Asian Case Reports in Oncology
Vol.2 No.3(2013), Article ID:12125,4 pages DOI:10.12677/ACRPO.2013.23004
A Case Report on Primary Ovarian Leiomyo-Sarcoma and Some Related Documents Review
Internal Medicine-Oncology Department, Fujian Provincial Hospital, Fuzhou
Email: *fjslctj@163.com
Received: May 13th, 2013; revised: May 20th, 2013; accepted: Jul. 8th, 2013
Copyright © 2013 Xin Lin et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT:
A 55-year-old woman received a health screening and ultrasound examination revealed a mass of the right ovary on September, 2010. The patient underwent a “right oophorectomy” on the local hospital. Histopathological examination of the right ovary showed that the tumor was composed of dysplasia spindle cells, and mitosis can easily be seen, then a diagnosis of Primary Ovarian Leiomyo-Sarcoma was made. There was neither chemotherapy nor radiotherapy for her after the surgery. The patient consulted in our hospital for suffering from her right upper abdominal pain on February, 2013. The whole-body PET-CT examination revealed that multiple metastasis lesions were found in parenchymal of liver and adrenal, bones and systemic soft tissues. Further a fine-needle biopsy of the liver was performed. Pathological diagnosis demonstrated a metastasis of leiomyosarcoma. Thus 2 cycles of the “CAP” 3-week chemotherapy were carried out as the first-line systemic palliative chemotherapy. After that the patient’s status was assessed as progressing with PD, according to the standard of “Response evaluation criteria in solid tumors” (RECIST). Then 2 cycles of “GP” 3-week chemotherapy were schemed as the second-line systemic palliative chemotherapy. After that the tumor was shrinked, and evaluated as stable with SD. To date, the patient has received the third period of systemic palliative second-line chemotherapy. And according to the standard of RECIST, the tumor was evaluated as stable again.
