Medical Diagnosis
Vol.
14
No.
01
(
2024
), Article ID:
82342
,
10
pages
10.12677/MD.2024.141001
颈动脉体瘤多排探测器CTA诊断
潘碧涛1,胡美玉2,潘希敏2,赖英荣3,江 波1*
1中山大学附属第一医院放射诊断科,广东 广州
2中山大学附属第六医院放射科,广东 广州
3中山大学附属第一医院病理科,广东 广州
收稿日期:2023年12月8日;录用日期:2024年3月4日;发布日期:2024年3月8日

摘要
背景:探讨颈动脉体瘤多排探测器CTA (MDCTA)表现特征与诊断意义。方法:观察26个颈动脉体瘤MDCTA的平扫、增强动脉期及静脉期三期扫描图,基于动脉期强化表现将瘤体分为显著强化的I区和轻微强化的II区。分别比较动、静脉期I区、II区间的强化程度,分别比较I区、II区动、静脉期间强化程度,比较动脉期I区和颈动脉间密度增加值及实际密度值,根据瘤体内部I区、II区的构成及分布进行CBT分型,比较CBT瘤体轴位最大径与纵向最大径,CT-病理对照比较I区、II区镜下表现。结果:MDCTA动、静脉期,I区的强化率均高于II区,差异有显著性(t = 7.95, P < 0.001; t = 4.07, P < 0.005)。I区动脉期强化率高于静脉期,差异有显著性(t = 10.38, P < 0.001);II区动、静脉期强化率差异无显著性(t = 0.53, P > 0.5)。MDCTA动脉期I区的密度增加值及密度值均低于颈动脉,差异有显著性(t = 11.06, P < 0.001; t = 11.13, P < 0.001)。CBT分型:A型11个,B型10个,C型5个。26个瘤体纵向最大径(53.2 ± 16.8 cm)均大于轴位最大径(38.7 ± 10.3 cm),差异有显著性(t = 8.43, P < 0.001)。组织学上,I区瘤细胞和血管丰富、纤维成分少;II区胶原纤维丰富、瘤细胞少。结论:MDCTA反映了颈动脉体瘤内部组织结构的异质性,在其诊断及鉴别诊断中有着重要意义。
关键词
颈动脉体瘤,计算机辅助断层成像血管造影,诊断,颈总动脉分叉

Multi-Detector Computed Tomography Angiography Diagnosis of Carotid Body Tumor
Bitao Pan1, Meiyu Hu2, Ximin Pan2, Yingrong Lai3, Bo Jiang1*
1Department of Diagnostic Radiology, Sun Yat Sen University First Affiliated Hospital, Guangzhou Guangdong
2Department of Radiology, Sun Yat Sen University Sixth Affiliated Hospital, Guangzhou Guangdong
3Department of Pathology, Sun Yat Sen University First Affiliated Hospital, Guangzhou Guangdong
Received: Dec. 8th, 2023; accepted: Mar. 4th, 2024; published: Mar. 8th, 2024

ABSTRACT
Background: To assess the multi-detector computed tomography angiography (MDCTA) features of carotid body tumor (CBT) and its diagnostic significance. Methods: The three-phase MDCTA images, pre-contrast, post-comtrast arterial phase and venous phase, of 26 cases of CBT were observed, and the tumoral mass was divided into 2 regions: Markedly enhancing region I and mildly enhancing region II based upon the enhancement pattern on the arterial phase of MDCTA. The enhancement degrees were compared between region I and region II in both arterial and venous phases, as well those compared between arterial and venous phases in both region I and region II. Both density increment and real density were compared between region I and carotid in the arterial phase of MDCTA. The MDCTA categorization of CBT was conducted based on the distribution of region I and region II in the tumoral mass. The maximal diameters were compared in the axial plane and in the longitudinal plane. CT-pathologic correlation was performed to identify the histopathology of both region I and region II. Results: The enhancing ratios of region I exceeded those of region II in both arterial and venous phases of MDCTA (t = 7.95, P < 0.001; t = 4.07, P < 0.005, respectively). The enhancing ratios of region I were higher in arterial phase than that in venous phase (t = 10.38, P < 0.001). No significant difference was shown in region II between arterial and venous phases (t = 0.53, P > 0.5). The density increment in region I was lower than that of carotid in the arterial phase of MDCTA, as well as the real density (t = 11.06, P < 0.001; t = 11.13, P < 0.001, respectively). Eleven CBTs of type A, 10 of type B and 5 of type C were noted in the MDCTA categorixation of the 26 CBTs. The maximal diameters in the longitudinal plane (53.2 ± 16.8 cm) surpassed that in the axial plane (38.7 ± 10.3 cm) among the 26 CBTs (t = 8.43, P < 0.001). Histologically, region I was composed of abundance of tumoral cellular nests and blood vessels and rarity of fibers, while region II was composed abundance of collagens and rarity of tumoral cellular nests. Conclusion MDCTA reveals the heterogeneity of intra-tumoral texture and configuration of CBT, which possesses vital significance in the diagnosis and differential diagnosis of this tumor.
