Pharmacy Information
Vol.
13
No.
01
(
2024
), Article ID:
78914
,
9
pages
10.12677/PI.2024.131001
小分子EGFR-TKIs在非小细胞肺癌治疗中的 研究进展
胡亚南
南京知和医药科技有限公司,江苏 南京
收稿日期:2023年12月3日;录用日期:2024年1月3日;发布日期:2024年1月10日

摘要
小分子表皮生长因子受体酪氨酸激酶抑制剂(epithelial growth factor receptor tyrosine kinase inhibitor, EGFR-TKI)在治疗伴有EGFR突变的非小细胞肺癌(non-small cell lung cancer, NSCLC)中获得了巨大的临床收益。但患者经过一段时间EGFR-TKIs治疗,不可避免的出现获得性耐药。本文主要对小分子EGFR-TKIs在NSCLC治疗中的研究进展及进行综述,为该类药物的临床使用及未来的研究方向提供参考。
关键词
表皮生长因子受体,酪氨酸激酶抑制剂,非小细胞肺癌,突变,耐药

Research Advances of Small Molecule EGFR-TKIs in NSCLC
Yanan Hu
Nanjing Zhihe Pharmaceutical Technology Co. Ltd., Nanjing Jiangsu
Received: Dec. 3rd, 2023; accepted: Jan. 3rd, 2024; published: Jan. 10th, 2024

ABSTRACT
Small molecule epithelial growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) has achieved great clinical benefits in the treatment of non-small cell lung cancer (NSCLC) with EGFR mutation. However, after a period of EGFR-TKIs treatment, patients will inevitably acquire drug resistance. Thereview summarizes the research progress of EGFR-TKIs in the treatment of NSCLC which will provide reference for the clinical application and the future of EGFR-TKIs discovery.
Keywords:Epidermal Growth Factor Receptor, Tyrosine Kinase Inhibitor, Non-Small Cell Lung Cancer, Mutation, Drug Resistance

Copyright © 2024 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
癌症是威胁人类健康且致死率很高的重大疾病,根据《AACR Cancer Progress Report 2022》 [1] 报告显示,预计到2040年,全球癌症患者总数将达到2800万,同时约有1620万患者将因癌症去世。在中国,每年新发癌症高达数百万例,排在第一位的是肺癌,其中NSCLC占据约80%。自从2001年第一个针对BCR-ABL酪氨酸激酶开发的小分子抑制剂伊马替尼(Imatinib)成功上市后,针对的EGFR的酪氨酸激酶抑制剂吉非替尼(Gefitinib,艾瑞莎)和埃罗替尼(Erlotinib,特罗凯)相继被批准用于治疗非小细胞肺癌(NSCLC)。虽然EGFR-TKI表现出了强大的抑制肿瘤的效果,但这些药物并不能完全治愈肿瘤,大多数只是延迟肿瘤进展,最终肿瘤都会找到逃避靶点抑制作用的途径,从而产生耐药性,而有效地解决这一问题已成为一项重大挑战 [2] 。在过去20年里,科学家针对其耐药机制,开发新的EGFR酪氨酸激酶抑制剂,或研究采用联合治疗的方法来提高治疗效果。基于近年来EGFR-TKI在治疗NSCLC中的应用,本文将对该类抑制剂研究进展进行概述。
2. EGFR的结构、与癌症的关系和耐药形成机制
EGFR相对分子质量为1.7 × 105 Da,其结构分为胞内区、跨膜区和胞外区三个部分。胞外区EGFR胞外包含4个结构域:结构域Ⅰ/L1 (氨基酸1~165)、富含半胱氨酸的结构域Ⅱ/CR2 (166~309)、结构域Ⅲ/L2 (310~481)和富含半胱氨酸的结构域Ⅳ/CR2 (482~621);(L1, CR1, L2, CR2)为配体结合区,能够与具有激动功能的配体相合(配体如EGF、转化生长因子2-α以及双向调节因子等);跨膜区是由23个氨基酸残基构成的疏水区,具有单链α螺旋结构,可将受体固定在细胞膜上;胞内区分为三个亚区:近膜亚区(JM, 645~682),酪氨酸激酶亚区(683~958)和C端(959~1186),其中近膜亚区(JM)前13个氨基酸(645~657)可介导胞内二聚化,其自身磷酸化位点在C端(图1) [3] [4] 。

Figure 1. The structure of epidermal growth factor receptor [2]
图1. 表皮生长因子受体(EGFR)结构示意图 [2]
