Journal of Organic Chemistry Research
Vol.04 No.04(2016), Article ID:19131,7
pages
10.12677/JOCR.2016.44013
Study on the Condensation Reaction of Hydrazide with Benzylideneacetophenone Catalyzed by Phosphotungstic Acid
Yang Wu, Xuejian Xing, Liuzhuang Xing, Yadong Hou, Jinghui Yang, Yonghai Hui*
College of Chemistry and Chemical Engineering, Xinjiang University, Urumqi Xinjiang
Email: *hyhai97@126.com
Received: Nov. 1st, 2016; accepted: Nov. 28th, 2016; published: Dec. 2nd, 2016
Copyright © 2016 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



ABSTRACT
The condensation of hydrazide with benzylideneacetophenone was studied by using phosphotungstic acid as catalyst. After a series of reaction conditions, the optimal reaction conditions were established, and the universality of the substrate was investigated. A series of acylhydrazones were obtained with the high yields, up to 99%. The reaction was simple and mild, which provided a new method for the synthesis of chalcone hydrazone.
Keywords:Heteropoly Acid, Condensation Reaction, Acylhydrazone

磷钨酸促进的酰肼与查尔酮缩合反应的研究
吴阳,邢雪建,邢刘桩,侯亚东,杨敬辉,惠永海*
新疆大学化学化工学院,新疆 乌鲁木齐
Email: *hyhai97@126.com
收稿日期:2016年11月1日;录用日期:2016年11月28日;发布日期:2016年12月2日

摘 要
本文以杂多酸–磷钨酸为催化剂,对酰肼与查尔酮的缩合反应进行了研究。经过一系列反应条件的筛选,确立了最佳反应条件,并对底物的普适性进行了考察,得到了一系列高产率的酰腙类目标产物,最高产率达到99%。本反应操作简单,条件温和,为查尔酮酰腙合成提供了一种新的方法。
关键词 :杂多酸,缩合反应,酰腙

1. 引言
酰腙是一类含有−CONHN = CH−基团的人工合成的化合物,通过酰肼与醛或酮缩合反应制得。因其分子结构中含有亚胺基(−CH = N−)故又属于席夫碱。在生物活性体系中体现出突出的抗菌、抗真菌、抗癌、脲酶抑制、抗氧化和抗糖化等良好的生理活性 [1] - [7] 。另外,酰腙类化合物与过渡金属、稀土金属等有着很强的配位能力,可以衍生出很多具有较高生物活性的金属配合物 [8] [9] [10] 。所以,在农药、医药、催化、分析和材料等方面有着广泛应用 [11] [12] [13] [14] [15] ,多年来一直备受人们的广泛关注。也引起了很多化学和生物学工作者们的极大兴趣,成为越来越活跃的研究领域之一。
本文以苯甲酰肼和查尔酮为原料,通过条件筛选得到最佳反应条件。在最佳条件下,合成了一系列收率较好的查尔酮苯甲酰腙衍生物。为合成酰腙的衍生物寻找一种简单的合成方法。
2. 实验部分
2.1. 试剂与仪器
薄层层析硅胶用GF254硅胶和300-400目柱层析硅胶(青岛海洋化工厂)。常见的显色方式有:ZF-2型三用紫外仪,碘缸,酸性溶液,茚三酮等,熔点是由X-4数字显示显微熔点仪测定。元素分析用EA-1110元素分析仪测定。核磁共振是有VARIAN INOVA-400型核磁共振波谱仪测定,核磁氢谱的内标为TMS (δ = 0.00),核磁碳谱的内标为CDCl3 (δ = 77.00)。常用试剂:石油醚、乙酸乙酯、甲醇、无水乙醇和二氯甲烷等分析纯试剂是由市售购买而来,未经处理直接使用。苯甲醛、苯乙酮、取代芳香醛、取代芳香酮和芳香胺等是购买于阿拉丁化学厂家,其中对有些不纯的底物在做反应时经过了纯化。
2.2. α,β-不饱和酮的合成
α,β-不饱和酮的合成参照文献 [16] 。
2.3. 目标化合物4a~4q的合成及结构分析
化合物4a~4q的合成反应如图1所示。以化合物4a为例,向反应管中依次加入查尔酮0.0208 g (0.10 mmol),
