Advances in Analytical Chemistry
Vol.08 No.02(2018), Article ID:24506,6
pages
10.12677/AAC.2018.82005
A Rapid and Low-Cost Synthesis of Kesterite Cu2ZnSnS4 Microparticles by Microwave Irradiation
Xianhua Chai, Hongmei Qing, Tao Shen, Shuhong Sun, Yan Zhu*
Kunming University of Science and Technology, Kunming Yunnan

Received: Mar. 23rd, 2018; accepted: Apr. 17th, 2018; published: Apr. 25th, 2018

ABSTRACT
With a special solvent, the mixture of ethylene glycol (EG) and triethylenetetramine (TETA), plate-like kesterite Cu2ZnSnS4 (CZTS) microparticles were successfully synthesized by a rapid and low-cost microwave-assisted method. In comparison with the traditional solution process for preparing Cu2ZnSnS4 powders, the reaction time was merely 3 minutes. The samples were characterized by X-ray diffraction, Raman spectroscopy, scanning electronic microscope, transmission electron microscopy and UV–vis spectrophotometer. The results showed that the kesterite Cu2ZnSnS4 microparticles have single phase and stoichiometric composition with plate-like diameters ranging from 300 nm to 1000 nm. The CZTS microparticles have an optical band gap of 1.5 eV, which matches well with optimal direct band gap (~1.5 eV).
Keywords:Kesterite, CZTS, Microwave
微波法快速低成本制备锌黄锡矿铜锌锡硫
柴鲜花,青红梅,沈韬,孙淑红,朱艳*
昆明理工大学,云南 昆明

收稿日期:2018年3月23日;录用日期:2018年4月17日;发布日期:2018年4月25日

摘 要
本文以一定比例的乙二醇(EG)和三乙烯四胺(TETA)混合物作为溶剂,通过快速、低成本的微波辅助方法成功合成了锌黄锡矿铜锌锡硫(Cu2ZnSnS4,CZTS)微粒。与传统制备Cu2ZnSnS4粉末的溶液法相比,反应时间仅为3分钟,是目前已知文献报道的最快合成速度。文中通过X射线衍射、拉曼光谱、扫描电子显微镜、透射电子显微镜和紫外可见分光光度计对样品进行表征。结果表明,锌黄锡矿铜锌锡硫微粒为纯相,其颗粒大小范围为300~1000 nm。铜锌锡硫微粒具有1.5 eV的光学带隙,其与最佳直接带隙(~1.5 eV)匹配良好。
关键词 :锌黄锡矿,铜锌锡硫,微波

Copyright © 2018 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
近年来,薄膜太阳能电池在全球范围内得到大力发展,其具有成本低、重量轻和易弯曲等优点。在用于薄膜太阳能电池的不同类型的吸收材料中,铜铟镓硒(CuInGaSe2,CIGS)因其性能稳定,转换效率达20%以上而成为最佳太阳能吸收材料之一 [1] [2] 。然而,铜铟镓硒的成分中镓有毒且可用性低,铟和镓较为昂贵从而阻碍了铜铟镓硒进一步大规模推广。铜锌锡硫(CZTS)具有光学直接带隙(~1.5 eV)和较大吸收系数(>104 cm−1)并且其原料无毒,锌和锡地壳含量丰富价格低廉,适用于替代CIGS 薄膜太阳能电池 [3] 。此外,通过Shockley-Queisser光子平衡计算,铜锌锡硫薄膜太阳能电池的理论极限接近32.2%,因此有很大的改进空间 [4] 。由于铜锌锡硫这些经济优势和优良的性能,近年越来越多的研究者研究铜锌锡硫在太阳能电池中的应用 [5] [6] 。