EGFR家族共有ErbB-1 (也称EGFR/HER-1)、ErbB-2 (HER-2)、ErbB-3 (HER-3)和ErbB-4 (HER-4) 4个亚型,当胞外区与生长因子或其他配体结合时发生同源或异源二聚化,无论是同源二聚化还是异源二聚化都会导致胞内酪氨酸激酶磷酸化,通过将ATP的γ磷酸基转移到功能蛋白的酪氨酸残基上,从而激活EGFR下游的各种信号通路,如MAPK、PI3K/Akt、STAT3与ATT5等通路,最终导致肿瘤细胞的增殖、分化、逃避细胞凋亡、侵袭及血管生成 [5] 。研究表明,非小细胞肺癌EGFR表达阳性率为40%~80%,并与不良预后有关 [6] 。EGFR-TKI通过与细胞内酪氨酸激酶结构域上的ATP位点竞争性结合,抑制受体自身磷酸化,阻止酪氨酸激酶的活化,从而抑制肿瘤细胞进程,加速肿瘤细胞凋亡,抑制新生血管形成、侵袭和转移 [7] [8] [9] 。
目前,EGFR-TKI最常见的耐药机制为EGFR的激酶区发生点突变(如L858R、DEL19、T790M和C797S) [10] [11] [12] [13] [14] ,当然肿瘤突变(如p53基因突变和ROS1基因突变) [15] [16] [17] [18] [19] 、信号旁路通路的激活(如MET信号通路和ROHA2信号通路) [20] [21] [22] [23] 也是产生耐药的途径之一。
3. 第一代和第二代EGFR-TKIs结构及治疗特点
在早期,人们发现NSCLC中EGFR结构突变主要为第858位处亮氨酸转变为精氨酸(L858R)和外显子19的缺失突变(DEL19) [10] 。第一、第二代EGFR-TKI在临床上主要用于EGFR-L858R和EGFR-Del19单突变和双突变的治疗,是ATP的竞争性抑制剂。第一代代表药物有吉非替尼(Gefitinib)和埃罗替尼(Erlotinib);第二代为不可逆的EGFR-TKIs,代表药物如阿法替尼(Afatinib)、达克替尼(Dacomitinib)。
第一、第二代EGFR-TKI,如吉非替尼、埃罗替尼、阿法替尼和达克替尼都是苯胺基喹唑啉类衍生物,如图2所示。
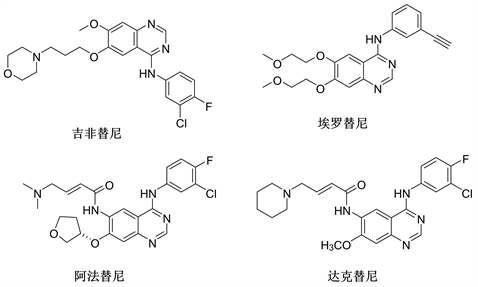
Figure 2. The structure of the first and second generation EGFR-TKIs
图2. 第一代和第二代EGFR-TKIs化学结构
WJOG 5108LFS [24] 研究对比了埃罗替尼和吉非替尼在治疗EGFR-L858R和EGFR-Del19晚期NSCLC患者中位生存时间(MST)和5年生存率(OS),埃罗替尼治疗组和吉非替尼治疗组整体患者的MST和5年生存率分别为31.97个月和27.98个月以及25%和20%,其中EGFR-Del19患者的MST为37.49个月和28.91个月,EGFR-L858R患者的MST为22.21个月和28.16个月。
ARCHER 1050 [25] 研究则比较了达克替尼和吉非替尼尼在EGFR突变晚期NSCLC一线治疗中的疗效,达克替尼组与吉非替尼组的无进展生存期(PFS)分别为14.7个月和9.2个月(P < 0.0001),该研究为达克替尼作为一线治疗药物提供了数据支持。LUX-Lung 7 II期 [26] 研究对比了阿法替尼和吉非替尼治疗EGFR-L858R和EGFR-Del19患者的OS (27.9个月和24.5个月),其结果并无显著差异,但在临床用药选择时可能会参考两种药物不良反应。在一项旨在评估阿法替尼治疗III期NSCLC突变的II期试验(NCTO4201756)中,阿法替尼治疗展示了良好的客观肿瘤反应和临床实践中可接受的毒性,并且观察到肿瘤微环境的变化,这可能对确定EGFR-TKI治疗的预测标志物和指导未来的临床试验具有一定意义 [27] 。
在联合治疗方面,吉非替尼联合化疗在治疗EGFR-L858R/Del19突变的IV期NSCLC患者中能有效地降低患者肿瘤标志物水平,提高生存质量且安全性较高 [28] 。秦冉冉等 [29] 使用埃罗替尼替尼联合奈达铂、培美曲塞治疗IIIB、IV期NSCLC,能延长生存期,提高生存率,且安全性较高,可广泛使用。在一项最新的厄罗替尼与吉西他滨联合顺铂辅助治疗IIIA-N2期EGFR突变型NSCLC:EMERGING-CTONG 1103随机II期试验的最终总生存率分析中,厄罗替尼联合化疗能够改善肿瘤患者的无进展生存期(PFS)和病理完全缓解(Pathological complete remission, pCR)率 [30] 。
4. 第三代EGFR-TKIs结构及治疗特点
随着治疗的不断进行,第一、第二代EGFR-TKI最常见的耐药机制是EGFR 20号外显子获得性T790M突变,导致抑制剂与EGFR的ATP口袋结合力减弱。另外,T790M突变增加了EGFR-L858R突变体与ATP的亲和力 [11] [12] 。专门针对EGFR-T790M突变开发出第三代EGFR-TKIs,如奥希替尼(Osimertinib, AZD9291)、拉泽替尼(Lazertinib, YH25448)、阿美替尼(Aumolertinib, HS10296)和伏美替尼(Furmonertinib, AST2818),第三代EGFR-TKI药物(图3)可以选择性的识别T790M突变的EGFR。
从结构上看,第三代EGFR-TKI如:奥希替尼、拉泽替尼、阿美替尼和伏美替尼为苯胺基嘧啶类衍生物,候选药物DBPR112为呋喃嘧啶类衍生物,但它们都含有丙烯酰氨这一特殊基团。该结构能与ATP结合位点的C797位残基形成不可逆的共价键来降低与ATP的亲和力 [31] [32] 。
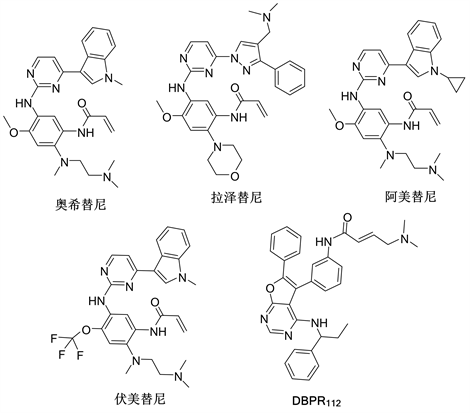
