure 5. CVs obtained on Nafion/GCE (a), TH/Nafion/GCE (b), Nafion/OMC/GCE (c) and TH/Nafion/OMC/GCE (d), in the absence (dotted line) and presence ( solid line) of
图5. 在0.1 mol∙L−1 PBS (pH 7.0)中的循环伏安图。(a) Nafion/GCE;(b) TH/Nafion/GCE;(c) Nafion/OMC/GCE;(d) TH/Nafion/OMC/GCE。实线,有NADH;虚线,没有NADH
3.5. 干扰试验
如图7所示,对TH/OMC/GCE进行NADH检测的干扰试验,待背景电流稳定后分别加入
3.6. TH/OMC修饰电极的重现性和稳定性
用该TH/OMC/GCE电极对NADH (浓度为1 mM)的PBS (0.1 mol∙L−1, pH 7.0)溶液重复测定8次,相对标准偏差为2.1%,表明该电极有良好的重现性。同时将此电极对NADH (浓度为1 mM)进行连续测定15天(每天平均测两次,不使用时将电极保存在4℃的冰箱中),其响应电流在前10天的偏差 < 5%,15天后对NADH的响应电流仍为初始电流的80% (图8),这表明该电极具有良好的稳定性。
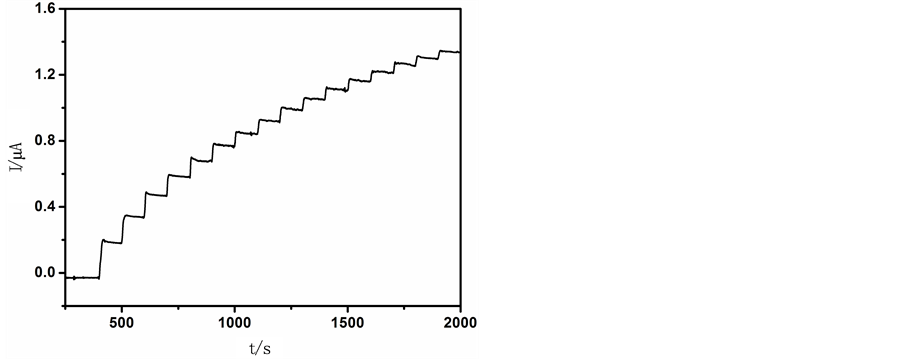
Figure 6. Current-time curves obtained at TH/OMC/GCE to successive addition of NADH into
图6. NADH的计时电流图,工作电位0 V
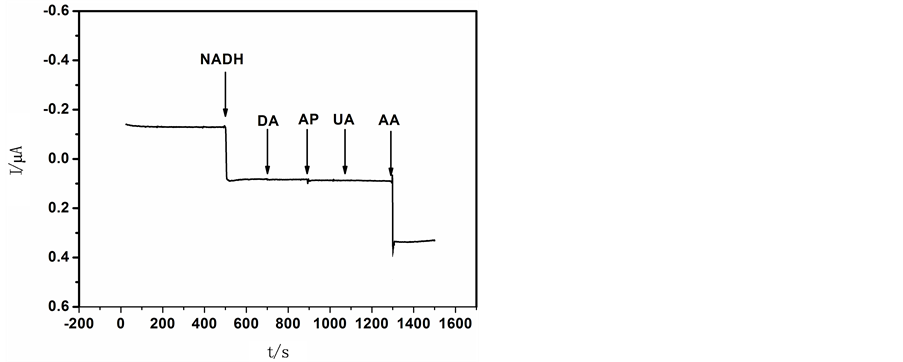
Figure 7. Effect of
图7. NADH、DA、AP、UA、AA的计时电流响应图。
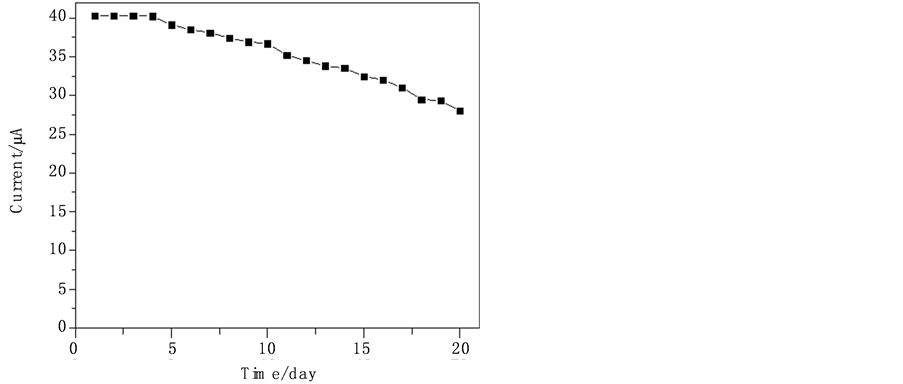
Figure 8. Current-time curve obtained from continuously detection of
图8. 对
4. 结论
在本工作中,我们用离子交换法将硫堇修饰到有序介孔碳修饰电极表面,进而制备出了一种新型的TH/OMC修饰电极,这种电极不仅具有有序介孔碳的特性,而且还兼有硫堇电子媒介体的性质。实验表明,TH/OMC复合材料修饰电极能明显降低NADH的过电位,对NADH的检测线性范围为1 × 10−6 mol∙L−1~6 × 10−4 mol∙L−1,检出限达到1 × 10−7 mol∙L−1。并且在检测过程中可以消除多巴胺、AP和尿酸干扰。此外,该修饰电极稳定性、重现性好,具有实际应用价值。
致谢
本文获得吉林省科技厅科技发展计划项目(20130102003JC),国家科技计划项目政策引导类科技计划国际科技合作计划(2014DFA31740),国家大学生创新创业训练计划项目资助。
文章引用
税 月,吉 艳,梁羽佳,魏倾鹤,唐 悦,高 翔,齐 斌, (2015) 基于Nafion与硫堇离子交换行为的NADH生物传感器的构建与性能评价
Construction and Evaluation of NADH Biosensor Based on Ion-Exchange Behavior between Nafion and Thionine. 传感器技术与应用,03,25-32. doi: 10.12677/JSTA.2015.33004
参考文献 (References)
- 1. Jaegfeldt, H. (1980) Adsorption and electrochemical oxidation behaviour of NADH at a clean platinum electrode. Journal of Electroanalytical Chemistry, 110, 295-302. http://dx.doi.org/10.1016/S0022-0728(80)80381-0
- 2. Moiroux, J. and Elving, P.J. (1978) Effects of adsorption, electrode material, and operational variables on the oxidation of dihydronicotinamide adenine dinucleotide at carbon electrodes. Analytical Chemistry, 50, 1056-1062. http://dx.doi.org/10.1021/ac50030a015