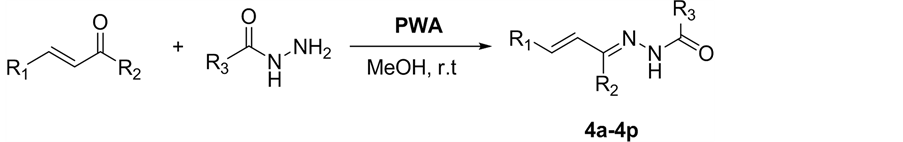
Figure 1. Synthesis of hydrazone derivatives of 1,3-diphenylallylidene)benzohydrazide (4a-4p)
图1. 查尔酮苯甲酰腙衍生物(4a~4p)的合成
苯甲酰肼0.0204 g (0.15 mmol),磷钨酸0.0042 g (0.15 mmol%),0.5 mL甲醇,在室温反应24 h,TLC跟踪反应情况,反应完毕后减压浓缩,得粗产物,经柱层析分离纯化,得到白色固体(洗脱液为V1(石油醚):V2(乙酸乙酯) = 1:30,1:20,1:10,1:5)。目标化合物的表征如下:
4a: (Z)-Nˊ-((E)-1,3-diphenylallylidene) benzohydrazide, White solid; 96% yield; m.p. 154~157˚C; 1H NMR (400 MHz, CDCl3): δ 8.98 (s, 1H), 7.66~7.27 (m, 15H), 6.42 (d, J = 16.4 Hz, 1 H). Anal. Calcd. (%) for C22H18N2O:C, 80.96; H, 5.56; N, 8.59. Found (%): C, 81.07; H, 5.52; N, 8.49.
4b: (Z)-Nˊ-((E)-1-(4-chlorophenyl)-3-phenylallylidene)benzohydrazide, Yellow solid; 92% yield; m.p. 161-163˚C; 1H NMR (400 MHz, CDCl3):δ 8.89(s, 1 H), 7.58~7.26 (m, 14 H), 6.38 (d, J = 16.4 Hz, 1 H). Anal. Calcd. (%) for C22H17ClN2O:C, 73.23; H, 4.75; N, 7.76. Found (%): C, 73.44; H, 4.71; N, 7.69.
4c: (Z)-Nˊ-((E)-1-(4-bromophenyl)-3-phenylallylidene) benzohydrazide, White solid; 94% yield; m.p. 173-175˚C; 1H NMR (400 MHz, CDCl3):δ 8.89 (s, 1 H), 7.87~7.19 (m, 14 H), 6.37 (d, 1 H, J = 16.8 Hz). Anal. Calcd. (%) for C22H17BrN2O: C, 65.20; H, 4.23; N, 6.91. Found (%): C, 65.37; H, 4.19; N, 6.84.
4d: (Z)-Nˊ-((E)-3-phenyl-1-(p-tolyl) allylidene) benzohydrazide, Yellow oil; 83% yield; 1H NMR (400 MHz, CDCl3): δ 9.03 (s, 1 H), 7.56 (d, J = 7.2 Hz, 1 H), 7.48~7.38 (m, 10 H ), 7.34~7.27 (m, 2 H), 6.44 (d, J = 16.2 Hz, 1H), 2.49 (s, 3 H).
4e: (Z)-Nˊ-((E)-1-(4-methoxyphenyl)-3-phenylallylidene) benzohydrazide, Yellow oil; 90% yield; 1H NMR (400 MHz, CDCl3): δ 9.07 (s, 1 H), 7.59-7.57 (m, 2 H), 7.52-7.25 (m, 10 H), 7.15-7.13 (m, 2 H), 6.46 (d, J = 16.4 Hz, 1 H), 3.90 (s, 3 H). Anal. Calcd. (%) for C23H20N2O2: C, 77.51; H, 5.66; N, 7.86. Found (%): C, 77.75; H, 5.57; N, 7.73.
4f: (Z)-Nˊ-((E)-1-(3-chlorophenyl)-3-phenylallylidene) benzohydrazide, White solid; 75% yield; m.p. 122-124˚C; 1H NMR (400 MHz, CDCl3): δ 8.88 (s, 1 H), 7.99~7.83 (m, 2 H), 7.74~7. 57 (m, 3 H), 7.53~7.26 (m, 8 H), 7.28~7.26 (m, 1 H), 6.40 (d, J = 16 Hz, 1 H). Anal. Calcd. (%) for C22H17ClN2O: C, 73.23; H, 4.75; N, 7.76. Found (%): C, 73.38; H, 4.74; N, 7.71.
