Hans Journal of Ophthalmology
Vol.05 No.04(2016), Article ID:19176,10
pages
10.12677/HJO.2016.54017
The Effect of Arsenic Trioxide on the Expression of Telomerase Reverse Transcriptase mRNA in ACC-2 Cells of Adenoid Cystic Carcinoma
Tao Jiang1*, Jing Jiang2, Yang Zhou1, Renping Wang3, Yi Wang4, Lihua Xiao4
1Department of Ophthalmology, The Affiliated Hospital of Qingdao University, Qingdao Shandong
2The Infectious Diseases Department, Eastern Branch of the Affiliated Hospital of Qingdao University, Qingdao Shandong
3Health Examination Center, The Affiliated Hospital of Qingdao University, Qingdao Shandong
4Institute of Orbital Disease, General Hospital of Armed Police Forces, Beijing

Received: Nov. 1st, 2016; accepted: Dec. 6th, 2016; published: Dec. 9th, 2016
Copyright © 2016 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



ABSTRACT
AIM: To investigate the effect of As2O3 on the expression of telomerase reverse transcriptase mRNA in ACC-2 cells. METHODS: ACC-2 cells were cultured. The different drug concentration gradient (0, 1.0, 2.0, 4.0, 8.0 μmol/L) of As2O3 were applied to ACC-2 cells for 24 h and 48 h respectively. The hTERT mRNA expression of ACC-2 cells before and after As2O3’s induction was detected by RT-PCR. RESULTS: RT-PCR results showed that hTERT mRNA expression was significantly higher in the control group, while after As2O3 action to ACC-2 cell for 24 h and 48 h, with the increase of As2O3 concentration (1.0 μmol/L, 2.0 μmol/L, 4.0 μmol/L, 8.0 μmol/L), hTERT mRNA expression decreased gradually (P < 0.01). CONCLUSION: As2O3 can induce apoptosis of ACC-2 cells by downregulating hTERT mRNA transcript expression.
Keywords:Arsenic Trioxide, Adenoid Cystic Carcinoma ACC-2 Cells, Apoptosis, hTERT mRNA

三氧化二砷对腺样囊性癌ACC-2细胞的端粒酶逆转录酶mRNA表达的影响
姜涛1*,姜靖2,周杨1,王仁萍3,王毅4,肖利华4
1眼科,青岛大学附属医院,山东 青岛
2东区感染科,青岛大学附属医院,山东 青岛
3健康查体中心,青岛大学附属医院,山东 青岛
4北京武警总医院眼眶病研究所,北京

收稿日期:2016年11月1日;录用日期:2016年12月6日;发布日期:2016年12月9日

摘 要
目的:探讨As2O3对ACC-2细胞端粒酶hTERT mRNA表达的影响。方法:进行ACC-2细胞培养,将As2O3建立不同药物浓度梯度(0、1.0、2.0、4.0、8.0 μmol/L)分别作用于ACC-2细胞24 h,48 h,应用RT-PCR方法,检测As2O3作用前后,ACC-2细胞的hTERT mRNA表达水平。结果:RT-PCR结果显示,在未经药物作用、作为对照组的正常培养的ACC-2细胞中,hTERT mRNA呈现明显高表达;在As2O3作用于ACC-2细胞后24 h与48 h时,随As2O3药物浓度的增高(1.0 μmol/L、2.0 μmol/L、4.0 μmol/L、8.0 μmol/L),hTERT mRNA表达逐渐降低(P < 0.01)。结论:As2O3可下调ACC-2细胞的hTERT mRNA转录表达,来诱导ACC-2细胞凋亡。
关键词 :三氧化二砷(As2O3),腺样囊性癌ACC-2细胞,凋亡,端粒酶hTERT mRNA

1. 引言
腺样囊性癌(adenoid cystic carcinoma, ACC)为恶性肿瘤,其特征为缓慢地局部浸润性生长,且很容易局部复发与远处转移 [1] 。ACC恶性程度比较高,属上皮性肿瘤,可发生于全身各处,于眼眶部好发于泪腺 [2] [3] 。泪腺ACC的平均发病年龄为40岁左右,女性稍多见,是一种浸润性极强预后很差的肿瘤,很难根治,多数会局部复发,死亡率较高,其15年存活率不到20% [4] 。ACC的临床治疗一直以来就是一个难点,化疗的反应率不高,有针对性的靶向治疗可能是一种选择 [5] ,寻找有效的化疗药物成为临床基础研究的当务之急。