Figure 3. The structure of the third generation EGFR-TKIs
图3. 第三代EGFR-TKIs化学结构
奥希替尼在与T790M结合时,倾向于自身吲哚环旋转180˚的翻转结合,该构像可以赋予化合物对T790M更高的亲和力,也能促进化合物丙烯酰氨结构与Cys797形成共价键 [33] 。拉泽替尼的吡唑取代基比较独特,其中不仅含有亲水性的N,N-二甲基氨基,还含有疏水性的苯环,苯环可以与T790M作用,而N,N-二甲基氨基可以与丙烯酰氨形成分子内氢键,对比奥希替尼,这种结构即稳定自身构象,也提高突变体选择性和改善药物化学性质 [34] 。
奥希替尼在临床上主要用于EGFR-T790M患者治疗,也有报道其对既往未经治疗的EGFR-L858R和EGFR-Del19晚期NSCLC患者显示出比第一、第二代更好的效果。针对全球人群的FLAURA [35] 研究结果显示奥希替尼组与吉非替尼或埃罗替尼组的PFS分别为18.9个月和10.2个月(P < 0.0001),不良事件的发生率也低于对照组。而AURA3 [36] 研究评估了奥希替尼在治疗EGFR-T790M晚期患者的安全性、有效性,为其临床使用的推广奠定了基础。
拉泽替尼是2021年韩国食品药品管理局批准上市用于治疗EGFR-T790M晚期和转移性NSCLC患者的药物,能有效靶向EGFR-L858R/T790M,并在较小程度上靶向HER-2 [34] 。根据其I/II期临床研究更新(MA26.09) [37] 显示,患者总体的客观缓解率(ORR)可达65%,其中EGFR-T790M患者的ORR为69%,并且在NSCLC患者治疗过程中表现出安全、耐受性良好的治疗效果。在1/2期LASER201 [38] 研究中拉泽替尼在治疗EGFR-L858R、Del19突变显示了显著的临床活性。
阿美替尼和伏美替尼都是通过保留奥希替尼苯氨基密啶和丙烯酰胺结构的基础上优化的得到的。阿美替尼是通过将吲哚环上甲基替换为环丙基得到的化合物,环丙基使得该化合物具有更好的选择性,可以有效抑制EGFR-T790M耐药突变,同时又增加血脑屏障穿透性,可有效控制颅内转移灶 [39] [40] 。AENEAS [41] 研究结果显示,阿美替尼组与吉非替尼组的PFS分别为19.3个月和9.9个月(P < 0.0001),在治疗结果上能够与奥希替尼相媲美。伏美替尼则是将苯环上甲氧基取代进行优化,引入三氟乙氧基吡啶结构。FURLONG [42] 研究结果显示,伏美替尼组与吉非替尼组的PFS分别为20.8个月和11.1个月(P < 0.0001),在安全性方面,伏美替尼组的不良事件发生率也低于对照组。
DBPR112中含有的(S)-2-苯基甘氨酸结构为生物活性关键片段,其上的羟基可以与Asp858残基形成额外的氢键,丙烯酰氨末端引入的N,N-二甲基氨基也能提高活性,自身的呋喃嘧啶结构和三个苯基也能与多个残基产生疏水相互作用 [43] 。DBPR112不仅对EGFR-L858R/T790M显示出有效的抑制性,还表现出比奥希替尼优越10倍的药效,目前正在台湾进行I期临床试验(NCT03246854) [43] 。当然在开发过程中还有许多失败的药物,如Naquotinib (ASP8273) [44] 、罗希替尼(Rociletinib, CO-1686) [45] 都因活性和安全性等问题而停止了进一步的临床研究。
5. 第四代EGFR-TKIs结构及治疗特点
虽然以奥希替尼为主的第三代药物在治疗EGFR-T790M上展现出优越的疗效,但患者在用药后也出现了继发耐药,其中最常见的是EGFR-C797S突变,研究显示EGFR上第20号外显子编码的酪氨酸结构域797位半胱氨酸突变为丝氨酸(C797S),该突变阻止了不可逆共价键的形成,是产生耐药的主要机制 [13] [14] 。针对这种耐药性,目前已研发出一批具有代表性的化合物进行治疗,其中部分化合物正在进行临床实验中 [46] [47] 。
EAI045是第一个针对T790M和C797S突变的选择性小分子变构抑制剂,单药治疗时疗效不明显,与西妥昔单抗联用对EGFR-Del19/T790M/C797S三重突变荷瘤小鼠NSCLC模型有效 [48] 。由于联合使用的不良反应较大,且对EGFR-Del19/T790M疗效不佳,目前该药已停止研发。
在EAI045结构的基础上进行结构优化,得到了开发出对EGFR突变有效的新型变构抑制剂JBJ-04-125-02,它作为单药可以在体外和体内抑制细胞增殖和EGFR-L858R/T790M/C797S突变,奥希替尼与JBJ-04-125-02联用的疗效更高 [49] 。对JBJ-04-125-02进行改造,得到更高效的EGFR变构抑制剂JBJ-09-063,相比于JBJ-04-125-02和奥希替尼(第三代EGFR-TKI),JBJ-09-063对EGFR-L858R/T790M和EGFR-L858R/T790M/C797S的抑制作用更强,且不受EGFR-C797S突变的影响 [50] 。
