Asian Case Reports in Veterinary Medicine
Vol.05 No.01(2016), Article ID:16860,6
pages
10.12677/ACRPVM.2016.51002
Identification and Isolation of a Wild Strain of Infectious Bursal Disease Virus
Mengjiao Fu, Yongqiang Wang, Xiaoqi Li, Hong Cao, Fuyong Chen, Shijun Zheng*
College of Veterinary Medicine, China Agricultural University, Beijing

Received: Jan. 5th, 2016; accepted: Jan. 26th, 2016; published: Jan. 29th, 2016
Copyright © 2016 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



ABSTRACT
The kidney, spleen and bursa of fabricius samples were collected from diseased chickens on a farm in
Keywords:Infectious Bursal Disease Virus, VP2, Virulence

一株传染性法氏囊病毒野毒株的分离与鉴定
付梦姣,王永强,李晓齐,曹红,陈福勇,郑世军*
中国农业大学动物医学院,北京

收稿日期:2016年1月5日;录用日期:2016年1月26日;发布日期:2016年1月29日

摘 要
笔者从北京延庆地区一鸡场采集发病鸡的肾脏、脾、法氏囊样品,通过鸡胚接种及RT-PCR技术初步诊断其病原为传染性法氏囊病毒,暂命名为BY-2015-1。本文设计IBDV VP2的引物,以反转录得到的cDNA为模板进行扩增,并对扩增得到的序列进行序列分析,序列分析显示该分离株的核苷酸序列与标准强毒株OKYM的同源性为97.7%,分析VP2高变区的氨基酸序列发现,此分离株在VP2高变区关键氨基酸位点的变异符合强毒株的特点,但又不完全相同。该毒株的分离和鉴定,为传染性法氏囊病防控和流行病学调查提供了依据。
关键词 :传染性法氏囊病毒,VP2,毒力