Keywords:Carotid Body Tumor, Computed Tomography Angiography, Diagnosis, Common Carotid Bifurcation

Copyright © 2024 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
颈动脉体瘤(Carotid Body Tumor, CBT),是颈部常见肿瘤,起源于颈总动脉分叉(Common Carotid Bifurcation, CCB) [1] [2] [3] [4] 。CBT富于血供,治疗主要靠手术切除,术前多采用颈动脉DSA下栓塞肿瘤供血动脉以减轻术中失血 [5] [6] 。邻近CCB结构的软组织肿瘤,如神经鞘瘤、血管瘤等,有时易误为CBT [1] [2] [7] [8] [9] [10] 。因此,术前的正确诊断裨益CBT治疗方案的制订。CT、MRI及超声成像等已普遍应用于CBT的诊断 [1] [3] [4] [11] [12] [13] 。MRI能准确显示瘤体的位置、形态与瘤内血管,但扫描时间长,强化效应类似常规CT,未能捕捉动脉期变化 [1] [3] [4] [11] 。超声成像,包括彩色多普勒成像(Color Doppler Ultrasound, CDU)及对比增强成像,可多体位、多角度探测CBT部位、形态及瘤内血流 [3] [11] [12] [13] ,但对于检测CBT的供血动脉及近颅底病变能力不足 [12] 。49例CBT的CDU研究显示,CBT瘤体的检出率为96%,位于上颈部的2例CBT未能发现,瘤体供血动脉检出率为76% [12] 。随着多排探测器CTA (Multi-Detector Computed Tomography Angiography, MDCTA)的临床应用,亚秒级的扫描速度使获取CBT动脉期、静脉期密度变化及同步颈动脉造影成为现实。应用MDCTA进行Shamblin分级、CBT容积预测术中失血量及术前瘤周动脉形态学评估等研究已有报道 [2] [14] [15] ,但较大样本的CBT内部结构的多排探测器MDCTA研究尚未见报道,本文就多排探测器MDCTA的CBT强化特征及其组织学基础与诊断意义做一探讨。
2. 材料与方法
2.1. 临床资料
收集中山大学附属第一医院2012年1月至2022年12月间24例经手术病理证实的CBT患者的MDCTA与临床病理资料。纳入标准:1) 肿瘤发生于CCB;2) 颈部MDCTA三期图像完整且动脉期、静脉期时相匹配准确;3) 手术病理资料完整。排除标准:1) 复发性肿瘤;2) 移动伪影及假牙伪影等影响图像质量。24例CBT,均因颈部肿物就诊,病程1个月~10年不等,其中10例10个肿物呈搏动性。男性13例,女性11例,年龄11~60岁,平均34.9岁,男性平均33.5岁,女性36.6岁。
2.2. MDCTA扫描参数
TOSHIBA Aquilion One 320排探测器螺旋CT,探测器宽度160 mm。MDCTA扫描方案如下:依次平扫、动脉期和静脉期三期扫描,动脉期与静脉期间隔35~40 s。动脉期扫描由Sure start辅件启动,ROI瞄定降主动脉起始段,动脉CT值 > 220 HU或管腔密度增加40~60 HU触发扫描。MDCTA扫描管电压120 KV,管电流250 mAs~300 mAs,球管转速0.27 s/圈,螺距1,层厚0.5 mm,层间距0,像素0.468 mm × 0.468 mm~0.625 mm × 0.625 mm,扫描范围为主动脉弓至鼻咽,上下径25~30 cm。经肘静脉以4 mL/s~5 mL/s速率注射对比剂碘普罗胺(碘浓度300 mg/mL) 60 mL~100 mL,紧接30 mL生理盐水冲管,全程由自动高压注射器(Ulrich Medical)控制。
2.3. 图像后处理
三期原始图像,轴位、冠状及矢状面的MPR处理产生3 mm~5 mm层厚图。动脉期原始图,经MIP、VR重组产生颈部动脉图,以最佳展示双侧颈总动脉及颈内外动脉为目标。强化程度的测定为(Dpost-Dpre)/Dpre,Dpre平扫密度,Dpost增强后密度,ROI置于测量区中心。MDCTA动脉期的密度增加值Dinc为Dpost-Dpre,实际密度值Drea为Dpost。
2.4. 研究方案