4g: (Z)-Nˊ-((E)-3-phenyl-1-(m-tolyl)allylidene) benzohydrazide, Yellow oil; 67% yield; 1H NMR (400 MHz, CDCl3): δ 9.01 (s, 1 H), 7.59 (d, J = 7.2 Hz, 1 H), 7.42-7.38 (m, 10 H ), 7.30~7.24 (m, 2 H), 6.42 (d, J = 16.6 Hz, 1 H), 2.42 (s, 3 H). Anal. Calcd. (%) for C23H20N2O: C, 81.15; H, 5.92; N, 8.23. Found(%): C, 81.26; H, 5.85; N, 8.17.
4h: (Z)-Nˊ-((E)-3-(4-fluorophenyl)-1-phenylallylidene) benzohydrazide, White solid; 85% yield; m.p. 114~116˚C; 1H NMR (400 MHz, CDCl3): δ 8.98 (s, 1 H), 7.98~7.88 (m, 1 H), 7.74~7.27 (m, 9 H), 7.12~6.99 (m, 2 H), 6.38 (d, J = 16.4 Hz, 1 H), 2.42 (s, 3 H). Anal. Calcd.(%) for C22H17FN2O: C, 76.73; H, 4.98; N, 8.13. Found (%):C, 76.88; H, 4.74; N, 8.21.
4i: (Z)-Nˊ-((E)-3-(4-chlorophenyl)-1-phenylallylidene)benzohydrazide A White solid; 87% yield; m.p. 118~121˚C; 1H NMR (400 MHz, CDCl3): δ 8.99 (s, 1 H), 8.01~7.94 (m, 1 H), 7.74~7.58 (m, 2 H), 7.53~7.52 (m, 9 H), 6.37 (d, J = 16.4 Hz, 1 H). Anal. Calcd.(%) for C22H17ClN2O: C, 73.23; H, 4.75; N, 7.76. Found(%): C, 73.41; H, 4.66; N, 7.62.
4j: (Z)-Nˊ-((E)-3-(4-bromophenyl)-1-phenylallylidene) benzohydrazide, Yellow solid; 65% yield; m.p. 112~114˚C; 1H NMR (400 MHz, CDCl3): δ 8.99 (s, 1 H), 7.65-7.62 (m, 3 H), 7.55~7.53 (m, 2 H), 7.50~7.44 (m, 3 H), 7.39-7.33 (m, 4 H), 7.27~7.25 (m, 2 H), 6.35 (d, J = 16.8 Hz, 1 H). Anal. Calcd. (%) for C22H17BrN2O: C, 65.20; H, 4.23; N, 6.91. Found (%): C, 65.34; H, 4.12; N, 6.88.
4k: (Z)-Nˊ-((E)-3-(4-methoxyphenyl)-1-phenylallylidene) benzohydrazide, Yellow solid; 88% yield; m.p. 106~109˚C; 1H NMR (400 MHz, CDCl3): δ 8.94 (s, 1 H), 7.65~7.46 (m, 6 H), 7.38~7.27 (m, 6 H), 6.86 (d, J = 8.8 Hz, 2 H), 6.37 (d, J = 16 Hz, 1 H), 3.86 (s, 3H). Anal. Calcd. (%) for C23H20N2O2: C, 77.51; H, 5.66; N, 7.86. Found (%): C, 77.68; H, 5.59; N, 7.78.
4l: (E)-Nˊ-((E)-4-(4-methoxyphenyl)but-3-en-2-ylidene)benzohydrazide, White solid; 92% yield; m.p. 202~204˚C; 1H NMR (400 MHz, CDCl3) δ: 9.01 (s, 1 H), 7.85 (s, 2 H), 7.55-7.36 (m, 5 H), 7.16-7.01 (m, 2 H), 6.99~6.88 (m, 2 H), 3.84 (s, 3 H), 2.18 (s, 3 H). 13C NMR (100 MHz, CDCl3) δ: 165.21, 161.20, 153.58, 134.42, 133.04, 129.90, 127.87, 128.49, 127.53, 126.76, 114.47, 55.36, 29.34. MS (ESI m/z) 317.1 [(M + Na+, 100%)]. Anal. Calcd. (%) for C18H18N2O2: C, 73.45; H, 6.16; N, 9.52. Found (%): C, 73.52; H, 6.09; N, 9.39.