三氧化二砷(arsenic trioxide, As2O3)是最古老的毒物之一,无臭无味,外观为白色霜状粉末,故称砒霜。作为抗肿瘤药物,在治疗急性早幼粒细胞白血病方面已较为成熟,尤其对于复发病例 [6] [7] [8] 。另外,还可用于肝癌 [9] 、肺癌 [10] 等的治疗。对于ACC的治疗目前仅限于实验室的研究中 [11] 。
本研究旨在探询端粒酶hTERT-mRNA表达对As2O3诱导人腺样囊性癌ACC-2细胞凋亡的影响,为临床应用提供可靠的理论依据。端粒(telomere)是真核细胞染色体末端的特殊结构,可稳定染色体的功能,防止染色体DNA降解、末端融合,保护染色体结构基因DNA,调节正常细胞生长。人端粒是由6个碱基重复序列(TTAGGG)和结合蛋白组成。正常细胞由于线性DNA复制5’末端消失,随着体细胞不断增殖,端粒逐渐缩短,当细胞端粒缩至一定程度,细胞停止分裂,处于静止状态。Counter CM称端粒为正常细胞的“有丝分裂钟”(mitotic clock) [12] ,端粒长短和稳定性决定了细胞寿命,并与细胞衰老和癌变密切相关。端粒酶(telomerase)是使端粒延伸的反转录DNA合成酶,是由RNA和蛋白质组成的核糖核酸-蛋白复合物,其中RNA为模板,蛋白具有催化活性,以端粒3’末端为引物,合成端粒重复序列。主要特征是用自身携带的RNA作模板,以dNTP为原料,通过逆转录催化合成模板链5’端DNA片段或外加重复单位。端粒酶的活性在真核细胞中可检测到,其功能是合成染色体末端的端粒,使因每次细胞分裂而逐渐缩短的端粒长度得以补偿,进而稳定端粒长度。值得注意的是,恶性肿瘤细胞具有高活性的端粒酶,能维持癌细胞端粒的长度,使其无限制扩增。而正常体细胞的增殖能力则非常有限,其端粒越来越短,端粒酶的活性不高。在端粒结合蛋白质方面,人端粒酶蛋白质部分的催化亚基编码基因也已经被克隆鉴定,命名为人端粒酶逆转录酶(human Telomerase Reverse Transcriptase, hTERT)基因。该基因含有一个端粒酶特异基序(telomerase-specific motif),翻译48个氨基酸的蛋白质序列。hTERT基因仅在肿瘤细胞——永生化的(immortal)细胞中表达,因此,hTERT基因更显示出肿瘤特异性诊断和治疗的潜在应用价值 [13] [14] 。
我们前面的研究已经表明,As2O3作用于ACC-2细胞,可通过降低线粒体膜电位从而引起细胞凋亡 [15] 。本部分实验我们针对端粒酶hTERT mRNA表达对As2O3诱导人腺样囊性癌ACC-2细胞凋亡的影响进行研究,用RT-PCR检测端粒酶hTERT亚基的表达,从端粒酶角度对As2O3诱导腺样囊性癌ACC-2细胞凋亡可能的分子机制进行研究。
2. 材料与方法
2.1. 实验标本、主要试剂、实验仪器
2.1.1. 实验标本
腺样囊性癌ACC-2细胞株由青岛大学附属医院中心实验室提供,经细胞复苏,RPMI-1640 + 10%胎牛血清培养液常规细胞培养,2~3天换液或传代一次,取指数生长期细胞用于实验。
2.1.2. 主要试剂
注射用三氧化二砷(Arsenic Trioxide for Injection):北京双鹭药业股份有限公司。
PrimeScript RT-PCR试剂盒:日本Takara公司。
引物合成:由上海生物工程公司合成。
2.1.3. 主要实验仪器
PCR仪GeneAMP PCR System 9600:美国PE公司。
Vilber Lourmat凝胶成像系统:法国VILBER LOURMAT公司。
2.2. 实验分组
用50 ml (25 cm2)培养瓶培养ACC-2细胞,分为对照组(0 μmol/L As2O3处理组)、1.0 μmol/L、2.0 μmol/L、4.0 μmol/L和8.0 μmol/L As2O3处理组,分别培养24 h、48 h。
2.3. RT-PCR检测端粒酶hTERT mRNA表达的实验步骤
2.3.1. 总RNA抽提
用不含小牛血清的RPMI 1640培养液洗涤收集的ACC-2细胞2次,计数5 × 106细胞,加入Eppendorf管,离心沉淀后加Trizol试剂1 ml,抽提总RNA。
2.3.2. cDNA链合成
按逆转录试剂盒说明操作。终体积20 μl,其中总RNA 4 μl,随机引物1 μl,5 × buffer 4 μl,核糖核酸酶抑制剂(RNasin) 1 μl,10 mmol/L dNTP 2 μl,鼠白血病病毒反转录酶(M-MuLVRT) 1 μl,ddH2O 7 μl。PCR扩增仪上运行:25℃ 10分钟,42℃ 60分钟,70℃ 10分钟;4℃保存。
2.3.3. PCR
1) 引物:hTERT和作为内参照的hGAPDH PCR反应寡核苷酸引物由上海Sangon合成。
2) PCR反应体系为50 μl,在200 μl反应管中加入以下试剂
3) PCR仪GeneAMP PCR System 9600:美国PE公司。
4) PCR反应程序如下
5) Agarose电泳及结果分析
取PCR扩增产物5 μl在1.5%琼脂凝胶(含0.5 μg/ml溴化乙锭),90 V电压下电泳20~30 min,用Vilber Lourmat凝胶成像系统进行目的基因表达水平分析。
将电泳后的琼脂糖凝胶置于琼脂糖凝胶自动图像分析仪下进行扫描,然后用Quantity one图像分析软件对电泳图进行定量分析,将所得出的各电泳条带光密度值与同一样品所测出的内参电泳条带的光密度值OD进行比较。
2.4. 统计学处理
采用SPSS19.0统计学软件处理数据。数据用 表示,显著性检验水准α = 0.05,P < 0.05有统计学意义,组间比较用方差分析。
表示,显著性检验水准α = 0.05,P < 0.05有统计学意义,组间比较用方差分析。
3. 结果
RT-PCR结果显示,在未经药物作用、作为对照组的正常培养的ACC-2细胞中,端粒酶hTERT亚基的mRNA呈现明显高表达;在As2O3作用于ACC-2细胞后24 h与48 h时,随As2O3药物浓度的增高(1.0 μmol/L、2.0 μmol/L、4.0 μmol/L、8.0 μmol/L),端粒酶hTERT亚基的mRNA表达逐渐降低(图1,图2)。
以hTERT基因表达的相对光密度值代表hTERT基因的表达强度。不同浓度、处理时间hTERT mRNA表达量差异有显著统计学意义(24 h,F = 322.38,P < 0.01;48 h,F = 1730.23,P < 0.01;表1)。