合成铜锌锡硫颗粒的主要方法包括热注入 [4] [7] [8] 、溶剂热 [9] [10] 、球磨 [11] [12] 和一锅煮 [13] [14] 等。以上均为非真空法,相比于真空法来说其具有低成本和高产量的优势 [15] 。然而溶剂热和球磨法的设备相对复杂且设备要求较高;溶剂热反应需要高温高压条件才能获得所需产物,并且难以控制。同时,这些方法的反应时间为30分钟至48小时,反应时间较长。铜锌锡硫作为薄膜太阳能电池是一种商业型应用半导体材料,所以降低反应成本和时间也是目前的商业需求。微波辐射在学术界和工业界是广泛使用的成熟技术,它具有制备简单,反应时间大大缩短的优点 [16] [17] 。微波加热是微波与物质分子相互作用并被吸收而产生的热效应,也就是说一般微波作用时,使极性分子产生偶极矩转向极化,因为微波形成的交变电场以每秒高达数亿次的高速变向,偶极矩转向极化不能够跟上交变电流改变的速度,导致偶极矩极化落后于电场,从而导致材料内部功率耗散,一部分微波能转化为热能,从而使材料自身得以升温加热。所以微波加热较传统加热更加的高效快速,通过体加热的方式使物料迅速升温,达到快速加热的效果。Hong等报道了微波辐射法制备CZTS纳米晶,反应时间减少到21分钟左右 [18] 。Kim等和Sarswat等通过采用微波法反应合成铜锌锡硫使反应时间进一步缩短到10~15分钟 [19] [20] 。此外,许多研究小组利用商业微波炉来有效降低设备成本 [18] [19] [20] 。Shen等采用微波法制备了铜锌锡硫颗粒,反应时间缩短到5分钟,但有杂质ZnS的存在 [21] 。
因此,本文采用乙二醇(EG)和三亚乙基四胺(TETA) (EG:TETA的体积比为1:6) [22] 的特殊混合溶剂在大气条件下通过微波照射合成铜锌锡硫颗粒,其具有快速、简便、环保且高效的特点。我们研究了一种简单的微波辅助方法,使用商用微波炉在不退火的情况下研究纯相锌黄锡矿铜锌锡硫微粒,反应时间仅需3分钟。
2. 试验材料与方法
制备方法如下:将2 mmol氯化铜(II)二水合物(CuCl2·2H2O),1.25 mmol乙酸锌(II)二水合物(Zn(CH3COO)2·2H2O),1 mmol氯化锡(II)二水合物(SnCl2·2H2O),6 mmol硫脲(CH4N2S)溶解于6 mL乙二醇(EG)和36 mL三亚乙基四胺(TETA)中。将原料完全溶解在有机混合溶剂中得到前驱体溶液,然后放入商用微波炉(2.45 GHz,最大功率800 W)中。前驱体在微波炉反应加热3分钟,在反应过程中溶液颜色从蓝色变为黑色。反应完成后通过离心(8000 rpm,6 min)清洗(乙醇和去离子水)数次。最后将洗净后的黑色沉淀物在60℃的真空干燥箱中干燥6小时。整个实验是在无任何气体保护的环境下进行的。
3. 试验结果及讨论
通过X射线衍射(XRD)和拉曼光谱研究合成的铜锌锡硫微粒的结晶度和相纯度。使用能谱扫描电镜(EDS)和扫描电子显微镜(SEM)分析合成产物的形态和组成。使用紫外可见分光光度计研究所得粉末的光学性质。
3.1. 物相分析
图1(a)显示了在800 W、3 min的条件下微波制备的产物的XRD图谱。图中显示2θ为18.21˚,28.40˚,32.93˚,47.30˚,56.16˚,69.24˚,76.42˚的衍射峰对应锌黄锡矿铜锌锡硫结构(JCPDS No. 26-0575)的(101),(112),(200),(220),(312),(008)和(332)晶面 [23] 。该结构与立方ZnS和四方Cu2SnS3类似 [24] 。所以为了进一步确认合成的铜锌锡硫微粒的相纯度,采用拉曼光谱进一步表征。如图1(b)所示,样品在327 cm−1出现一个主峰,这是由于锌黄锡矿铜锌锡硫在这个位置对拉曼有作用。然而,立方ZnS拉曼峰位在351 cm−1和274 cm−1,四方Cu2SnS3的拉曼峰位为318 cm−1和348 cm−1 [25] [26] 。而所测拉曼峰与两者均不吻合,所以产物为纯相锌黄锡矿铜锌锡硫,无其他杂质。
3.2. 形貌分析
图2(a)显示了在800 W、3 min条件下合成的铜锌锡硫粉末的SEM图像。图中可看出其形貌为类平板状,直径大小范围为300 nm到1000 nm。基于EDS光谱的结果表明(图2(b)),产物的平均组成为Cu:Zn:Sn:S = 2.3:0.58:1:3.61,锌含量少可能是由于反应时间太快从而使锌不能完全进入反应体系中。