4m: (Z)-4-chloro-Nˊ-((E)-1,3-diphenylallylidene)benzohydrazide, Yellow solid; 99% yield; m.p. 175~176˚C; 1H NMR (400 MHz, CDCl3) δ: 8.91 (s, 1 H), 8.02 (s, 1 H), 7.65~7.60 (m, 3 H), 7.46-7.39 (m, 5 H), 9.34~7.26 (m, 5 H), 7.23 (s, 1 H), 7.43 (d, J = 16 Hz, 1 H). Anal. Calcd. (%) for C22H17ClN2O: C, 73.23; H, 4.75; N, 7.76. Found (%): C, 73.36; H, 4.68; N, 7.65.
4n: (Z)-4-bromo-Nˊ-((E)-1,3-diphenylallylidene) benzohydrazide, Yellow solid; 98% yield; m.p. 124~126˚C; 1H NMR (400 MHz, CDCl3) δ: 8.91 (s, 1 H), 8.02 (s, 1 H), 7.71~7.55 (m, 3 H), 7.53~7.50 (m, 2 H), 7.44~7.27 (m, 8 H), 6.43 (d, J = 16.4 Hz, 1 H). Anal. Calcd. (%) for C22H17BrN2O: C, 65.20; H, 4.23; N, 6.91. Found (%): C, 65.33; H, 4.18; N, 6.82.
4o: (Z)-Nˊ-((E)-1,3-diphenylallylidene)-4-methoxybenzohydrazide, Yellow solid; 78% yield; m.p. 204~207˚C; 1H NMR (400 MHz, CDCl3) δ: 8.92 (s, 1 H), 7.66-7.57 (m, 3 H), 7.52-7.49 (m, 2 H), 7.44-7.39 (m, 3 H), 7.35~7.27 (m, 4 H), 6.86 (d, J = 6 Hz, 2 H), 6.40 (d, J = 16.4 Hz, 1 H). Anal. Calcd. (%) for C23H20N2O2: C, 77.51; H, 5.66; N, 7.86. Found (%): C, 77.61; H, 5.53; N, 7.69.
4p: (Z)-2-chloro-Nˊ-((E)-1,3-diphenylallylidene) benzohydrazide, White solid; 86% yield; m.p. 163~165˚C; 1H NMR (400 MHz, CDCl3) δ: 9.26 (s, 1 H), 7.87~7.84 (m, 1 H), 7.60~7.52 (m, 4 H), 7.47~7.36 (m, 3 H), 7.32-7.29 (m, 6 H), 6.91 (d, J = 16.4 Hz, 1 H). Anal. Calcd.(%) for C22H17ClN2O: C, 73.23; H, 4.75; N, 7.76. Found(%): C, 73.41; H, 4.57; N, 7.59.
4q: (Z)-Nˊ-((E)-1,3-diphenylallylidene)-2-methylbenzohydrazide, Yellow oil; 85% yield; 1H NMR (400 MHz, CDCl3) δ:8.57 (s, 1 H), 7.60~7.51 (m, 3 H), 7.46~7.24 (m, 9 H), 7.21~7.13 (m, 2 H), 6.40 (d, J = 16.4 Hz, 1 H). Anal. Calcd. (%) for C23H20N2O: C, 81.15; H, 5.92; N, 8.23. Found (%): C, 81.31; H, 5.79; N, 8.12.