Keywords: Ovary; Leiomyo-Sarcoma; Diagnose; Treatment
原发性卵巢平滑肌肉瘤1例并文献复习
林 鑫,刘振华,黄 颖,崔同建*
福建省立医院肿瘤内科,福州
Email: *fjslctj@163.com
摘 要:
患者,女性,55岁,于2010年9月在当地医院体检时彩超发现右卵巢占位,行“右侧卵巢切除术”。术后病理:(右卵巢)镜下见肿瘤细胞呈梭形,可见核分裂。病理诊断:卵巢平滑肌肉瘤。术后无化放疗。至2013年2月因右上腹痛求诊我院,查全身PET-CT提示全身多处软组织、内脏实质(包括肝、肾上腺等)、骨骼转移(metastasis,MT)。进一步行肝占位穿刺活检病理报告提示符合平滑肌肉瘤。遂行CAP 3周方案全身姑息一线化疗2个周期,按RECIST标准((Response evaluation criteria in solid tumors)评估病情为进展,之后改为GP 3周方案全身姑息二线化疗2个周期,按RECIST标准评估病情为稳定(缩小趋势)。至写稿时间患者完成第3周期全身姑息二线化疗,按RECIST标准评估病情仍为稳定。
收稿日期:2013年5月13日;修回日期:2013年5月20日;录用日期:2013年7月8日
关键词:卵巢;平滑肌肉瘤;诊断;治疗
1. 引言
患者,女性,55岁,于2010年9月因在当地医院体检时彩超发现右卵巢占位,考虑恶性肿瘤,遂在该院行“右侧卵巢切除术”。术中见右卵巢增大约10 × 9 × 8 cm,质硬,与子宫后壁形成粘连,左卵巢萎缩,子宫正常大小,浆膜光滑,未见腹水。术后病理(图1)报告:(右卵巢)镜下可见异型细胞,肿瘤细胞呈梭形,免疫组化Actin(sm)(+++),Desmin(+),CD117(−),PDGFR(−),CK5/6(−),Vimentin(−),inhinbin-a(−);诊断:右卵巢平滑肌肉瘤。术后患者无接受化放疗治疗,随后因经济原因自行在当地医院定期复查,未发现肿瘤复发。2013年2月因“右上腹胀痛1周”求诊我院,查血CEA、CA125、CA153、CA199均正常水平。全身PET-CT提示(图2(a)~(d)):1) 肝右叶后段、左肾上腺、双肺、右胸膜、全身多处肌肉、骨骼多发高代谢占位,考虑转移瘤;2) 网膜、肠系膜多发结节影,代谢增高,考虑种植MT。进一步行肝占位穿刺活检,病理(图3)报告:镜下可见异型细胞,肿瘤细胞呈梭形,免疫组化SM(++),Desmin(+),CD117(−),PDGFR(−),CK5/6(−),Ki67(70%阳性),ER(−),PR(−);符合平滑肌肉瘤。临床诊断:右卵巢平滑肌肉瘤术后复发并全身多发转移,排除相关禁忌后,于2013年2月、2013年3月分别行CAP(异环磷腺胺2.0 g d1~5 + 表柔比星90 mg d1 + 顺铂 40 mg d1~3)3周方案全身姑息一线化疗2个周期,按RECIST(1.1版)标准评估病情为进展(图4(a)~(d)),后改为GP(吉西他滨1.4 g d1,8 + 洛铂50 mg d2)3周方案全身姑息二线化疗2个周期,按RECIST(1.1版)标准评估病情为稳定(图5(a)~(d)),肿瘤有缩小趋势,至写稿时间患者完成第3周期全身姑息二线化疗,耐受情况可,按RECIST标准评估病情仍为稳定(图6(a)~(d))。
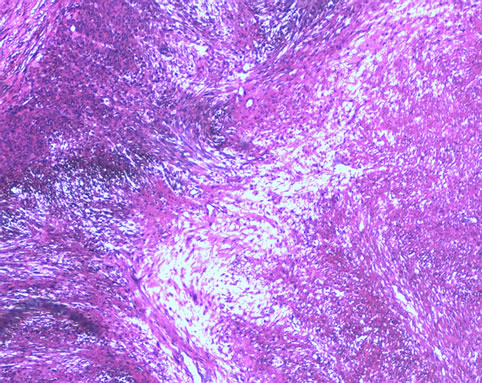
Figure 1. Shows the patient’s right ovarian histopathology that the dysplasia spindle cells and mitosis can be seen