1. 引言
传染性法氏囊病(IBD)是一种主要危害雏鸡的急性高度传染性病毒病,最早报道于1962年[1] ,又称“岗博罗病”。我国于1979年开始出现此病,并于上世纪90年代流行超强毒株[2] 。其病原为传染性法氏囊病病毒(IBDV),该病毒通过损伤其靶细胞造成免疫抑制,从而使患病鸡增强了对其他病原的易感性[3] 。IBDV属于双RNA病毒科禽双RNA病毒属[4] ,基因组由A和B两个片段组成,A片段由两个部分重叠的开放阅读框组成。第一个开放阅读框编码病毒的非结构蛋白VP5,第二个编码约100 kDa的PVP2-VP4-VP3前体,后被VP4蛋白水解为病毒蛋白VP2、VP3、VP4 [5] [6] 。B片段编码病毒蛋白VP1,是一种RNA聚合酶[7] 。
高强度的免疫压力下,RNA病毒的高突变性往往使得病毒出现一些新属性以维持它们在免疫群体中的存在[8] 。IBDV的突变主要体现在抗原变异,致病性和毒力改变,这种变异与IBDV基因组VP2高变区有关[9] [10] 。本研究从北京一发病鸡场中分离了一株IBDV,并对其进行了初步的鉴定。
2. 材料与方法
2.1. 病料来源与主要试剂
病料来源于北京延庆某鸡场发病鸡的肾脏、脾脏、法氏囊。实验所用主要试剂:
2.2. 病料处理与病毒分离
取病鸡的法氏囊于灭菌的PBS中研磨,−80℃反复冻融三次,5000 rpm/min离心10 min,无菌取上清,加入10,000 U/mL的青链霉素,于4℃作用4 h,经0.22 μm孔径的滤器过滤后冻于−80℃。取处理后的病毒液接种于10日龄SPF鸡胚的绒毛尿囊膜上,待鸡胚死亡后观察绒毛尿囊膜及胚体的病变。
2.3. 毒株的鉴定
2.3.1. 引物合成与RT-PCR检测
根据GeneBank上IBDV SegA片段(NO: EU595672)的序列设计IBDV VP2引物,预计扩增片段为1469 bp,上游引物:5’ATGACAAACCTGCAAGATCAAACCC3’,下游引物:5’CCTTATGGCCCGGATTATGTCTTT3’。提取病变组织及绒毛尿囊膜的RNA并反转录,以得到的cDNA为模板,用VP2引物进行特异性扩增。RNA提取及反转录过程均按照试剂盒说明书进行。
2.3.2. 序列分析及氨基酸比对
将扩增所得到的VP2片段进行测序,利用DNAMAN生物学相关软件,将BY-2015-1株与IBDV超强毒株UK661、OKYM、HK46,经典毒株,变异毒株,血清II型OH等的VP2核苷酸、氨基酸序列进行比对,并绘制进化树。
3. 结果
3.1. 病毒分离与鉴定
病料处理液接种鸡胚后,鸡胚的绒毛尿囊膜较对照组明显增厚,出现IBDV接种后的典型病变(见图1)。提取病变组织及绒毛尿囊膜的RNA进行RT-PCR,扩增出与预计大小相符的特异条带(见图2)。
3.2. 核苷酸及氨基酸序列分析
IBDV超强毒株在222、256、294、299具有四个特征性氨基酸,分别为A、I、I、S,这4个氨基酸使得超强毒株的亲和性发生变化,从而导致毒力大大增强 [11] 。分析分离株VP2高变区氨基酸序列发现,其关键氨基酸位点均符合超强毒株的要求(见图3)。进化树分析也显示分离株与标准强毒株OKYM、UK661、HK46等属于同一进化分支(见图4)。
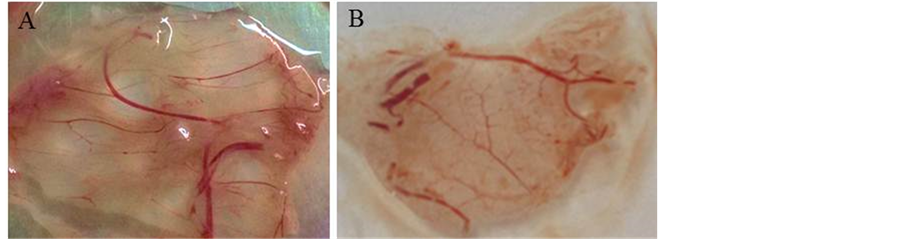
Figure 1. Inoculation of SPF chicken embryos with samples via chorioallantoic membrane. (A) SPF chicken embryo chorioallantoic membrane inoculated with samples and cultured for 72 h, (B) normal SPF chicken embryo chorioallantoic membrane inoculated with sterile PBS as controls
图1. 病毒感染鸡胚结果。(A) 感染72小时后的SPF鸡胚绒毛尿囊膜,(B) 正常SPF鸡胚绒毛尿囊膜

Figure 2. Amplification of IBDV vp2 by PCR. M: DNA Marker III; 1-3: Cloning of VP2; 4: Negative control
图2. PCR结果。M:DNA Marker III;1-3:VP2基因扩增产物;4:阴性对照

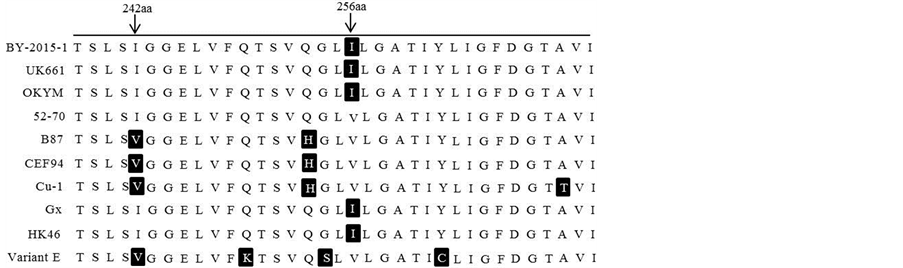


Figure 3. The amino acid sequence analysis of VP2 hypervariable region
图3. IBDV VP2高变区氨基酸序列分析
4. 讨论
传染性法氏囊病是危害鸡群的一类重要免疫抑制性疾病,目前免疫接种和卵黄抗体的特异性治疗是预防和控制该疾病的主要手段。但近几年也经常出现变异株、地方强毒株等流行,造成免疫失败[12] 。IBDV VP2的206到350位氨基酸的差异占整个多聚前体蛋白中氨基酸差异的50%,这一区域称为VP2高变区,并且被认为是决定IBDV毒力的关键区域[13] [14] 。我们通过比较分析分离株病毒VP2基因的高变区来