3. 结果与讨论
3.1. 最优反应条件的筛选
以查尔酮与苯甲酰肼反应为标准反应,分别进行了催化剂种类和用量、反应溶剂种类和用量、底物配比和反应时间等条件进行了优化,结果见表1。
从表1中可以看出,在没有加入催化剂时,反应不发生(表1,Entry 1);用杂多酸磷钨酸和磷钼酸分别催化时,磷钨酸表现出了较好的产率(表1,Entry 2);当用MCM-41固载的磷钨酸(磷钼酸)催化反应时,产率有所降低,所以我们选定磷钨酸作为催化剂。然后进行了催化剂的量筛选,实验结果表明磷钨酸量为0.15 mmol%产率最高。在对反应溶剂筛选时,发现甲醇作为溶剂,反应产率最高,89%。为了得到更高的产率,随后考察了其它溶剂对产率的影响。结果表明其它溶剂没有醇类溶剂的效果好,而在醇类溶剂中,反应产率依然在甲醇中得到最高。确定上述反应条件后,我们对底物比例进行了考察,分别对底物查尔酮:苯甲
Table 1. Optimization of reaction conditionsa
表1. 反应条件的优化a
a反应条件:查尔酮0.1 mmol,酰肼0.15 mmol催化剂量为0.15 mmol%在0.5 mL甲醇中室温反应24 h。b柱层析产率。cN.R = No Reaction。
酰肼为1:1,1:1.2,1:1.5,1:2等比例下进行了筛选,结果见表1的Entries 14-17。从表中可以看到,随着酰肼量的增加,产率有所上升,在1:1.5时,达到96%的产率,当继续增加酰肼的量(比例为1:2)时,产率有所下降,所以最有底物比例为1:1.5。实验在常温条件下进行,这属于理想反应条件范畴。最后对反应时间进行了考察,结果列于表1的Entries 17-20。反应中,当反应时间延长到24 h时,反应产率得到最高值96%,继续延长反应时间,产率处于下降趋势。通过对实验条件的筛选,最佳反应条件为:室温下以0.15 mmol%的磷钨酸为催化剂,0.5 mL甲醇为溶剂,底物配比(查尔酮:苯甲酰肼)为1:1.5,反应24 h。
3.2. 底物结构对反应的影响
在最佳反应条件下,对底物进行了普适性的研究,结果详见表1。
从表2中,可以看到R2上的取代基无论是吸电子基团还是供电子基团,都能够很好地得到相应的目
Table 2. Substrate scopea
表2. 底物结构的拓展a
a反应条件:查尔酮0.1 mmol,酰肼0.15 mmol催化剂量为0.004 g在0.5 mL甲醇中室温反应24 h。b柱层析产率。
标产物;当同种取代基苯环上的位置不同时,其产率也有很大的变化,而且对位取代的产率要高于间位取代,如R2,氯取代对位时的产率要高于其间位取代(Entries 2, 6),对甲基比间甲基的产率高(Entries 4, 7)。对于R1苯环上的取代基,除了Br取代产率较低外,其它产率都能达到85%以上。在酰肼R3取代基的改变中,从表中可以看出,对位和邻位取代的酰肼都获得了较高的产率。
4. 结论
本文研究了查尔酮和酰肼的缩合反应。通过优化实验,最终得出了最优反应条件:0.004 g磷钨酸为催化剂、底物配比为1:1.5(查尔酮:酰肼),甲醇为溶剂,室温下反应24 h。在该反应条件下,获得了一系列高产率的酰腙类目标产物,最高产率达到99%。本反应具有反应条件温和,催化剂廉价易得等优点。
基金项目
国家自然科学基金(Nos. 21161026, 21362036)。
文章引用
吴 阳,邢雪建,邢刘桩,侯亚东,杨敬辉,惠永海. 磷钨酸促进的酰肼与查尔酮缩合反应的研究
Study on the Condensation Reaction of Hydrazide with Benzylideneacetophenone Catalyzed by Phosphotungstic Acid[J]. 有机化学研究, 2016, 04(04): 93-99. http://dx.doi.org/10.12677/JOCR.2016.44013
参考文献 (References)
- 1. Rollas, S. and Küçükgüzel, S.G. (2007) Biological Activities of Hydrazone Derivatives. Molecules, 12, 1910-1939. https://doi.org/10.3390/12081910
- 2. Vicini, P., Zani, F., Cozzini, P. and Doytchinova, I. (2002) Hydrazones of 1, 2-Benzisothiazole Hydrazides: Synthesis, Antimicrobial Activity and QSAR Investigations. European Journal of Medicinal Chemistry, 37, 553-564. https://doi.org/10.1016/S0223-5234(02)01378-8
- 3. Jayabharathi, J., Thangamani, A., Padmavathy, M. and Krishnakumar, B. (2007) Synthesis and Microbial Evaluation of Novel n (1)-arilidene-n (2)-t (3)-methyl-r(2), c (6)-diaryl-piperidin-4-one Azine Derivatives. Medicinal Chemistry Research, 15, 431-442. https://doi.org/10.1007/s00044-006-0014-0
- 4. Ragavendran, J.V., Sriram, D., Patel, S.K., Reddy, I.V., Bharathwajan, N. and Stables, J. (2007) Design and Synthesis of Anticonvulsants from a Combined Phthalimide-Gaba-Anilide and Hydrazone Pharmacophore. European Journal of Medicinal Chemistry, 42, 146-151. https://doi.org/10.1016/j.ejmech.2006.08.010
- 5. El-Hawash, S.A.M., Wahab, A.E.A. and El-Demellawy, M.A. (2006) Cyanoacetic Acid Hydrazones of 3-(and 4-)acetylpyridine and Some Derived Ring Systems as Potential Antitumor and Anti-HCV Agents. Archiv Der Pharmazie, 339, 14-23. https://doi.org/10.1002/ardp.200500161