图3为锌黄锡矿铜锌锡硫的TEM,其中包含选区电子衍射(SAED)和高分辨透射电子显微镜(HRTEM)。图3(a)中的粒子尺寸约为350 nm,与SEM显示粉末的粒径范围为300~1000 nm吻合。SAED图(图3(a)中插图)表明微粒是单晶,因此CZTS粒子具有良好的结晶度。图3(b)显示产物具有明确的晶格条纹,晶格间距为0.27 nm对应于锌黄锡矿结构铜锌锡硫的(200)面。
3.3. 光学性能
合成的铜锌锡硫的紫外-可见光吸收光谱如图4(a)所示。由图可以看出该材料在可见光范围内对光吸收有所不同,在400 nm时吸收较弱,随着波长增加吸收逐渐增强,到波长为680 nm为一个波峰。由于铜锌锡硫为直接带隙材料,吸收系数满足关系式(αhν)2 = A(hν – Eg),(α = 吸光度,h = 普朗克常数,ν =频率,Eg = 带隙能量,A为常数)。通过绘制(αhν)2和光子能量hν的函数关系图,然后采用外推法得到两
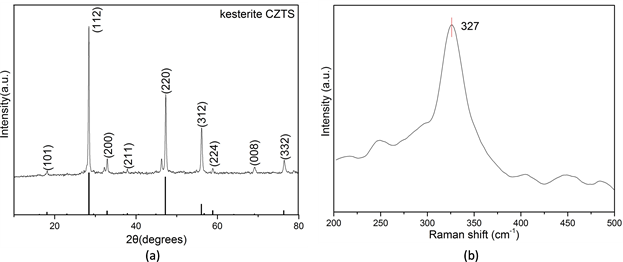
Figure 1. (a) XRD patterns and (b) Raman spectra of the typical sample synthesized at 800 W for 3 min
图1. 在800 W、3 min条件下合成产物的:(a) XRD图谱,(b) 拉曼光谱

Figure 2. (a) An SEM image; (b) The EDS spectrum of the CZTS microparticles synthesized at 800 W for 3 min
图2. 在800 W、3 min条件下合成的铜锌锡硫微粒的:(a) SEM图谱;(b) EDS光谱
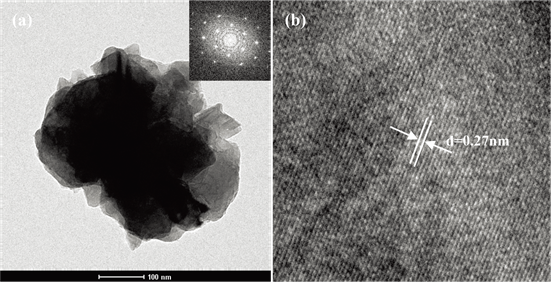
Figure 3. (a) The TEM and (b) HRTEM images of the kesterite CZTS (the inset of Figure 3(a) shows the SAED of the microparticles)
图3. 锌黄锡矿铜锌锡硫的:(a) TEM图谱,(b) HRTEM光谱(图3(a)中嵌入图为微粒的衍射花样)
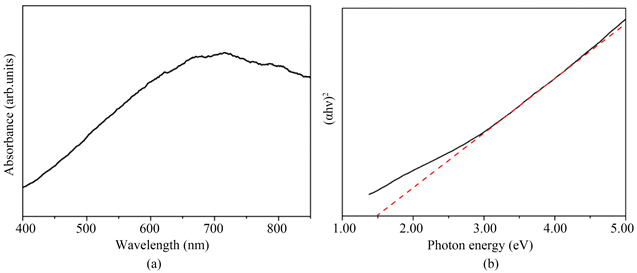
Figure 4. (a) The UV-vis absorption spectrum; (b) The plot of (αhν)2 versus photon energy of the kesterite CZTS microcrystals
图4. 锌黄锡矿铜锌锡硫微粒的:(a) 紫外光学图谱;(b) (αhν)2 ~hν曲线图
者的线性拟合交点,取该值得到产物的光学带隙。如图4(b)所示所得光学带隙能量为1.5 eV,这与文献中报道的光学带隙(1.4~1.56 eV)相符 [4] [9] [10] 。
4. 结论
综上所述,采用EG和TETA一定比例混合溶剂作为环境友好型溶剂,在常压条件下800 W微波辐射,合成了大小为300~1000 nm的类板状锌黄锡矿铜锌锡硫微粒。采用这种微波辅助加热方法加速了铜锌锡硫的反应,反应时间仅需要3分钟,是目前已知文献报道的最短反应时间。通过紫外吸收测量铜锌锡硫微粒的带隙为1.5 eV,这符合太阳能电池最佳光学带隙范围。
基金项目
国家自然科学基金(No.61764010)。
文章引用
柴鲜花,青红梅,沈 韬,孙淑红,朱 艳. 微波法快速低成本制备锌黄锡矿铜锌锡硫
A Rapid and Low-Cost Synthesis of Kesterite Cu2ZnSnS4 Microparticles by Microwave Irradiation[J]. 分析化学进展, 2018, 08(02): 33-38. https://doi.org/10.12677/AAC.2018.82005
参考文献
- 1. Jackson, P., Hariskos, D., Lotter, E., Paetel, S., Wuerz, R., Menner, R., Wischmann, W. and Powalla, M. (2011) New World Record Efficiency for Cu(In,Ga)Se2 Thin-Film Solar Cells beyond 20%, Progress in Photovoltaics. Research and Applications, 19, 894-897.