Figure 4. The systemic cladogram analysis of VP2 genes between IBDV strains
图4. 分离株BY-2015-1与IBDV其他毒株的进化树分析
初步判定此分离株为变异强毒株。
但IBDV病毒毒力并不完全决定于VP2基因,Boot等[15] 人通过反向遗传操作证明VP2并不是决定IBDV毒力的唯一因素。Schroder [16] ,Brandt [13] 等学者分别通过拯救嵌合病毒认为IBDV的非编码区及VP3、VP4蛋白都不是IBDV毒力的决定性因素。然而,Boot等[17] 的研究发现VP3的C端对病毒的毒力有一定影响。VP5蛋白可以影响病毒复制,并在细胞凋亡中扮演重要角色[18] ,但目前还无研究报道证实其与病毒毒力有关。Cyril Le Nouën等[19] 发现非强毒B节段与强毒A节段构成的重组病毒的毒力比A、B节段均来自强毒的病毒毒力要低。Yu等[20] 也证实了VP1蛋白与病毒的毒力有关。但是,B节段究竟是如何影响 IBDV的毒力及其具体功能位点均未有定论。所以目前大家还是主要通过对比VP2高变区的关键氨基酸位点来判定病毒毒力。本研究所分离的BY-2015-1株VP2高变区的A222、I242、I256、I294、S299氨基酸均符合超强毒株的特点,另外在第一亲水区212位氨基酸由D变成了N,与其他株均不同,其生物学意义还需进一步探讨。
本研究所分离的IBDV BY-2015-1株属于超强毒株的范围,但又与标准毒株存在一定差异,为传染性法氏囊病的流行病学研究提供一定依据。
基金项目
本项目由中国国家自然科学基金项目(#31272543)和现代农业产业技术体系建设专项资金(#NYCYTX-41)资助。
文章引用
付梦姣,王永强,李晓齐,曹 红,陈福勇,郑世军. 一株传染性法氏囊病毒野毒株的分离与鉴定
Identification and Isolation of a Wild Strain of Infectious Bursal Disease Virus[J]. 亚洲兽医病例研究, 2016, 05(01): 5-10. http://dx.doi.org/10.12677/ACRPVM.2016.51002
参考文献 (References)
- 1. Cosgrove, A.S. (1962) An Apparently New Disease of Chickens: Avian Nephrosis. Avian Diseases, 6, 385-389. http://dx.doi.org/10.2307/1587909
- 2. 李成洪, 李英伦, 周晓容. 鸡传染性法氏囊病研究进展[J]. 黑龙江畜牧兽医, 2004(2): 47-49.
- 3. Stricker, R.L., Behrens, S.E. and Mundt, E. (2010) Nuclear Factor NF45 Interacts with Viral Proteins of Infectious Bursal Disease Virus and Inhibits Viral Replication. Journal of Virology, 84, 10592-10605. http://dx.doi.org/10.1128/JVI.02506-09
- 4. Bottcher, B., et al. (1997) Three-Dimensional Structure of Infectious Bursal Disease Virus Determined by Electron Cryomicroscopy. Journal of Virology, 71, 325-330.
- 5. Hudson, P.J., et al. (1986) Genomic Structure of the Large RNA Segment of Infectious Bursal Disease Virus. Nucleic Acids Research, 14, 5001-5012. http://dx.doi.org/10.1093/nar/14.12.5001
- 6. Jagadish, M.N., et al. (1988) Birnavirus Precursor Polyprotein Is Processed in Escherichia coli by Its Own Virus-En- coded Polypeptide. Journal of Virology, 62, 1084-1087.
- 7. von Einem, U.I., et al. (2004) VP1 of Infectious Bursal Disease Virus Is an RNA-Dependent RNA Polymerase. Journal of General Virology, 85, 2221-2229. http://dx.doi.org/10.1099/vir.0.19772-0
- 8. van den Berg, T.P., et al. (2004) Assessment of Genetic, Antigenic and Pathotypic Criteria for the Characterization of IBDV Strains. Avian Pathology, 33, 470-476. http://dx.doi.org/10.1080/03079450400003650
- 9. Oppling, V., Muller H. and Becht, H. (1991) Heterogeneity of the Antigenic Site Responsible for the Induction of Neutralizing Antibodies in Infectious Bursal Disease Virus. Archives of Virology, 119, 211-223. http://dx.doi.org/10.1007/BF01310671