图1. 该例患者右卵巢组织病理图,可见肿瘤细胞呈梭形及核分裂
2. 讨论
原发性卵巢平滑肌肉瘤(primary ovarian leiomyosarcoma, POLMS)临床上罕见,发生率仅约卵巢恶性肿瘤的0.1%[1],属高度恶性且预后差的肿瘤,是非特异性间质细胞肿瘤中的一类。该病早期无明显症状,术前难诊断,当出现临床症状就诊时多已至中晚期。目前原发性卵巢平滑肌肉瘤的病因尚不完全清楚,
 (a)
(a)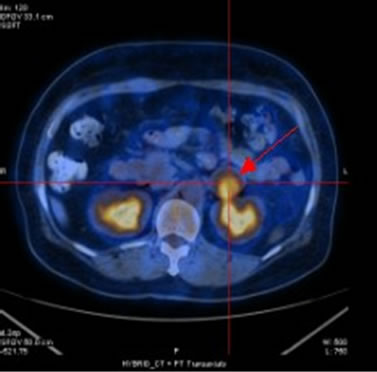 (b)
(b)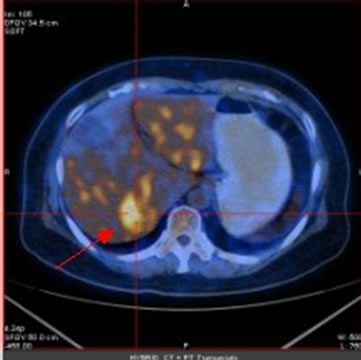 (c)
(c) (d)
(d)
Figure 2. (a) Shows the left adrenal space-occupying lesion before chemotherapy by PET-CT, and the maximum diameter of it is about 2.9 cm (arrow); (b) Shows the right pleural lesion before chemotherapy by PET-CT, and the maximum diameter of it is about 2.0 cm (arrow); (c) Shows the right abdominal soft tissue lesion before chemotherapy by PET-CT, and the maximum diameter of it is about 1.8 cm (arrow); (d) Shows the right hepatic space-occupying lesion before chemotherapy by PET-CT, and the maximum diameter of it is about 4.6 cm (arrow)
图2. (a)为化疗前PET-CT提示左肾上腺占位性病变,最大直径约2.9 cm(箭头);(b)为化疗前PET-CT提示右胸膜占位性病变,最大直径约2.0 cm(箭头);(c)为化疗前PET-CT提示右侧腹肌软组织占位性病变,最大直径约1.8 cm(箭头);(d)为化疗前PET-CT提示右肝占位性病变,最大直径约4.6 cm(箭头)
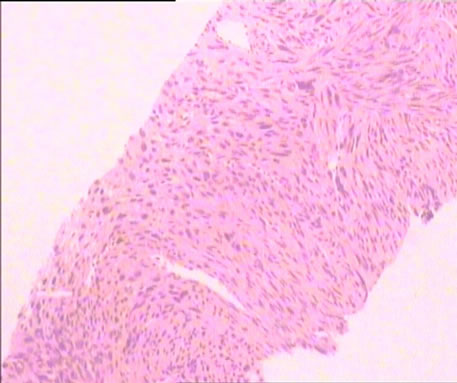
Figure 3. Shows the patient’s liver histopathology that the dysplasia spindle cells and mitosis can be seen
图3. 该例患者肝组织病理图,可见肿瘤细胞呈梭形及核分裂
 (a)
(a)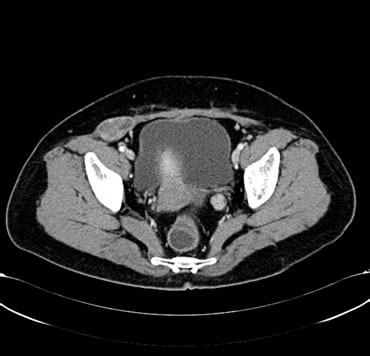 (b)
(b)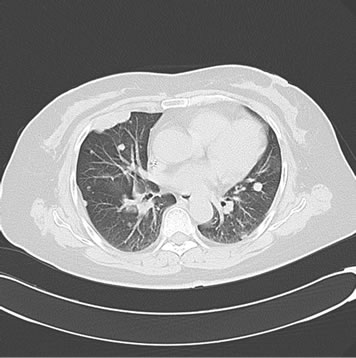 (c)
(c)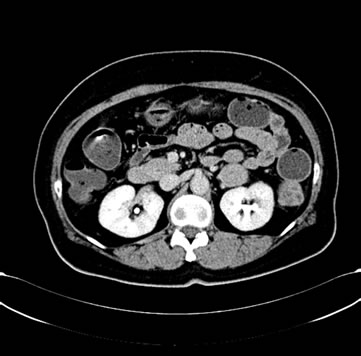 (d)
(d)
Figure 4. (a) Shows the left adrenal space-occupying lesion after the first-line systemic palliative chemotherapy by CT, and the maximum diameter of it is about 2.9 cm; (b) Shows the right pleural lesion after the first-line systemic palliative chemotherapy by CT, and the maximum diameter of it is about 2.8 cm; (c) Shows the right abdominal soft tissue lesion after the first-line systemic palliative chemotherapy by CT, and the maximum diameter of it is about 2.8 cm; (d) Shows the right hepatic space-occupying lesion after the first-line systemic palliative chemotherapy by CT, and the maximum diameter of it is about 4.9 cm