https://doi.org/10.1002/pip.1078 - 2. Guchhait, A., Dewi, H.A., Leow, S.W., Wang, H., Han, G., Suhaimi, F.B., Mhaisalkar, S., Wong, L.H. and Mathews, N. (2017) Over 20% Efficient CIGS-Perovskite Tandem Solar Cells. ACS Energy Letters, 2, 807-812.
https://doi.org/10.1021/acsenergylett.7b00187 - 3. Riha, S.C., Parkinson, B.A. and Prieto, A.L. (2009) Solu-tion-Based Synthesis and Characterization of Cu2ZnSnS4 Nanocrystals. Journal of the American Chemical Society, 131, 12054-12055.
https://doi.org/10.1021/ja9044168 - 4. Guo, Q., Hillhouse, H.W. and Agrawal, R. (2009) Syn-thesis of Cu2ZnSnS4 Nanocrystal Ink and Its Use for Solar Cells. Journal of the American Chemical Society, 131, 11672-11673.
https://doi.org/10.1021/ja904981r - 5. Steinhagen, C., Panthani, M.G., Akhavan, V., Goodfellow, B., Koo, B. and Korgel, B.A. (2009) Synthesis of Cu2ZnSnS4 Nanocrystals for Use in Low-Cost Photovoltaics. Journal of the American Chemical Society, 131, 12554-12555.
https://doi.org/10.1021/ja905922j - 6. Katagiri, H., Jimbo, K., Maw, W.S., Oishi, K., Yamazaki, M., Araki, H. and Takeuchi, A. (2009) Development of CZTS-Based Thin Film Solar Cells. Thin Solid Films, 517, 2455-2460.
https://doi.org/10.1016/j.tsf.2008.11.002 - 7. Xia, D., Zheng, Y., Lei, P. and Zhao, X. (2013) Characterization of Cu2ZnSnS4 Thin Films Prepared by Solution-Based Deposition Techniques. Physics Procedia, 48, 228-234.
https://doi.org/10.1016/j.phpro.2013.07.036 - 8. Nguyen, D.-C., Ito, S. and Dung, D.V.A. (2015) Effects of Annealing Conditions on Crystallization of the CZTS Absorber and Photovoltaic Properties of Cu(Zn,Sn)(S,Se)2 Solar Cells. Journal of Alloys and Compounds, 632, 676-680.
https://doi.org/10.1016/j.jallcom.2015.01.258 - 9. Cao, M. and Shen, Y. (2011) A Mild Solvothermal Route to Kesterite Quaternary Cu2ZnSnS4 Nanoparticles. Journal of Crystal Growth, 318, 1117-1120.
https://doi.org/10.1016/j.jcrysgro.2010.10.071 - 10. Gong, Z., Han, Q., Li, J., Hou, L., Bukhtiar, A., Yang, S. and Zou, B. (2016) A Solvothermal Route to Synthesize Kesterite Cu2ZnSnS4 Nanocrystals for Solution-Processed Solar Cells. Journal of Alloys and Compounds, 663, 617-623.
https://doi.org/10.1016/j.jallcom.2015.12.181 - 11. Pani, B., Singh, R.K. and Singh, U.P. (2015) Impact of Pre-Annealing Temperature on the Formation of Cu2ZnSnS4 Absorber Layer. Journal of Alloys and Compounds, 648, 332-337.
https://doi.org/10.1016/j.jallcom.2015.05.207 - 12. Pareek, D., Balasubramaniam, K.R. and Sharma, P. (2015) Synthesis and Characterization of bulk Cu2ZnSnX4(X: S, Se) via Thermodynamically Supported Mecha-no-Chemical Process. Materials Characterization, 103, 42-49.
https://doi.org/10.1016/j.matchar.2015.03.014 - 13. Zou, Z., Gao, Y., Long, F., Wang, J. and Zhang, J. (2015) One-Pot Solvothermal Synthesis of Wurtzite Cu2ZnSnS4 Nanocrystals. Materials Letters, 158, 13-16.
https://doi.org/10.1016/j.matlet.2015.05.063 - 14. Zhong, J., Xia, Z., Zhang, C., Li, B., Liu, X., Cheng, Y.-B. and Tang, J. (2014) One-Pot Synthesis of Self-Stabilized Aqueous Nanoinks for Cu2ZnSn(S,Se)4 Solar Cells. Chemistry of Materials, 26, 3573-3578.