Ulrike等 [51] 通过对EAI045进一步优化开发出的化合物,经进一步证实与ATP竞争性抑制剂奥希替尼联合使用可以获得更好的疗效。BLU-945是一种高效、高选择性的第四代EGFR-TKI (图4),在奥希替尼耐药的EGFR突变移植模型中展现出强大的抗肿瘤活性,可以有效地抑制EGFR-L858R/T790M、EGFR-L858R/T790M/C797S、EGFR-Del19/T790M/C797S突变 [52] ,目前其I/II临床试验(NCT04862780)正在进行中。CH7233163被证明能有效抑制EGFR-Del19/T790M/C797S三突变和EGFR-L858R/T790M/C797S三突变、双突变和单突变,极大的有益于奥希替尼耐药患者,具有临床评估潜力 [53] 。TQB3804显示出对EGFR-T790M/C797S强有效的抑制活性,并将进行相应临床研究(NCT04128085) [47] 。BBT-176对EGFR-Del19/T790M/C797S和 EGFR-L858R/T790M/C797S三重突变均具有显著的抑制作用,目前处于I期临床研究 [54] 。
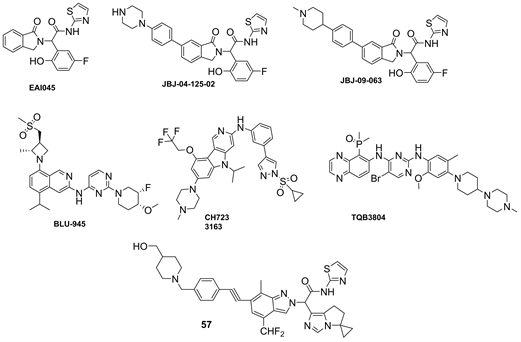
Figure 4. The structure of the fourth generation EGFR-TKIs
图4. 第四代EGFR-TKIs化学结构
6. 小结与展望
从第一个EGFR-TKI研制出来到现在几十年,已有十多种EGFR-TKIs应用于临床治疗NSCLC。但分子靶向药物治疗出现的耐药现象,为癌症患者后续治疗带来了挑战。充分了解耐药机制设计开发新一代EGFR-TKIs潜力巨大,如第四代变构抑制剂的一个潜在优势是避免了与细胞内ATP竞争与催化位点的结合,从而增加了开发更强效和更特异抑制剂的可能性。EGFR-TKIs与其它药物的联合使用可以阻断多个信号通路,具有协同增效的作用,但联合用药临床获益还有待更多的研究论证。
文章引用
胡亚南. 小分子EGFR-TKIs在非小细胞肺癌治疗中的研究进展
Research Advances of Small Molecule EGFR-TKIs in NSCLC[J]. 药物资讯, 2024, 13(01): 1-9. https://doi.org/10.12677/PI.2024.131001
参考文献
- 1. American Association for Cancer Research, Philadelphia, Pennsylvania (AACR) (2022) AACR Cancer Progress Report 2022. https://cancerprogressreport.aacr.org/progress/
- 2. Cohen, P., Cross, D. and Janne, P. (2021) Kinase Drug Discovery 20 Years after Imatinib: Progress and Future Directions. Nature Reviews Drug Discovery, 20, 551-569. https://doi.org/10.1038/s41573-021-00195-4
- 3. Bishayee, S. (2000) Role of Conformational Alteration in the Epidermal Growth Factor Receptor (EGFR) Function. Biochemical Pharmacology, 60, 1217-1223. https://doi.org/10.1016/S0006-2952(00)00425-1
- 4. Heon, S., Yeap, B.Y., Lindeman, N.I., et al. (2012) The Im-pact of Initial Gefitinib or Erlotinib versus Chemotherapy on Central Nervous System Progression in Advanced Non-Small Cell Lung Cancer with EGFR Mutations. Clinical Cancer Research, 18, 4406-4414. https://doi.org/10.1158/1078-0432.CCR-12-0357
- 5. Mitsudomi, T. and Yatabe, Y. (2010) Epidermal Growth Factor Receptor in Relation to Tumor Development: EGFR Gene and Cancer. FEBS, 277, 301-308. https://doi.org/10.1111/j.1742-4658.2009.07448.x
- 6. Krause, D.S. and Vanetten, R.A. (2005) Tyrosine Kinases as Targets for Cancer Therapy. The New England Journal of Medicine, 353, 172-187. https://doi.org/10.1056/NEJMra044389
- 7. Nguyen, K.S.H. and Neal, J.W. (2012) First-Line Treatment of EGFR-Mutant Non-Small-Cell Lung Cancer: The Role of Erlotinib and Other Tyrosine Kinase Inhibitors. Biology, 6, 337-345. https://doi.org/10.2147/BTT.S26558
- 8. Min, H.Y. and Lee, H.Y. (2022) Molecular Targeted Therapy for Anticancer Treatment. Experimental & Molecular Medicine, 54, 1670-1694. https://doi.org/10.1038/s12276-022-00864-3