1) 分析CBT瘤内部组织结构的差别,根据三期扫描图上CBT强化效应的不同,将瘤组织分为2区。I区:动脉期显著强化,II区:动脉期轻微强化或中度强化,2区密度强烈对比。分别比较动脉期、静脉期I区、II区间的强化程度差异,分别比较I区、II区动脉期、静脉期间的强化程度差异。比较MDCTA动脉期I区和同层面颈动脉间Dinc及Drea的差异。2) CBT分型,根据瘤体内部I区、II区的构成比例及分布,CBT分为3型。A型,I区集中于瘤体周围区域,II区位于瘤体中央;B型,I区、II区片状或小灶状交错分布;C型,基本上由I区构成,II区成分少且强化较明显。3) 探讨CBT的空间生长特点,观察瘤体位置、大小及形态,比较CBT瘤体轴位最大径与纵向最大径;观察MPR、MIP及VR图上CAB形态变化、肿瘤包埋颈动脉及瘤体显现,按Shamblin标准进行CBT分级 [2] [4] [16] 。4) CT-病理对照,观察比较I区、II区与大体标本、镜下HE染色及免疫组化表现,明确2区的组织学差异性。5) 所有评阅由两名工作10年以上的放射科医师完成,不一致时由两人协商解决。
2.5. 统计学处理
应用SPSS 25.0软件进行:配对t检验统计分析。瘤体最大径以均数 ± 标准差(x ± s)表示,CBT瘤体轴位最大径与纵向最大径的差异性,以P < 0.05为差异有统计学意义。I区、II区的强化程度以均数 ± 标准差(x ± s)表示,2区之间及2区在动脉期、静脉期之间强化程度的差异性,以P < 0.05为差异有统计学意义。动脉期I区和颈动脉的Dinc、Drea以均数 ± 标准差(x ± s)表示,二者间的Dinc差异性和Drea差异性,以P < 0.05为差异有统计学意义。
3. 结果
3.1. 手术治疗情况
CBT单侧的21例,双侧的3例,共27个瘤体,26个行手术切除。其中位于左侧12个,右侧14个。15例术前行颈动脉DSA下CBT供血动脉栓塞,栓塞后1~4天手术切除瘤体,其中1例双侧CBT的间隔4个月先后行双侧栓塞后瘤体切除。24个瘤体完整切除,2个因瘤体周围严重粘连颈内外动脉做了部分切除。术中3例因颈内动脉壁破裂行动脉壁修补术,6例放置颈总动脉–颈内动脉人工血管,3例行静脉替代颈内动脉,22个结扎颈外动脉。
3.2. CBT内部分区
以颈部肌群为对照,瘤体平扫呈等或稍低密度,无钙化或脂肪,类圆形或长圆形,边缘光整、分叶。CTA动脉期,瘤体I区显著强化,密度低于同层面颈内动脉;II区轻度至中度强化,密度低于I区;I区、II区密度强对比。至静脉期,I区强化消退,II区强化轻微消退或轻微进展,二者密度对比度弱(图1)。25个CBT出现I区的这种动态密度变化,仅1例静脉期密度稍高于动脉期。II区在动、静脉期间的密度变化小,强化进展与消退的例数接近。CTA动脉期I区强化均高于II区,静脉期I区强化高于II区的有22个,仅4个低于II区。CTA三期扫描26个CBT 2区的密度变化,见表1、表2。
3.3. CBT分型
26个CBT,A型11个(图2),B型10个(图3),C型5个(图4)。A型中,瘤体中央的II区呈圆形、类圆形或星芒状,整体上类似环形强化。B型瘤体内2区组织交错排列,呈花斑状或融冰状改变。C型均质强化,II区成分很少。
3.4. CBT的三维侵犯
26个CBT均发生于CCB区,主体以CCB为中心的21个,位于CCB外侧的1个,位于CCB内侧的4个。26个CBT均见CCB角不同程度膨胀、扩大,呈U形状。25个CBT包绕颈总动脉远端,推压及包埋颈内外动脉近端,仅1例瘤体局限于CCB角内。26个CBT瘤体的纵向最大径,均超过轴位最大径,比较见表3。动脉期MPR、3D MIP/VR图上,21个可见供血动脉,呈单条短直或数条迂曲的动脉丛穿行于团块,另5个未见显示。3D MIP/VR图上与颈动脉显影同步展示瘤体显现的有19个,表现为包绕颈总动脉远端及颈内外动脉近端的显著强化团块;未见瘤体显现的有7个,呈扩大CCB角内的充盈缺损样改变(图5、图6)。根据MDCTA表现,CBT的Shamblin分级为1级1个、2级3个、3级22个,与术中所见吻合。
3.5. CT-病理联系