 (M:Marker 0:对照组1:1.0 μmol/L As2O3组,2:2.0 μmol/L As2O3组,4:4.0 μmol/L As2O3组,8:8.0 μmol/L As2O3组) (左图:hTERT;右图:GAPDH)
(M:Marker 0:对照组1:1.0 μmol/L As2O3组,2:2.0 μmol/L As2O3组,4:4.0 μmol/L As2O3组,8:8.0 μmol/L As2O3组) (左图:hTERT;右图:GAPDH)
Figure 1. Expression levels of hTERT mRNA in ACC-2 cells before and after As2O3 treatment (24 h)
图1. As2O3作用前、后(24 h)的ACC-2细胞hTERT的mRNA表达水平
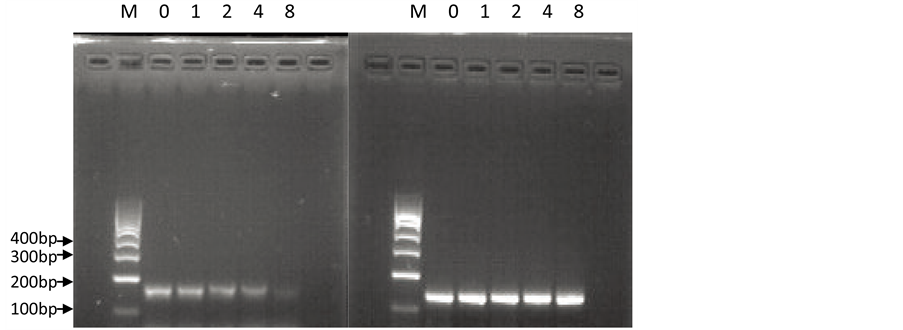 (M:Marker 0:对照组 1:1.0 μmol/L As2O3组, 2:2.0 μmol/L As2O3组, 4:4.0 μmol/L As2O3组,8:8.0 μmol/L As2O3组) (左图:hTERT;右图:GAPDH)
(M:Marker 0:对照组 1:1.0 μmol/L As2O3组, 2:2.0 μmol/L As2O3组, 4:4.0 μmol/L As2O3组,8:8.0 μmol/L As2O3组) (左图:hTERT;右图:GAPDH)
Figure 2. Expression levels of hTERT mRNA in ACC-2 cells before and after As2O3 treatment (48 h)
图2. As2O3作用前、后(48 h)的ACC-2细胞hTERT的mRNA表达水平
Table 1. Expression of hTERT mRNA in different concentration and treatment time ()
表1. 不同浓度、处理时间hTERT mRNA表达量( )
)
经方差分析,*不同浓度As2O3作用24 h的ACC-2细胞hTERT mRNA表达水平差异有显著统计学意义(F = 322.38,P < 0.01),#不同浓度As2O3作用48 h的ACC-2细胞hTERT mRNA表达水平差异有显著统计学意义(F = 1730.23,P < 0.01)
4. 讨论
端粒酶(Telomerase)是一种核糖核蛋白复合物,在维持端粒长度方面有重要作用。正常人大部分体细胞中检测不到端粒酶 [16] 。一些良性病变组织 [17] ,体外培养的成纤维细胞中也测不到端粒酶活性。但在生殖细胞睾丸 [18] 、卵巢 [19] 、胎盘及胎儿细胞中此酶为阳性。恶性肿瘤细胞具有很高的端粒酶活性,端粒酶阳性的肿瘤有卵巢癌 [20] 、淋巴瘤 [16] 、急性白血病 [21] 、乳腺癌 [22] 、结肠癌 [23] 、肺癌 [24] 等等。端粒酶异常高表达与恶性肿瘤发生、发展及预后密切相关,端粒酶的活性被认为是肿瘤细胞增殖与肿瘤进展的关键因素,还有一些疾病如骨髓衰竭综合征、先天性角化不良、再生障碍性贫血、特发性肺纤维化、肝硬化等也与端粒酶基因突变有关 [25] [26] ,端粒酶活性的调控可能成为这些疾病尤其是恶性肿瘤的治疗方法。人端粒酶包括三种组分:端粒酶RNA(human telomerase RNA, hTR)、端粒酶逆转录酶(human telomerase reverse transcriptase, hTERT) [27] [28] 及连结二者的端粒酶连结蛋白(telomerase associated protein 1, TP1) [29] 。其中hTERT与端粒酶活性密切相关,被认为是大多数细胞端粒酶激活的限速酶 [30] 。
本研究结果发现,As2O3可以诱导腺样囊性癌ACC-2细胞的凋亡,RT-PCR结果显示,在未经药物作用、作为对照组的正常培养的ACC-2细胞中,端粒酶hTERT亚基的mRNA呈现明显高表达;在As2O3作用于ACC-2细胞后24 h与48 h时,随As2O3药物浓度的增高(1.0 μmol/L、2.0 μmol/L、4.0 μmol/L、8.0 μmol/L),端粒酶hTERT亚基的mRNA表达逐渐降低。端粒酶hTERT亚基在ACC-2细胞的转录水平高表达,与报道的恶性肿瘤中hTERT的高表达相一致 [20] [21] [22] [23] [24] 。As2O3作为抗肿瘤药物,在转录水平可明显抑制ACC-2细胞的hTERT表达,并随着药物浓度的增高,ACC-2细胞的hTERT表达逐渐降低。推论As2O3可通过抑制ACC-2细胞的hTERT表达,抑制端粒酶激活,抑制甚至缩短其端粒的长度,引起ACC-2细胞生长能力下降,并伴有细胞周期阻滞及细胞凋亡。
端粒酶的活化是一个多步骤、多因素参与的复杂的过程,其活性调控不仅与生长因素有关,而且与一系列癌基因、抑癌基因、蛋白酶类等有关 [31] 。Bashash D等 [32] 的研究证实,抗肿瘤药物、端粒酶抑制剂BIBR 1532对急性早幼粒细胞白血病(APL)NB4细胞的直接短期细胞毒性,就是通过p21的诱导、bax/bcl-2平衡比率失衡以及下调c-myc和hTERT的转录来实现的。Huang ST等 [33] 的研究发现,黄芩中的活性化合物-汉黄芩素可诱导人粒细胞性白血病细胞(HL-60细胞)凋亡,他们认为,这种抑制HL-60细胞生长的作用就是部分通过bax/bcl-2细胞凋亡诱导途径,以及通过抑制hTERT启动子介导的c-myc基因来抑制端粒酶活性而实现的。Park SE等 [34] 的研究发现,蛹虫草水提取物可诱导人肺癌A549细胞凋亡,通过下调人端粒酶逆转录酶(hTERT),c-myc和Sp1表达,产生剂量依赖性端粒酶活性抑制。他们认为,蛹虫草水提取物通过外在的信号级联的死亡受体介导途径和内在的线粒体介导的caspase途径诱导A549细胞凋亡。也可得出蛹虫草水提取物所致的凋亡是通过抑制hTERT的转录活性,从而降低端粒酶活性所致的结论。Yang SM等 [35] 的研究则从另一个角度提出了治疗恶性肿瘤的思路,他们在体外,用反义hTERT基因(ahTERT)真核表达载体,通过基因重组技术转染人胃癌SGC-7901细胞株,结果表明,ahTERT转染后,SGC-7901细胞的增殖显著受到抑制。