- 9. Yang, Y., Li, S., Wang, Y., et al. (2022) Protein Tyrosine Kinase Inhibitor Resistance in Malignant Tumors: Molecular Mechanisms and Future Perspective. Signal Transduction Targeted Therapy, 7, Article No. 329. https://doi.org/10.1038/s41392-022-01168-8
- 10. Rosell, R., Moran, T., Queralt, C., et al. (2009) Screening for Epidermal Growth Factor Receptor Mutations in Lung Cancer. The New England Journal of Medicine, 361, 958-967. https://doi.org/10.1056/NEJMoa0904554
- 11. Pao, W., Miller, V.A., Politi, K.A., et al. (2005) Acquired Re-sistance of Lung Adenocarcinomas to Gefitinib or Erlotinib Is Associated with a Second Mutation in the EGFR Kinase Domain. PLOS Medicine, 2, 225-235. https://doi.org/10.1371/journal.pmed.0020073
- 12. Kobayashi, S., Boggon, T.J., Dayaram, T., et al. (2005) EGFR Mutation and Resistance of Non-Small-Cell Lung Cancer to Gefitinib. The New England Journal of Medicine, 352, 786-792. https://doi.org/10.1056/NEJMoa044238
- 13. Chen, L.F., et al. (2018) Recent Progress of Small-Molecule Epidermal Growth Factor Receptor (EGFR) Inhibitors against C797S Resistance in Non-Small-Cell Lung Cancer. Journal of Medicinal Chemistry, 61, 4290-4300. https://doi.org/10.1021/acs.jmedchem.7b01310
- 14. Park, S., Bo, M.K., Jung, H.A., et al. (2020) EGFR C797S as a Resistance Mechanism of Lazertinib in Non-Small Cell Lung Cancer with EGFR T790M Mutation. Cancer Research and Treatment, 52, 1288-1290. https://doi.org/10.4143/crt.2020.278
- 15. Ho, T.L.F., et al. (2023) Domain-Specific p53 Mutants Activate EGFR by Distinct Mechanisms Exposing Tissue-Independent Therapeutic Vulnerabilities. Nature Communication, 14, Article No. 1726. https://doi.org/10.1038/s41467-023-37223-3
- 16. Vaughan, C.A., Singh, S., Subler, M.A., et al. (2021) The On-cogenicity of Tumor-Derived Mutant p53 Is Enhanced by the Recruitment of PLK3. Nature Communication, 12, Article No. 704. https://doi.org/10.1038/s41467-021-20928-8
- 17. Meinhardt, A.L., Munkhbaatar, E., Höckendorf, U., et al. (2022) The BCL-2 Family Member BOK Promotes KRAS-Driven Lung Cancer Progression in a p53-Dependent Manner. Oncogene, 41, 1376-1382. https://doi.org/10.1038/s41388-021-02161-1
- 18. Schneider, J.L., Shaverdashvili, K., Mino-Kenudson, M., et al. (2023) Lorlatinib and Capmatinib in a ROS1-Rearranged NSCLC with MET-Driven Resistance: Tumor Response and Evolution. Precision Oncology, 7, Article No. 116. https://doi.org/10.1038/s41698-023-00464-y