图4. (a) 为一线化疗后CT提示左肾上腺占位性病变,最大直径约2.9 cm;(b) 为一线化疗后CT提示右胸膜占位性病变,最大直径约2.8 cm;(c) 为一线化疗后CT提示右侧腹肌软组织占位性病变,最大直径约2.8 cm;(d) 为一线化疗后CT提示右肝占位性病变,最大直径约4.9 cm
 (a)
(a)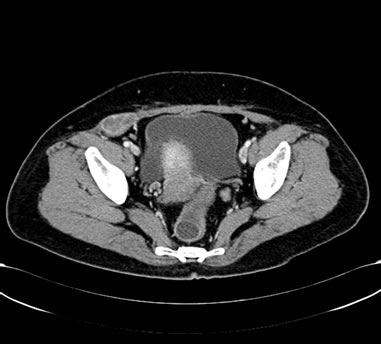 (b)
(b) (c)
(c) (d)
(d)
Figure 5. (a) Shows the left adrenal space-occupying lesion after 2 cycles of second-line systemic palliative chemotherapy by CT, and the maximum diameter of it is about 2.9 cm; (b) Shows the right pleural lesion after 2 cycles of second-line systemic palliative chemotherapy by CT, and the maximum diameter of it is about 1.8 cm; (c) Shows the right abdominal soft tissue lesion after 2 cycles of second-line systemic palliative chemotherapy by CT, and the maximum diameter of it is about 2.8 cm; (d) Shows the right hepatic space-occupying lesion after 2 cycles of second-line systemic palliative chemotherapy by CT, and the maximum diameter of it is about 4.9 cm
图5. (a) 为二线化疗2个周期后复查CT提示左肾上腺占位性病变,最大直径约2.9 cm;(b) 为二线化疗2个周期后复查CT提示右胸膜占位性病变,最大直径约1.8 cm;(c) 为二线化疗2个周期后复查CT提示右侧腹肌软组织占位性病变,最大直径约2.8 cm;(d) 为二线化疗2个周期后复查CT提示右肝占位性病变,最大直径约4.9 cm
 (a)
(a) (b)
(b)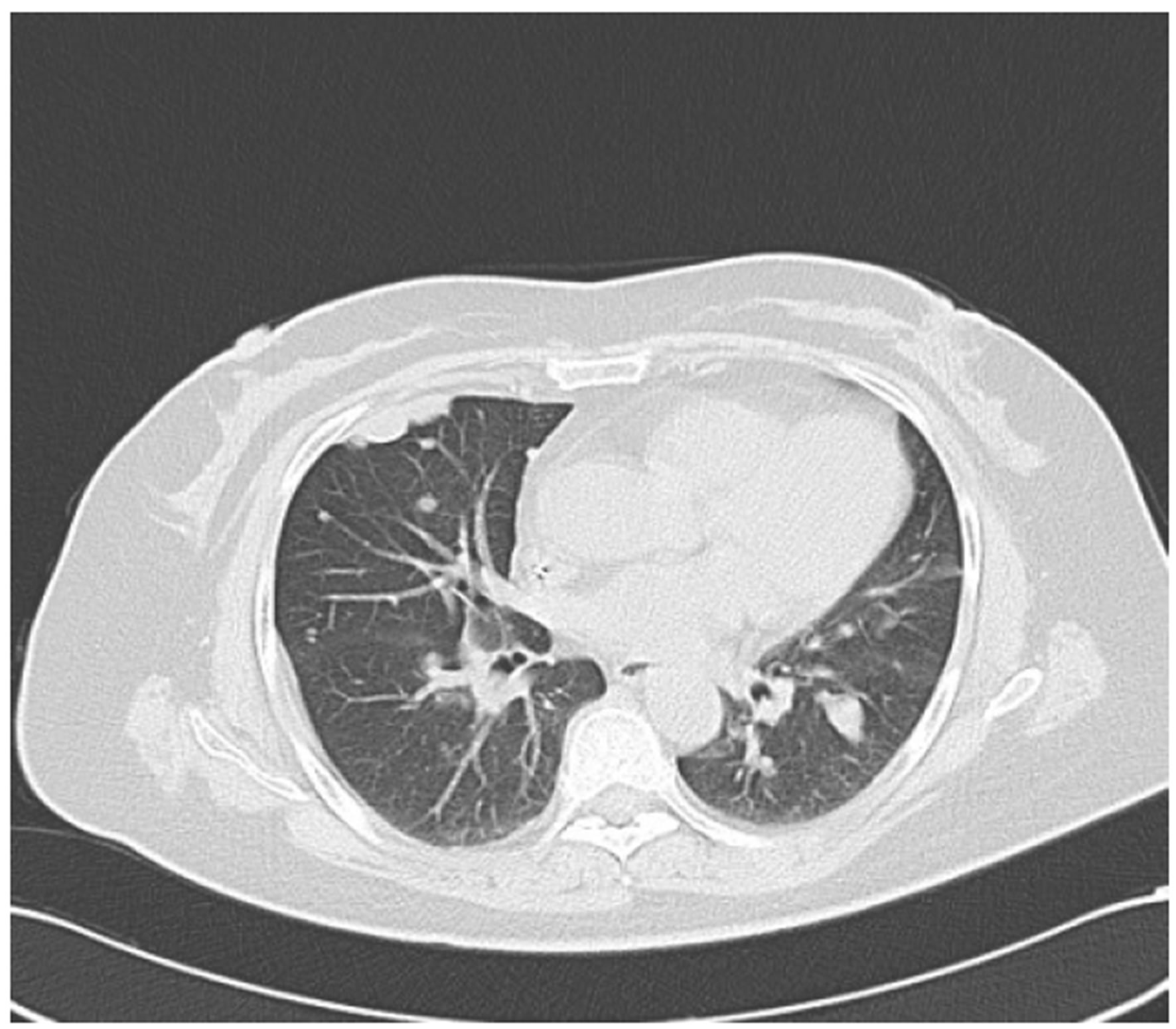 (c)
(c) (d)
(d)
Figure 6. (a) Shows the left adrenal space-occupying lesion after 3 cycles of second-line systemic palliative chemotherapy by CT, and the maximum diameter of it is about 3.0 cm; (b) Shows the right pleural lesion after 3 cycles of second-line systemic palliative chemotherapy by CT, and the maximum diameter of it is about 2.2 cm; (c) Shows the right