- 3. Alvarez, G.M.I., Saidman, S.B. and Jesus, L.C. (2000) Electrocatalytic detection of NADH and glycerol by NAD+- modified carbon electrodes. Analytical Chemistry, 72, 520-527. http://dx.doi.org/10.1021/ac9908344
- 4. Grundig, B., Wittstock, G. and Rudel, U. (1995) Mediator-modified electrodes for electrocatalytic oxidation of NADH. Journal of Electroanalytical Chemistry, 395, 143-157. http://dx.doi.org/10.1016/0022-0728(95)04090-B
- 5. Jena, B.K. and Raj, C.R. (2005) Efficient electrocatalytic oxidation of NADH at gold nanoparticles self-assembled on three-dimensional sol-gel network. Chemical Communica-tions, 15, 2005-2007.
- 6. Mano, N. and Kuhn, A. (1999) Immobilized nitro-fluorenone derivatives as electrocatalysts for NADH oxidation. Journal of Electroanalytical Chemistry, 477, 79-88. http://dx.doi.org/10.1016/S0022-0728(99)00393-9
- 7. Wu, Q., Maskus, M. and Pariente, F. (1996) Electrocata-lytic oxidation of NADH at glassy carbon electrodes modified with transition metal complexes containing 1,10-phenanthroline-5,6-dione ligands. Analytical Chemistry, 68, 3688- 3696. http://dx.doi.org/10.1021/ac960395y
- 8. Akers, N.L., Moore, C.M. and Minteer, S.D. (2005) Development of alcohol/O2 biofuel cells using salt-extracted tetrabutylammonium bromide/Nafion membranes to immobilize dehydro-genase enzymes. Electrochimica Acta, 50, 2521- 2525. http://dx.doi.org/10.1016/j.electacta.2004.10.080
- 9. Ensafi, A.A. (2003) Determination of ascorbic acid by electrocatalytic voltammetry with methylene blue. Analytical Letters, 36, 591-604. http://dx.doi.org/10.1081/AL-120018250
- 10. Muhtanu, F.D., Marcus, M. and Albert, S. (2002) Fast-scan cyclic voltammetry and scanning electrochemical microscopy studies of the pH-dependent dissolution of 2-electron mediators immobilized on zirco nium phosphate containing carbon pastes. Electroanalysis, 14, 1479-1487. http://dx.doi.org/10.1002/1521-4109(200211)14:21<1479::AID-ELAN1479>3.0.CO;2-T
- 11. Maria, J.L. and Miranda, A.J. (1997) Amperometric biosensors based on NAD(P)-dependent dehydrogenase enzymes. Electroanalysis, 9, 191-202. http://dx.doi.org/10.1002/elan.1140090302
- 12. 周颖琳, 胡玉姣, 曾涌淮 (2002) 血红蛋白在双十二烷基铵-聚乙烯硫酸盐多双层复合薄膜电极上的电化学与电催化. 分析化学, 30, 262-266.