进一步研究表明,在ahTERT转染的SGC-7901细胞中,其端粒酶活性,端粒长度,hTERT基因、bcl-2基因和c-myc基因的mRNA和蛋白表达下降。但是,人类端粒酶RNA(HTR)和端粒酶相关蛋白1 (TP1)的转录在转染细胞和未转染细胞中无明显影响。流式细胞仪分析显示,在ahTERT转染的细胞,其G0/G1期积聚,增殖指数(PI)下降。此外,在裸鼠皮下注射ahTERT转染的细胞后发现无致瘤性,而在注射对照未转染的细胞的小鼠中,可观察到可触及的肿瘤。研究表明,外源性ahTERT可以抑制增殖,通过抑制端粒酶活性、端粒酶逆转录酶、c-myc和bcl-2表达,而部分逆转SGC-7901细胞的恶性表型。针对hTERT的反义技术,可能是一个潜在的胃癌治疗方法。我们的研究也证实,As2O3可通过下调ACC-2细胞的hTERT mRNA转录表达,来诱导ACC-2细胞凋亡。As2O3可能通过癌基因、抑癌基因、蛋白酶类的多步骤、多因素参与的复杂过程,来调控腺样囊性癌ACC-2细胞端粒酶活性,从而诱导ACC-2细胞凋亡,发挥其抗癌作用。
hTERT的调控因子很多,包括正相调节因子和负相调节因子,且这些调节因子间可能存在协同或拮抗作用。从上面的讨论中可以看出,bcl-2、c-myc可能是hTERT的正相调节因子,对hTERT起协同作用。
综上所述,As2O3可通过下调ACC-2细胞hTERT mRNA转录表达,来诱导ACC-2细胞凋亡。
基金项目
山东省自然科学基金资助项目(No. ZR2012HM062)。
文章引用
姜 涛,姜 靖,周 杨,王仁萍,王 毅,肖利华. 三氧化二砷对腺样囊性癌ACC-2细胞的端粒酶逆转录酶mRNA表达的影响
The Effect of Arsenic Trioxide on the Expression of Telomerase Reverse Transcriptase mRNA in ACC-2 Cells of Adenoid Cystic Carcinoma[J]. 眼科学, 2016, 05(04): 95-104. http://dx.doi.org/10.12677/HJO.2016.54017
参考文献 (References)
- 1. Jaso, J. and Malhotra, R. (2011) Adenoid Cystic Carcinoma. Archives of Pathology & Laboratory Medicine, 135, 511- 515.
- 2. Boukheris, H., Curtis, R.E., Land, C.E. and Dores, G.M. (2009) Incidence of Carcinoma of the Major Salivary Glands according to the WHO Classification, 1992 to 2006: A Population-Based Study in the United States. Cancer Epidemiology, Biomarkers & Prevention, 18, 2899-2906. https://doi.org/10.1158/1055-9965.EPI-09-0638
- 3. Saito, M., Nishiyama, H., Maruyama, S., Oda, Y., Saku, T. and Hayashi, T. (2008) Adenoid Cystic Carcinoma of Sublingual Gland Involving the Submandibular Duct. Dentomaxillofacial Radiology, 37, 421-424. https://doi.org/10.1259/dmfr/31299961
- 4. Al-Khateeb, T.H. and Ababneh, K.T. (2007) Salivary Tumors in North Jordanians: A Descriptive Study. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 103, e53-59. https://doi.org/10.1016/j.tripleo.2006.11.017 http://www.ncbi.nlm.nih.gov/pubmed/?term=Salivary+tumors+in+north+Jordanians%3A+a+descriptive+study
- 5. de Oliveira, F.A., Duarte, E.C., Taveira, C.T., Máximo, A.A., de Aquino, E.C., Alencar Rde, C. and Vencio, E.F. (2009) Salivary Gland Tumor: A Review of 599 Cases in a Brazilian Population. Head and Neck Pathology, 3, 271-275. https://doi.org/10.1007/s12105-009-0139-9
- 6. Fujii, Y., Masuda, M., Hirokawa, M., Matsushita, K. and Hasegawa, H. (1991) Bilateral Renal Metastases of Lung Adenoid Cystic carcinoma. Hinyokika Kiyo, 37, 1307-1311. http://www.ncbi.nlm.nih.gov/pubmed/1661561
- 7. Campistron, M., Rouquette, I., Courbon, F., Chabbert, V., Rochaix, P., Prévot, G., Laroumagne, S., Têtu, L., Didier, A. and Mazières, J. (2008) Adenoid Cystic Carcinoma of the Lung: Interest of 18FDG PET/CT in the Management of an Atypical Presentation. Lung Cancer, 59, 133-136. https://doi.org/10.1016/j.lungcan.2007.06.002 http://www.ncbi.nlm.nih.gov/pubmed/?term=Adenoid+cystic+carcinoma+of+the+lung%3A+interest+of+18FDG+PET%2FCT+in+the+management+of+an+atypical+presentation