abdominal soft tissue lesion after 3 cycles of second-line systemic palliative chemotherapy by CT, and the maximum diameter of it is about 2.8 cm; (d) Shows the right hepatic space-occupying lesion after 3 cycles of second-line systemic palliative chemotherapy by CT, and the maximum diameter of it is about 4.9 cm
图6. (a) 为二线化疗3个周期后复查CT提示左肾上腺占位性病变,最大直径约3.0 m;(b) 为二线化疗3个周期后复查CT提示右胸膜占位性病变,最大直径约2.2 cm;(c) 为二线化疗3个周期后复查CT提示右侧腹肌软组织占位性病变,最大直径约2.8 cm;(d) 为二线化疗3个周期后复查CT提示右肝占位性病变,最大直径约4.9 cm Clark-Knowles等[2]研究发现,条件性灭活鼠卵巢上等位基因Brcal、p53、Rb的异常表达会导致平滑肌肉瘤的发生。其组织来源可能有:1) 来源于中肾管遗迹、残件;2) 来源于卵巢间质血管的平滑肌组织,该亚型肿瘤生长相对较慢,较少侵袭周边组织,SMA(+)、CD117(−),且静脉发生率约为动脉的5倍,术后5年生存率(OS)可达33%~53%[3],但该亚型仅占POLMS患者的2%左右;3) 来源于卵巢韧带在卵巢端附着处的平滑肌;4) 来源于畸胎瘤中的多潜能间叶组织或异位子宫内膜间质组织。其病理特征为肿瘤横切面多呈鱼肉样,伴有出血坏死及囊性变。显微镜下可见细胞异型、核分裂象 ≥ 10个/10HPF并出现肿瘤性坏死[4]。免疫组化特征SMA(+)、Desmin(+)、p53(+),同时S-100(−)、ER约33%(+)、PR约50%(+)[5]。
查阅近15年来国内外文献报道,卵巢平滑肌肉瘤的患者发病年龄跨度范围较大,20~65岁首诊发现者均有,但大多集中在中年(40岁)以后。其临床表现可有腹胀、腹痛、便秘、体表局部肿块等,无明显特异性。例如本例患者,50多岁发病,早期无特殊的症状,通过体检影像检查时发现卵巢占位性疾病,并及时手术治疗,经术后病理方予证实。原发性卵巢平滑肌肉瘤应与纤维肉瘤、转移性胃肠间质瘤、卵巢神经纤维瘤、未成熟畸胎瘤及卵巢恶性中胚叶混合瘤等鉴别。病理免疫组化SMA、Desmin、S-100、CD117、DOG-1、inhinbin等有助于鉴别。同时肿瘤标记物CA125是卵巢上皮癌的相关性抗原,在卵巢肉瘤患者中大都也会异常升高。另彩超、MRI可协助诊断,放射性影像检查多表现为血管丰富和典型的无血管坏死中心区[6]。本例患者血清CA125无异常升高,组织免疫组化SM(++),Desmin(+),CD117(−),PDGFR(−),CK5/6(−),Ki67(70%阳性),ER(−),PR(−);符合卵巢原发性平滑肌肉瘤的诊断并助于排除其他恶性肿瘤可能。
原发性卵巢平滑肌肉瘤恶性程度高,在短期内易出现复发、转移,常见的有盆腔、淋巴结等。国际妇产科联盟(FIGO)对卵巢平滑肌肉瘤的分期与卵巢上皮癌相同。目前临床上对POLMS无标准的治疗方案。手术治疗是该病的最重要的治疗手段,手术应尽量减瘤,尽量清除肉眼可见病灶。而临床上,多数患者虽进行了手术,但实施的手术范围及术后是否必须接受辅助化放疗仍存在着争议,国际上及国内目前尚无标准的治疗方案。已报道的术后化疗方案有CAP、PEB、CVAD等;相关研究表明,联合化疗(尤其是含有铂类的化疗方案)比单一药物化疗疗效好。但也有相关研究
报道,大多数患者在接受辅助治疗后或在辅助治疗期间仍较短时间内出现复发(平均在术后3个月左右),且在1~2年内死亡,与不接受辅助治疗患者的生存期无统计学差异[7],平均生存期为6~12个月。而临床分期、残余灶的大小及是否接受手术治疗是影响生存期重要的因素[8]。同时治疗方式不同,其生存期亦有所不同。而病理分类对生存期的影响目前尚有争议。