- 19. Priest, K., Le, A., Gebregzabheir, A., et al. (2023) Evolution of Acquired Resistance in a ROS1+ KRAS G12C+ NSCLC through the MAPK Pathway. Precision Oncology, 7, Article No. 9. https://doi.org/10.1038/s41698-023-00349-0
- 20. Kim, J., Chang, I.Y. and You, H.J. (2022) Interactions between EGFR and EphA2 Promote Tumorigenesis through the Action of Ephexin1. Cell Death & Disease, 13, Article No. 528. https://doi.org/10.1038/s41419-022-04984-6
- 21. Pasquale, E.B. (2023) Eph Receptors and Ephrins in Cancer Progression. Nature Review Cancer, 24, 5-27. https://doi.org/10.1038/s41568-023-00634-x
- 22. Peters, T.L., Patil, T., Le, A.T., et al. (2021) Evolution of MET and NRAS Gene Amplification as Acquired Resistance Mechanisms in EGFR Mutant NSCLC. Precision Oncology, 5, Article No. 91. https://doi.org/10.1038/s41698-021-00231-x
- 23. Han, S., Tian, Z., Tian, H., et al. (2023) HDGF Promotes Ge-fitinib Resistance by Activating the PI3K/AKT and MEK/ERK Signaling Pathways in Non-Small Cell Lung Cancer. Cell Death & Differentiation, 9, Article No. 181. https://doi.org/10.1038/s41420-023-01476-0
- 24. Toyozawa, R., Iwamoto, Y., Yokoyama, T., et al. (2021) P76.61 Long Follow up Study of Comparing Erlotinib (ER) with Gefitinib (GE) for Previously Treated Advanced Non-Small Cell Lung Cancer: WJOG5108LFS. Journal of Thoracic Oncology, 16, 613-614. https://doi.org/10.1016/j.jtho.2021.01.1118
- 25. Wu, Y.L., Cheng, Y., Zhou, X., et al. (2017) Dacomitinib versus Gefitinib as First-Line Treatment for Patients with EGFR-Mutation-Positive Non-Small-Cell Lung Cancer (ARCHER 1050): A Randomized, Open-Label, Phase 3 Trial. The Lancet Oncology, 18, 1454-1466. https://doi.org/10.1016/S1470-2045(17)30608-3
- 26. Corral, J., Park, K., Yang, J.C., et al. (2017) Afatinib (A) vs Gefitinib (G) in Patients with EGFR Mutation-Positive (EGFRm+) NSCLC: Updated OS Data from the Phase IIb Trial LUX-Lung 7 (LL7). Annals of Oncology, 28, 34-34. https://doi.org/10.1093/annonc/mdx091.013
- 27. Bian, D., Sun, L., Hu, J., et al. (2023) Neoadjuvant Afatinib for Stage III EGFR-Mutant Non-Small Cell Lung Cancer: A Phase II Study. Nature Communication, 14, Article No. 4655. https://doi.org/10.1038/s41467-023-40349-z
- 28. 杨曼, 李国文, 邱文纯. 吉非替尼联合一线化疗方案治疗EGFR突变阳性的Ⅳ期非鳞非小细胞肺癌的临床疗效及安全性[J]. 临床合理用药杂志, 2022, 15(27): 95-97.