- 8. Yurut-Caloglu, V., Caloglu, M., Ozyilmaz, F., Saynak, M., Cosar-Alas, R., Karagol, H., Bayir-Angin, G. and Uzal, C. (2007) Lung, Bone, Skeletal Muscles and Cutaneous Metastases from Adenoid Cystic Carcinoma of the Parotid Gland: A Case Report and Review of the Literature. Medical Oncology, 24, 458-462. https://doi.org/10.1007/s12032-007-0016-x http://www.ncbi.nlm.nih.gov/pubmed/?term=Lung%2C+bone%2C+skeletal+muscles+and+cutaneous+metastases+from+adenoid+cystic+carcinoma+of+the+parotid+gland%3A+a+case+report+and+review+of+the+literature
- 9. Yokouchi, H., Otsuka, Y., Otoguro, Y., Takemoto, N., Ito, K., Uchida, Y., Okamoto, K., Nishimura, M., Kimura, K. and Kaji, H. (2007) Primary Peripheral Adenoid Cystic Carcinoma of the Lung and Literature Comparison of Features. Internal Medicine, 46, 1799-1803. http://www.ncbi.nlm.nih.gov/pubmed/?term=Primary+peripheral+adenoid+cystic+carcinoma+of+the+lung+and+literature+comparison+of+features
- 10. Aubry, M.C., Heinrich, M.C., Molina, J., Lewis, J.E., Yang, P., Cassivi, S.D. and Corless, C.L. (2007) Primary Adenoid Cystic Carcinoma of the Lung: Absence of KIT Mutations. Cancer, 110, 2507-2510. http://www.ncbi.nlm.nih.gov/pubmed/?term=Primary+adenoid+cystic+carcinoma+of+the+lung%3A+absence+of+KIT+mutations https://doi.org/10.1002/cncr.23075
- 11. Haresh, K.P., Prabhakar, R., Rath, G.K., Sharma, D.N., Julka, P.K. and Subramani, V. (2008) Adenoid Cystic Carcinoma of the Trachea Treated with PET-CT Based Intensity Modulated Radiotherapy. Journal of Thoracic Oncology, 3, 793-795. https://doi.org/10.1097/JTO.0b013e31817c9245
- 12. Counter, C.M. (1996) The Roles of Telomeres and Telomerase in Cell Life Span. Mutation Research, 366, 45-63. http://www.ncbi.nlm.nih.gov/pubmed/8921986 https://doi.org/10.1016/S0165-1110(96)90006-8
- 13. Kirkpatrick, K.L. and Mokbel, K. (2001) The Significance of Human Telomerase Reverse Transcriptase (hTERT) in Cancer. European Journal of Surgical Oncology, 27, 754-760. http://www.ncbi.nlm.nih.gov/pubmed/11735173
https://doi.org/10.1053/ejso.2001.1151 - 14. Ducrest, A.L., Szutorisz, H., Lingner, J. and Nabholz, M. (2002) Regulation of the Human Telomerase Reverse Transcriptase Gene. Oncogene, 21, 541-552. https://doi.org/10.1038/sj.onc.1205081
http://www.ncbi.nlm.nih.gov/pubmed/?term=Ducrest+AL%2C+Szutorisz+H%2C+Lingner+J%2C+Nabholz+M - 15. 欧阳艳艳, 姜涛, 高萌, 肖利华, 周杨, 顿月丽, 赵桂秋, 刘世海, 梁晔. △ψm和Caspase 3在As2O3诱导腺样囊性癌ACC-2细胞凋亡过程中的作用[J]. 国际眼科杂志, 2014, 14(2): 232-235.