3. 结论
本例原发性卵巢平滑肌肉瘤(POLMS)术后2年出现复发转移,既有腹腔广泛播散,同时也出现了血道转移和软组织浸润,恶性程度高,经全身姑息一线化疗治疗无效,ER、PR无阳性表达,无内分泌治疗指征,评估预后差。但其在早期无临床症状情况下经单纯手术治疗(单侧卵巢切除术)获得了超过2年的无疾病生存期,体现了早发现、早诊断、早治疗及手术治疗为主的临床意义。且目前该患者采用GP方案(含铂的联合化疗方案)二线治疗,肿瘤病情暂得以控制,治疗有效,临床观察患者耐受情况好。同时,结合该病例,我们应当反省到,患者虽早期接受了根治性手术治疗,但因各种原因,尤其在患者自身尚无异常症状情况下往往无法做到定期随访,从而延误诊疗,应当引起临床医务工作者的警惕。一方面,对POLMS患者应力求早发现、早手术,术中尽量减少肿瘤负荷。另一方面,对于根治术后患者虽并不要求必须接受辅助化放疗[7],但本病恶性程度高,术后仍应当密切随访,并努力提高患者医从性,督促其定期到医院复查,尤其在术后1~2年内,建议2~3个月复查一次;若患者ER、PR阳性表达,可建议手术创伤愈合后即开始内分泌治疗;提高随访率,尽早发现肿瘤复发征象,一旦发现肿瘤复发或对于无法进行根治性切除手术的患者,应尽快予以内科解救治疗。对于无局部急症危及生命患者而言,选择全身化疗可采用含有铂类的联合化疗方案治疗。当然,如何提高术前诊断率、寻找规范、有效的手术和化放疗方案、减少病理漏诊率等仍是肿瘤科医师和病理科医师共同协作、努力解决的课题。
参考文献 (References)
[1] 曹泽毅. 中华妇产科学[M]. 北京: 人民卫生出版社, 2005: 2233-2234.
[2] K. V. Clark-Knowles, M. K. Senterman, O. Collins, et al. Conditional inactivation of Brcal, p53 and Rb in mouse ovaries results in the development of leiomyosarcomas. PLoS One, 2009, 4(12): e8534.
[3] M. Chiarugi, E. Pressi, R. Mancini, et al. Leiomyosarcoma of the right ovarian vein. The American Journal of Surgery, 2009, 197(4): e36-e37.
[4] 陈乐真. 妇产科诊断病理学[M]. 2版, 北京: 人民军医出版社, 2010: 423.
[5] S. Taskin, E. A. Taskin, N. Uzum, et al. Primary ovarian leiomyosarcoma: A review of the clinical and immunohistochemical features of the rare tumor. Obstetrical & Gynecological Survey, 2007, 62(7): 480-486.
[6] S. H. Kim, H. J. Kwon, J. H. Cho, et al. Atypical radiological features of a leiomyosarcoma that arose from the ovarian vein and mimicked a vascular tumor. British Journal of Radiology, 2010, 83(989): e95-e97.
[7] 陈晨, 沈铿, 冯凤芝. 原发性卵巢平滑肌肉瘤诊断和治疗(附2例报告)[J]. 实用妇产科杂志, 2001, 17(1): 40-41.
[8] A. K. Sood, J. I. Sorosky, M. S. Gelder, et al. Primary ovarian sarcoma.Cancer, 1998, 82(1): 731-737.
NOTES
*通迅作者。