- 29. 秦冉冉, 李海银, 黄卷舒. 厄洛替尼联合奈达铂, 培美曲塞治疗IIIB, IV期非小细胞肺癌的临床效果[J]. 临床合理用药杂志, 2021, 14(10): 68-69.
- 30. Zhong, W.Z., Yan, H.H., Chen, K.N., et al. (2023) Erlotinib versus Gemcita-bine plus Cisplatin as Neoadjuvant Treatment of Stage IIIA-N2 EGFR-Mutant Non-Small-Cell Lung Cancer: Final Overall Survival Analysis of the EMERGING-CTONG 1103 Randomised Phase II Trial. Signal Transduction Targeted Therapy, 8, Article No. 76. https://doi.org/10.1038/s41392-022-01286-3
- 31. Wittlinger, F. and Laufer, S.A. (2021) The Pre-Clinical Discov-ery and Development of Osimertinib Used to Treat Non-Small Cell Lung Cancer. Expert Opinion on Drug Discovery, 16, 1091-1103. https://doi.org/10.1080/17460441.2021.1936496
- 32. Li, S., Zhang, T., Zhu, S.J., et al. (2022) Optimization of Brigatinib as New Wild-Type Sparing Inhibitors of EGFR (T790M/C797S) Mutants. ACS Medicinal Chemistry Letters, 13, 196-202. https://doi.org/10.1021/acsmedchemlett.1c00555
- 33. Yan, X.E., Ayaz, P., Zhu, S.J., et al. (2020) Structural Basis of AZD9291 Selectivity for EGFR T790M. Journal of Medicinal Chemistry, 63, 8502-8511. https://doi.org/10.1021/acs.jmedchem.0c00891
- 34. David, E.H., Florian, W., Tyler, S.B., et al. (2022) Structural Basis for Inhibition of Mutant EGFR with Lazertinib (YH25448). ACS Medicinal Chemistry Letters, 13, 1856-1863. https://doi.org/10.1021/acsmedchemlett.2c00213
- 35. Jeancharles, S., Johan, V., Yuichiro, O., et al. (2018) Osi-mertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. The New England Journal of Medicine, 378, 113-125. https://doi.org/10.1056/NEJMe1714580
- 36. Mok, T.S., Wu, Y.L., Ahn, M.J., et al. (2017) Osimertinib or Plati-num-Pemetrexed in EGFR T790M-Positive Lung Cancer. The New England Journal of Medicine, 376, 629-640. https://doi.org/10.1056/NEJMoa1612674
- 37. Cho, B.C., Han, J., Kim, S., et al. (2018) MA26.09 Lazertinib, a Third Generation EGFR-TKI, in Patients with EGFR-TKI-Resistant NSCLC: Updated Results of a Phase I/II Study. Journal of Thoracic Oncology, 13, 453-453. https://doi.org/10.1016/j.jtho.2018.08.544
- 38. Ahn, M.J., et al. (2019) Lazertinib in Patients with EGFR Muta-tion-Positive Advanced Non-Small-Cell Lung Cancer: Results from the Dose Escalation and Dose Expansion Parts of a First-in-Human, Open-Label, Multicentre, Phase 1-2 Study. The Lancet Oncology, 20, 1681-1690. https://doi.org/10.1016/S1470-2045(19)30504-2
- 39. Yang, C.H., Camidge, D.R., Yang, C.T., et al. (2020) Safety, Efficacy and Pharmacokinetics of Almonertinib (HS-10296) in Pretreated Patients with EGFR-mutated Advanced NSCLC: A Multicenter, Open-Label, Phase I Trial. Journal of Thoracic Oncology, 15, 1907-1918. https://doi.org/10.1016/j.jtho.2020.09.001
- 40. Lu, A.S., et al. (2022) Efficacy of Aumolertinib (HS-10296) in Pa-tients with Advanced EGFR T790M+ NSCLC: Updated Post-National Medical Products Administration Approval Re-sults from the APOLLO Registrational Trial. Journal of Thoracic Oncology, 17, 411-422. https://doi.org/10.1016/j.jtho.2021.10.024
- 41. Lu, S., Dong, X., Jian, H., et al. (2022) AENEAS: A Randomized Phase III Trial of Aumolertinib versus Gefitinib as First-Line Therapy for Locally Advanced or Metastatic Non-Small-Cell Lung Cancer with EGFR Exon 19 Deletion or L858R Mutations. Journal of Thoracic Oncology, 40, 3162-3171.