- 13. Razola, S.S., Ruizb, L. and Diez, N.M. (2002) Hydrogen peroxide sensitive ampermetric biosensor based on horseradish peroxidase entrapped polypyrrole eletrode. Biosensors and Bioelectronics, 17, 921-928. http://dx.doi.org/10.1016/S0956-5663(02)00083-0
- 14. 蔡称心, 鞠熀先, 陈洪渊 (1995) 聚硫堇修饰微带金电极的性质及对NADH的催化氧化. 高等学校化学学报, 16, 368-372.
- 15. Gao, Q., Cui, X.Q. and Yang, F. (2003) Preparation of poly(thionine) modified screen-printed carbon electrode and its application to determine NADH in flow injection analysis system. Biosensors and Bioelectronics, 19, 277-282. http://dx.doi.org/10.1016/S0956-5663(03)00212-4
- 16. Huang, M.H., Jiang, H., Zhai, J.F., et al. (2007) A simple route to incorporate redox mediator into carbon nanotubes/ Nafion composite film and its application to determine NADH at low potential. Talanta, 74, 132-139. http://dx.doi.org/10.1016/j.talanta.2007.05.042
- 17. Lei, C. and Lisdat, F. and Wollenberger, U. (1999) Cytochrome c/clay-modified electrode. Electroanalysis, 11, 274- 276. http://dx.doi.org/10.1002/(SICI)1521-4109(199904)11:4<274::AID-ELAN274>3.0.CO;2-G
- 18. Yu, J.H. and Ju, H.X. (2002) Preparation of porous titania sol-gel matrix for immobilization of horseradish peroxidase by a vapor depo-sition method. Analytical Chemistry, 74, 3579-3583. http://dx.doi.org/10.1021/ac011290k
- 19. Fan, C., Zhuang, Y. and Li, G. (2000) Direct electrochemistry and enhanced catalytic Activity for hemoglobin in a sodium montmorillonite film. Electroanalysis, 12, 1156-1158. http://dx.doi.org/10.1002/1521-4109(200010)12:14<1156::AID-ELAN1156>3.0.CO;2-4
- 20. Sallez, Y., Bianco, P. and Lojou, E. (2000) Electrochemical behavior of c-type cytochromes at clay-modified carbon electrodes: A model for the interaction between proteins and soils. Journal of Electroanalytical Chemistry, 493, 37-49. http://dx.doi.org/10.1016/S0022-0728(00)00280-1
- 21. Walcarius, A., Mandler, D.L. and Cox, J.A. (2005) Ex-citing new directions in the intersection of functionalized sol-gel materials with electrochemistry. Journal of Materials Chemistry, 15, 3663 -3689. http://dx.doi.org/10.1039/b504839g
- 22. Lee, G. and Pyun, S. (2006) Effect of mi-crocrystallite structures on electrochemical characteristics of mesoporous carbon electrodes for electric double-layer capacitors. Electrochimica Acta, 51, 3029-3038. http://dx.doi.org/10.1016/j.electacta.2005.08.037
- 23. Furukawa, H., Hibino, M. and Zhou, H. (2003) Synthesis of mesoporous carbon-containing ferrocene derivative and its electrochemical property. Chemistry Letters, 42, 132-133. http://dx.doi.org/10.1246/cl.2003.132
- 24. Vartufi, J.C., Kresge, C.T. and Leonowicz, M.E. (1994) Synthesis of mesoporous materials: Liquid-crystal templating versus intercalation of layered silicates. Chemical Material, 6, 2070-2077. http://dx.doi.org/10.1021/cm00047a029
- 25. Walcarius, A. (2005) Impact of mesoporous silica-based materials on electrochemistry and feedback from electrochemical science to the characterization of these ordered materials. Comptes Rendus Chimie, 8, 693-712. http://dx.doi.org/10.1016/j.crci.2004.10.003
- 26. Zhou, M., Guo, L.P. and Lin, F.Y. (2007) Electrochemistry and electrocatalysis of polyoxometalate-ordered mesoporous carbon modified electrode. Analytica Chimica Acta, 587, 124-131. http://dx.doi.org/10.1016/j.aca.2007.01.017
- 27. Zhou, M., Guo, L.P., Ding, J. and Shang, Q.K. (2007) Electrochemical behavior of L-cysteine and its detection at ordered mesoporous carbon-modified glassy carbon electrode. Analytical Chemistry, 79, 5328-5335. http://dx.doi.org/10.1021/ac0703707
- 28. Zhou, M., Guo, L.P., Hou, Y. and Peng, X.J. (2008) Immobilization of Nafion-ordered mesoporous carbon on a glassy carbon electrode: Application to the detection of epinephrine. Electrochim Acta, 53, 4176-4184. http://dx.doi.org/10.1016/j.electacta.2007.12.077
- 29. Ndamanisha, J.C., Guo, L.P. and Wang, G. (2007) Meso-porous carbon functionalized with ferrocenecarboxylic acid and its electrocatalytic properties. Microporous and Me-soporous Materials, 113, 114-121. http://dx.doi.org/10.1016/j.micromeso.2007.11.009