- 10. Schnitzler, D., et al. (1993) The Genetic Basis for the Antigenicity of the VP2 Protein of the Infectious Bursal Disease Virus. Journal of General Virology, 74, 1563-1571. http://dx.doi.org/10.1099/0022-1317-74-8-1563
- 11. Jackwood, D.J. and Sommer-Wagner, S. (2007) Genetic Characteristics of Infectious Bursal Disease Viruses from Four Continents. Virology, 365, 369-375. http://dx.doi.org/10.1016/j.virol.2007.03.046
- 12. 赵云英, 等. 一株鸡传染性法氏囊病病毒的分离鉴定[J]. 中国畜牧兽医, 2012, 39(6): 207-211.
- 13. Brandt, M., Yao, K., Liu, M., et al. (2001) Molecular Determinants of Viru-lence, Cell Tropism, and Pathogenic Phenotype of Infectious Bursal Disease Virus. Journal of Virology, 75, 11974-11982. http://dx.doi.org/10.1128/JVI.75.24.11974-11982.2001
- 14. Van, L.A.D.H., Zeyda, I. and Mundt, E. (2002) Al-teration of Amino Acids in VP2 of Very Virulent Infectious Bursal Disease Virus Results in Tissue Culture Adaptation and Attenuation in Chickens. Journal of General Virology, 83, 121-129.
- 15. Boot, H.J., ter Huurne, A.A., Hoekman, A.J., et al. (2000) Rescue of Very Virulent and Mosaic Infectious Bursal Disease Virus from Cloned cDNA: VP2 Is Not the Sole Determinant of the Very Virulent Phenotype. Journal of Virology, 74, 6701-6711. http://dx.doi.org/10.1128/JVI.74.15.6701-6711.2000
- 16. Schroder, A., van Loon, A.A., Goovaerts, D., et al. (2000) Chimeras in Noncoding Regions between Serotypes I and II of Segment A of Infectious Bursal Disease Virus Are Viable and Show Pathogenic Phenotype in Chickens. Journal of General Virology, 81, 533-540. http://dx.doi.org/10.1099/0022-1317-81-2-533
- 17. Boot, H.J., Ter Huurne, A.A., Peeters, B.P. and Gielkens, A.L. (1999) Efficient Rescue of Infectious Bursal Disease Virus from Cloned cDNA: Evidence for Involvement of the 3’-Terminal Sequence in Genome Replication. Virology, 265, 330-341. http://dx.doi.org/10.1006/viro.1999.0042
- 18. Li, Z., Wang, Y., Xue, Y., et al. (2012) Critical Role for Vol-tage-Dependent Anion Channel 2 in Infectious Bursal Disease Virus-Induced Apoptosis in Host Cells via Interaction with VP5. Journal of Virology, 86, 1328-1338. http://dx.doi.org/10.1128/JVI.06104-11
- 19. Le Nouen, C., Rivallan, G., Toquin, D., et al. (2006) Very Virulent Infectious Bursal Disease Virus: Reduced Pathogenicity in a Rare Natural Segment-B-Reassorted Isolate. Journal of General Virology, 87, 209-216. http://dx.doi.org/10.1099/vir.0.81184-0
- 20. Yu, F., Ren, X.G., Wang, Y.Q., et al. (2013) A Single Amino Acid V4I Substitution in VP1 Attenuates Virulence of Very Virulent Infectious Bursal Disease Virus (vvIBDV) in SPF Chickens and Increases Replication in CEF Cells. Virology, 440, 204-209. http://dx.doi.org/10.1016/j.virol.2013.02.026