- 42. Shi, Y., Chen, G., Wang, X., et al. (2022) Furmonertinib (AST2818) versus Gefitinib as First-Line Therapy for Chinese Patients with Locally Advanced or Metastatic EGFR Mutation-Positive Non-Small-Cell Lung Can-cer (FURLONG): A Multicentre, Double-Blind, Randomised Phase 3 Study. The Lancet Respiratory Medicine, 10, 1019-1028. https://doi.org/10.1016/S2213-2600(22)00168-0
- 43. Lin, S.Y., Hsu, Y.C., Peng, Y.H., et al. (2019) Discovery of a Furanopyrimidine-Based Epidermal Growth Factor Receptor Inhibitor (DBPR112) as a Clinical Candidate for the Treatment of Non-Small Cell Lung Cancer. Journal of Medicinal Chemistry, 62, 10108-10123. https://doi.org/10.1021/acs.jmedchem.9b00722
- 44. Kelly, R.J., Shepherd, F.A., Krivoshik, A., et al. (2019) A Phase 3, Randomized, Open-Label Study of ASP8273 versus Erlotinib or Gefitinib in Patients with Advanced Stage IIIB/IV Non-Small Cell Lung Cancer. Annals of Oncology, 30, 1127-1133. https://doi.org/10.1093/annonc/mdz128
- 45. Lola, A. and Chao, L. (2016) NDA 208542-Rociletinib FDA Presen-tation, Food and Drug Administration. https://www.fda.gov/media/97250/download
- 46. Enom, S., Brubaker, D., Campbell, E., et al. (2022) Discovery of BLU-945, a Reversible, Potent, and Wild-Type-Sparing Next-Generation EGFR Mutant Inhibitor for Treatment-Resistant Non-Small-Cell Lung Cancer. Journal of Medicinal Chemistry, 65, 9662-9677. https://doi.org/10.1021/acs.jmedchem.2c00704
- 47. Liu, X., Zhang, X., Yang, L., et al. (2019) Abstract 1320: Pre-clinical Evaluation of TQB3804, a Potent EGFR C797S Inhibitor. Proceedings: AACR Annual Meeting 2019, Atlanta, 29 March-3 April 2019, 1320-1320. https://doi.org/10.1158/1538-7445.AM2019-1320
- 48. To, C., Jang, J., Chen, T., et al. (2019) Single and Dual Targeting of Mutant EGFR with an Allosteric Inhibitor. Cancer Discovery, 9, 926-943. https://doi.org/10.1158/2159-8290.CD-18-0903
- 49. Jia, Y., Yun, C.H., Park, E., et al. (2016) Overcoming EGFR(T790M) and EGFR(C797S) Resistance with Mutant-Selective Allosteric Inhibitors. Nature, 534, 129-132. https://doi.org/10.1038/nature17960
- 50. To, C., Beyett, T.S., Jang, J., et al. (2022) An Allosteric Inhibitor against the Therapy Resistant Mutant Forms of EGFR in Non-Small Cell Lung Cancer. Nature Cancer, 3, 402-417. https://doi.org/10.1038/s43018-022-00351-8
- 51. Ulrike, O.S., Antonio, R., Bernd, K., et al. (2022) Discovery of Novel Allosteric EGFR L858R Inhibitors for the Treatment of Non-Small-Cell Lung Cancer as a Single Agent or in Combination with Osimertinib. Journal of Medicinal Chemistry, 65, 13052-13073. https://doi.org/10.1021/acs.jmedchem.2c00893
- 52. Schalm, S.S., Dineen, T., Lim, S.M., et al. (2020) 1296P BLU-945, a Highly Potent and Selective 4th Generation EGFR TKI for the Treatment of EGFR T790M/C797S Resistant NSCLC. Annals of Oncology, 31, 839-839. https://doi.org/10.1016/j.annonc.2020.08.1610
- 53. Kashima, K., Kawauchi, H., Tanimura, H., et al. (2020) CH7233163 Overcomes Osimertinib Resistant EGFR-Del19/T790M/C797S Mutation. Molecular Cancer Therapeutics, 19, 2288-2297. https://doi.org/10.1158/1535-7163.MCT-20-0229
- 54. Lim, S.M., Kim, D.W., Jung, J.E., et al. (2021) 1365TiP A Phase I/II, Open-Label Study of BBT-176, a Triple Mutation Targeting EGFR TKI, in Patients with NSCLC Who Pro-gressed after Prior EGFR TKI Therapy. Annals of Oncology, 32, 1035-1035. https://doi.org/10.1016/j.annonc.2021.08.1966