- 16. Bougel, S., Renaud, S., Braunschweig, R., Loukinov, D., Morse, H.C., Bosman, F.T., Lobanenkov, V. and Benhattar, J. (2010) PAX5 Activates the Transcription of the Human Telomerase Reverse Transcriptase Gene in B Cells. Journal of Pathology, 220, 87-96. https://doi.org/10.1002/path.2620
- 17. Dowdy, S.C., O’Kane, D.J., Keeney, G.L., Boyd, J. and Podratz, K.C. (2001) Telomerase Activity in Sex Cord- Stromal Tumors of the Ovary. Gynecologic Oncology, 82, 257-260. http://www.ncbi.nlm.nih.gov/pubmed/?term=Telomerase+activity+in+sex+cord-stromal+tumors+of+the+ovary https://doi.org/10.1006/gyno.2001.6293
- 18. Riou, L., Bastos, H., Lassalle, B., Coureuil, M., Testart, J., Boussin, F.D., Allemand, I. and Fouchet, P. (2005) The Telomerase Activity of Adult Mouse Testis Resides in the Spermatogonial Alpha6-Integrin-Positive Side Population Enriched in Germinal Stem Cells. Endocrinology, 146, 3926-3932. http://www.ncbi.nlm.nih.gov/pubmed/?term=The+telomerase+activity+of+adult+mouse+testis+resides+in+the+spermatogonial+alpha6-integrin-positive+side+population+enriched+in+germinal+stem+cells https://doi.org/10.1210/en.2005-0502
- 19. Misiti, S., Nanni, S., Fontemaggi, G., Cong, Y.S., Wen, J., Hirte, H.W., Piaggio, G., Sacchi, A., Pontecorvi ,A., Bacchetti, S. and Farsetti, A. (2000) Induction of hTERT Expression and Telomerase Activity by Estrogens in Human Ovary Epithelium Cells. Molecular and Cellular Biology, 20, 3764-3771. http://www.ncbi.nlm.nih.gov/pubmed/?term=Induction+of+hTERT+expression+and+telomerase+activity+by+estrogens+in+human+ovary+epithelium+cells https://doi.org/10.1128/MCB.20.11.3764-3771.2000
- 20. Gan, Y., Mo, Y., Johnston, J., Lu, J., Wientjes, M.G. and Au, J.L. (2002) Telomere Maintenance in Telomerase-Posi- tive Human Ovarian SKOV-3 Cells Cannot Be Retarded by Complete Inhibition of Telomerase. FEBS Letters, 527, 10-14. http://www.ncbi.nlm.nih.gov/pubmed/?term=Telomere+maintenance+in+telomerase-positive+human+ovarian+SKOV-3+cells+cannot+be+retarded+by+complete+inhibition+of+telomerase https://doi.org/10.1016/S0014-5793(02)03141-1
- 21. Nakajima, A., Tauchi, T., Sashida, G., Sumi, M., Abe, K., Yamamoto, K., Ohyashiki, J.H. and Ohyashiki, K. (2003) Telomerase Inhibition Enhances Apoptosis in Human Acute Leukemia Cells: Possibility of Antitelomerase Therapy. Leukemia, 17, 560-567. http://www.ncbi.nlm.nih.gov/pubmed/?term=Telomerase+inhibition+enhances+apoptosis+in+human+acute+leukemia+cells%3A+possibility+of+antitelomerase+therapy https://doi.org/10.1038/sj.leu.2402825