https://doi.org/10.1021/cm501270j - 15. Zhao, Y., Tao, W., Chen, X., Liu, J. and Wei, A. (2015) Synthesis and Characterization of Cu2ZnSnS4 Nanocrystals Prepared by Microwave Irradiation Method. Journal of Materials Science: Materials in Electronics, 26, 5645-5652.
https://doi.org/10.1007/s10854-015-3114-0 - 16. Singh, R., Rangari, V.K., Sanagapalli, S., Jayaraman, V., Ma-hendra, S. and Singha, V.P. (2004) Nano-Structured CdTe, CdS and TiO2 for Thin Film Solar Cell Applications. Solar Energy Materials and Solar Cells, 82, 315-330.
https://doi.org/10.1016/j.solmat.2004.02.006 - 17. Li, L., Qian, H. and Ren, J. (2005) Rapid Synthesis of Highly Luminescent CdTe Nanocrystals in the Aqueous Phase by Microwave Irradiation with Controllable Temperature. Chemical Communications, 528-530.
https://doi.org/10.1039/b412686f - 18. Saravana Kumar, R., Ryu, B.D., Chandramohan, S., Seol, J.K., Lee, S.-K. and Hong, C.-H. (2012) Rapid Synthesis of Sphere-Like Cu2ZnSnS4 Microparticles by Microwave Irradiation. Materials Letters, 86, 174-177.
https://doi.org/10.1016/j.matlet.2012.07.059 - 19. Shin, S.W., Han, J.H., Park, C.Y., Kim, S.R., Park, Y.C., Agawane, G.L., Moholkar, A.V., Yun, J.H., Jeong, C.H., Lee, J.Y. and Kim, J.H. (2012) A Facile and Low Cost Syn-thesis of Earth Abundant Element Cu2ZnSnS4 (CZTS) Nanocrystals: Effect of Cu Concentrations. Journal of Alloys and Compounds, 541, 192-197.
https://doi.org/10.1016/j.jallcom.2012.06.086 - 20. Sarswat, P.K. and Free, M.L. (2013) An Investigation of Rapidly Synthesized Cu2ZnSnS4 Nanocrystals. Journal of Crystal Growth, 372, 87-94.
https://doi.org/10.1016/j.jcrysgro.2013.03.022 - 21. Wang, W., Shen, H. and He, X. (2013) Study on the Synthesis and Formation Mechanism of Cu2ZnSnS4 Particles by Microwave Irradiation. Materials Research Bulletin, 48, 3140-3143.
https://doi.org/10.1016/j.materresbull.2013.04.078 - 22. Qing, H.M., Zhu, Y., Hu, Y.M., Hu, W., Zhou, W., Yi, J. and Shen, T. (2016) A Facile Two-Step-Heating Route to Synthesize Hierarchical Metastable Wurtzite Cu2ZnSnS4 Microcrystals under the Open-Air Condition. Materials Letters, 176, 177-180.
https://doi.org/10.1016/j.matlet.2016.04.084 - 23. Song, Y., Liu, A., Mu, C., Huo, W., Lv, W. and He, W. (2015) A Facile and Scalable Route to Nano-Crystallized Kesterite Cu2ZnSnS4 Fibers via Electrospinning/Sulfurization. Ma-terials Research Bulletin, 61, 504-510.
https://doi.org/10.1016/j.materresbull.2014.10.069 - 24. Guo, Q., Ford, G.M., Yang, W.C., Walker, B.C., Stach, E.A., Hillhouse, H.W. and Agrawal, R. (2010) Fabrication of 7.2% Efficient CZTSSe Solar Cells Using CZTS Nano-crystals. Journal of the American Chemical Society, 132, 17384-17386.
https://doi.org/10.1021/ja108427b - 25. Wang, W., Shen, H., Jiang, F., He, X. and Yue, Z. (2012) Low-Cost Chemical Fabrication of Cu2ZnSnS4 Microparticles and Film. Journal of Materials Science: Materials in Electronics, 24, 1813-1817.
https://doi.org/10.1007/s10854-012-1017-x - 26. Méndez-López, A., Morales-Acevedo, A., Acosta-Silva, Y.J. and Ortega-López, M. (2016) Synthesis and Characterization of Colloidal CZTS Nanocrystals by a Hot-Injection Method. Journal of Nanomaterials, 2016, Article ID: 7486094.
https://doi.org/10.1155/2016/7486094
NOTES
*通讯作者。