- 6. Todeschini, A.R., Miranda, A.L.P.D., Silva, K.C.M.D., Parrini, S.C. and Barreiro, E.J. (1998) Synthesis and Evaluation of Analgesic, Antiinflammatory and Antiplatelet Properties of New 2-Pyridylarylhydrazone Derivatives. European Journal of Medicinal Chemistry, 33, 189-199. https://doi.org/10.1016/S0223-5234(98)80008-1
- 7. Nath, M., Vats, M. and Roy, P. (2013) Tri- and Diorganotin(iv) Complexes of Biologically Important Orotic Acid: Synthesis, Spectroscopic Studies, In Vitro Anti-Cancer, DNA Fragmentation, Enzyme Assays and In Vivo Anti-Inflammatory Activities. European Journal of Medicinal Chemistry, 59, 310-321. https://doi.org/10.1016/j.ejmech.2012.11.023
- 8. Despaigne, A.A.R., Costa, F.B.D., Piro, O.E., Castellano, E.E., Louro, S.R.W. and Beraldo, H. (2012) Complexation of 2-Acetylpyridine- and 2-Benzoylpyridine-Derived Hydrazones to Copper (ii) as an Effective Strategy for Antimicrobial Activity Improvement. Polyhedron, 38, 285-290. https://doi.org/10.1016/j.poly.2012.03.017
- 9. Xu, Z.H., Zhang, X.W., Zhang, W.Q., Gao, Y.H. and Zeng, Z.Z. (2011) Synthesis, Characterization, DNA Interaction and Antibacterial Activities of Two Tetranuclear Cobalt (ii) and Nickel (ii) Complexes with Salicylaldehyde 2-Phenylquinoline-4-Carboylhydrazone. Inorganic Chemistry Communications, 14, 1569-1573. https://doi.org/10.1016/j.inoche.2011.06.005
- 10. El-Sayed, L., Iskander, M.F., Hawash, N.M. and Massoud, S.S. (1998) Synthesis and Characterization of Nickel(ii), Zinc(ii), Copper(ii), Cobalt(ii) and Cobalt(iii) complexes of α-Dicarbonylbis(aroylhydrazone). Polyhedron, 17, 199-206. https://doi.org/10.1016/S0277-5387(97)00191-5
- 11. Lal, R.A., Adhikari, S., Pal, A., Siva, A.N. and Ku-mar, A. (1997) Synthesis and Characterization of the Homobimetallic [bis(2-hydroxy-1-naphthaldehyde)oxaloyldihydrazonato]bis(dioxomolybdenum(vi)) Tetrahydrate Complex and Its Reactivity towards Proton and Electron Donor Reagents. Journal of Chemical Research, 4, 122-123. https://doi.org/10.1039/a506810j
- 12. Aboraia, A.S., Yee, S.W., Gomaa, M.S., Shah, N., Robotham, A.C. and Makowski, B. (2010) Synthesis and CYP24AL Inhibitory Activity of N-(2-(1H-imidazol-1-yl)-2-phenylethyl)arylamides. Bioorganic & Medicinal Chemistry, 18, 4939-4946. https://doi.org/10.1016/j.bmc.2010.06.011
- 13. Fatmawati, S., Kondo, R. and Shimizu, K. (2013) Structure-Activity Relationships of Lanostane-Type Triterpenoids from Ganoderma Lingzhi, as α-Glucosidase Inhibitors. Bioorganic & Medicinal Chemistry Letters, 23, 5900-5903. https://doi.org/10.1016/j.bmcl.2013.08.084
- 14. Ma, J., Shi, W., Feng, L., Chen, Y., Fan, K. and Hao, Y. (2016) A Highly Selective and Sensitive Acylhydrazone- Based Turn-On optical Sensor for Al3+. RSC Advances, 6, 28034-28037. https://doi.org/10.1039/C6RA01589A
- 15. Hu, J.H., Li, J.B., Qi, J. and Sun, Y. (2015) Acylhydrazone Based Fluorescent Chemosensor for Zinc in Aqueous Solution with High Selectivity and Sensitivity. Sensors & Actuators B Chemical, 208, 581-587. https://doi.org/10.1016/j.snb.2014.11.066
- 16. 李在国, 王清民, 黄君珉. 有机中间体制备[M]. 第二版. 北京: 化学工业出版社, 1996: 51.