- 22. Kammori, M., Izumiyama, N., Hashimoto, M., Nakamura, K., Okano, T., Kurabayashi, R., Naoki, H., Honma, N., Ogawa, T., Kaminishi, M. and Takubo, K. (2005) Expression of Human Telomerase Reverse Transcriptase Gene and Protein, and of Estrogen and Progesterone Receptors, in Breast Tumors: Preliminary Data from Neo-Adjuvant Chemotherapy. International Journal of Oncology, 27, 1257-1263. http://www.ncbi.nlm.nih.gov/pubmed/?term=Expression+of+human+telomerase+reverse+transcriptase+gene+and+protein%2C+and+of+estrogen+and+progesterone+receptors%2C+in+breast+tumors%3A+preliminary+data+from+neo-adjuvant+chemotherapy https://doi.org/10.3892/ijo.27.5.1257
- 23. Sanz-Casla, M.T., Vidaurreta, M., Sanchez-Rueda, D., Maestro, M.L., Arroyo, M. and Cerdán, F.J. (2005) Telomerase Activity as a Prognostic Factor in Colorectal Cancer. Onkologie, 28, 553-557. http://www.ncbi.nlm.nih.gov/pubmed/16249640 https://doi.org/10.1159/000088525
- 24. Hsu, C.P., Ko, J.L., Shai, S.E. and Lee, L.W. (2007) Modulation of Telomere Shelterin by TRF1 [Corrected] and TRF2 Interacts with Telomerase to Maintain the Telomere Length in Non-Small Cell Lung Cancer. Lung Cancer, 58, 310- 316. http://www.ncbi.nlm.nih.gov/pubmed/?term=Modulation+of+telomere+shelterin+by+TRF1+%5Bcorrected%5D+and+TRF2+interacts+with+telomerase+to+maintain+the+telomere+length+in+non-small+cell+lung+cancer https://doi.org/10.1016/j.lungcan.2007.06.019
- 25. Hartmann, D., Srivastava, U., Thaler, M., Kleinhans, K.N., N’kontchou, G., Scheffold, A., Bauer, K., Kratzer, R.F., Kloos, N., Katz, S.F., Song, Z., Begus-Nahrmann, Y., Kleger, A., von Figura, G., Strnad, P., Lechel, A., Günes, C., Potthoff, A., Deterding, K., Wedemeyer, H., Ju, Z., Song, G., Xiao, F., Gillen, S., Schrezenmeier, H., Mertens, T., Ziol, M., Friess, H., Jarek, M., Manns, M.P., Beaugrand, M. and Rudolph, K.L. (2011) Telomerase Gene Mutations Are Associated with Cirrhosis Formation. Hepatology, 53, 1608-1617. https://doi.org/10.1002/hep.24217
- 26. Calado, R.T., Regal, J.A., Kleiner, D.E., Schrump, D.S., Peterson, N.R., Pons, V., Chanock, S.J., Lansdorp, P.M. and Young, N.S. (2009) A Spectrum of Severe Familial Liver Disorders Associate with Telomerase Mutations. PLoS ONE, 4, e7926. https://doi.org/10.1371/journal.pone.0007926
- 27. Pendino, F., Tarkanyi, I., Dudognon, C., Hillion, J., Lanotte, M., Aradi, J. and Ségal-Bendirdjian, E. (2006) Telomeres and Telomerase: Pharmacological Targets for New Anticancer Strategies? Current Cancer Drug Targets, 6, 147-180. http://www.ncbi.nlm.nih.gov/pubmed/16529544 https://doi.org/10.2174/156800906776056482
- 28. Swanberg, S.E., Payne, W.S., Hunt, H.D., Dodgson, J.B. and Delany, M.E. (2004) Telomerase Activity and Differential Expression of Telomerase Genes and c-Myc in Chicken Cells in Vitro. Developmental Dynamics, 231, 14-21. http://www.ncbi.nlm.nih.gov/pubmed/?term=Telomerase+activity+and+differential+expression+of+telomerase+genes+and+c-myc+in+chicken+cells+in+vitro https://doi.org/10.1002/dvdy.20149
- 29. Bhattacharyya, S., Keirsey, J., Russell, B., Kavecansky, J., Lillard-Wetherell, K., Tahmaseb, K., Turchi, J. and Groden, J. (2009) Telomerase-Associated Protein 1, HSP90, and Topoisomerase IIAlpha Associate Directly with the BLM Helicase in Immortalized Cells Using ALT and Modulate Its Helicase Activity Using Telomeric DNA Substrates. Journal of Biological Chemistry, 284, 14966-14977. https://doi.org/10.1074/jbc.M900195200
- 30. Kim, H.R., Christensen, R., Park, N.H., Sapp, P., Kang, M.K. and Park, N. (2001) Elevated Expression of hTERT Is Associated with Dysplastic Cell Transformation during Human Oral Carcinogenesis in Situ. Clinical Cancer Research, 7, 3079-3086. http://www.ncbi.nlm.nih.gov/pubmed/11595698
- 31. Xu, D., Popov, N., Hou, M., Wang, Q., Bjrkholm, M., Gruber, A., Menkel, A. and Henriksson, M. (2001) Switch from Myc/Max to Mad1/Max Binding and Decrease in Histone Acetylation at the Telomerase Reverse Transcriptase Promoter during Differentiation of HL60 Cells. Proceedings of the National Academy of Sciences of the United States of America, 98, 3826-3831. http://www.ncbi.nlm.nih.gov/pubmed/?term=Switch+from+Myc%2FMax+to+Mad1%2FMax+binding+and+decrease+in+histone+acetylation+at+the+telomerase+reverse+transcriptase+promoter+during+differentiation+of+HL60+cells https://doi.org/10.1073/pnas.071043198
- 32. Bashash, D., Ghaffari, S.H., Zaker, F., Hezave, K., Kazerani, M., Ghavamzadeh, A., Alimoghaddam, K., Mosavi, S.A., Gharehbaghian, A. and Vossough, P. (2012) Direct Short-Term Cytotoxic Effects of BIBR 1532 on Acute Promyelocytic Leukemia Cells through Induction of p21 Coupled with Downregulation of c-Myc and hTERT Transcription. Cancer Investigation, 30, 57-64. https://doi.org/10.3109/07357907.2011.629378
- 33. Huang, S.T., Wang, C.Y., Yang, R.C., Chu, C.J., Wu, H.T. and Pang, J.H. (2010) Wogonin, an Active Compound in Scutellaria baicalensis, Induces Apoptosis and Reduces Telomerase Activity in the HL-60 Leukemia Cells. Phytomedicine, 17, 47-54. https://doi.org/10.1016/j.phymed.2009.06.005
- 34. Park, S.E., Yoo, H.S., Jin, C.Y., Hong, S.H., Lee, Y.W., Kim, B.W., Lee, S.H., Kim, W.J., Cho, C.K. and Choi, Y.H. (2009) Induction of Apoptosis and Inhibition of Telomerase Activity in Human Lung Carcinoma Cells by the Water Extract of Cordyceps militaris. Food and Chemical Toxicology, 47, 1667-1675. https://doi.org/10.1016/j.fct.2009.04.014
- 35. Yang, S.M., Fang, D.C., Yang, J.L., Chen, L., Luo, Y.H. and Liang, G.P. (2008) An-tisense Human Telomerase Reverse Transcriptase Could Partially Reverse Malignant Phenotypes of Gastric Carcinoma Cell Line in Vitro. European Journal of Cancer Prevention, 17, 209-217. https://doi.org/10.1097/CEJ.0b013e3282b71f0d
*通讯作者。