HE染色镜下为不同比例、不同数量的瘤细胞与间质。瘤细胞排列成巢状、小片状、条索状或器官样,圆形、卵圆形或多角形,胞浆丰富、红染或呈空泡状,异形性不明显。细胞核圆形,可见核仁,核分裂像不易见。瘤巢边缘可见支持细胞。瘤巢或瘤细胞团之间可见丰富的薄壁血管、血窦,及多少不等的纤维与继发胶原化、玻璃样变。免疫组化检测到Vimentin、CD56、Syn、NSE和CgA表达,而CK、GFAP和NF无表达。MDCTA动脉期与病理对照显示,I区成分为丰富的瘤细胞和血管、血窦,纤维成分少(图7);II区以胶原纤维为主,瘤细胞零星散布其中(图8)。
图1三期扫描与2区。1A,平扫;1B,动脉期,I区显著强化(箭),II区轻微强化(箭头);3C,静脉期,I区强化减退,II区强化轻度进展。
图2 A型。I区位于瘤体周围(箭),II区位于中央(星),类似环状强化。
图3 B型。瘤体内I区、II区交错分布,呈融冰状(白箭),左侧颈内动脉(粗黑箭)、颈外动脉(细黑箭)受压后外侧移位。
图4 C型。瘤体强化较均质,后部强化稍弱(白箭),位于颈动脉分叉中心,颈内动脉受压后外移位(粗黑箭)、颈外动脉受压前移(细黑箭)。
图5 3D VR与瘤体显现。A,动脉期,B型CBT (短箭),推压、包埋颈内(粗箭)、颈外动脉(细箭);B,VR冠状位,颈动脉分叉区瘤体(粗箭),肿块表面数条颈外动脉发出的供血动脉(细箭);C,VR斜矢状位,颈动脉分叉角受压、扩大(粗箭);D,DSA显示颈外动脉发出的供血动脉(细箭)。
图6 3D VR与MPR。A,动脉期,A型CBT (短箭),推压、包埋颈内(粗箭)、颈外动脉(细箭);B,VR斜矢状位,颈动脉分叉角受压、扩大(粗箭),分叉区未见瘤体显现;C,MPR图显示颈动脉分叉区环状强化瘤体边缘(细箭);D,DSA显示颈外动脉起源的细小供血动脉(细箭)。
图7 I区镜下表现。A,大片瘤细胞巢(粗黑箭)和其间血管纤维间质(细黑箭) (HE × 40);B,致密瘤细胞,核深染(粗黑箭),瘤细胞巢间纤维间隔(细黑箭) (HE × 400)。
图8 II区镜下表现。大片状、条索状胶原纤维(粗黑箭),其间夹杂小灶性瘤巢(细黑箭) (HE × 40)。
Table 1. Comparison of CBT’s enhancement rate between region I and region II in the tri-phasic MDCTA
表1. MDCTA三期扫描CBT I区、II区强化率比较
注:配对t检验,MDCTA动脉期、静脉期,I区的强化率均高于II区,差异有显著性(t = 7.95, P < 0.001; t = 4.07, P < 0.005)。I区动脉期强化率高于静脉期,差异有显著性(t = 10.38, P < 0.001);II区动、静脉期强化率差异无显著性(t = 0.53, P > 0.5)。
Table 2. Comparison of the density alteration between region I and carotid artery in the arterial phase of MDCTA (units: HU)
表2. MDCTA动脉期I区与颈动脉密度比较(单位:HU)
注:配对t检验,MDCTA动脉期I区的密度增加值及密度值均低于颈动脉,差异有显著性(t = 11.06, P < 0.001; t = 11.13, P < 0.001)。
Table 3. Comparison of CBT’s maximal diameter between axial and longitudinal orientations
表3. CBT瘤体轴位与纵向最大径比较(单位:mm)
注:配对t检验,26个CBT瘤体纵向最大径均大于轴位最大径,差异有显著性(t = 8.43, P < 0.001)。
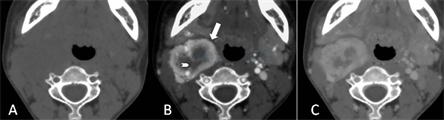
Figure 1. Tri-phase scans and 2 regions
图1. CBT三期扫描与2区
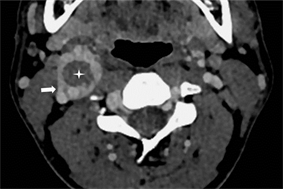
Figure 2. Type A
图2. A型
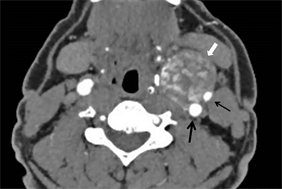
Figure 3. Type B
图3. B型
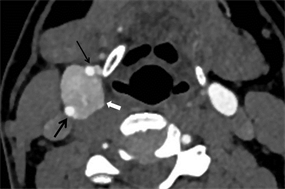
Figure 4. Type C
图4. C型
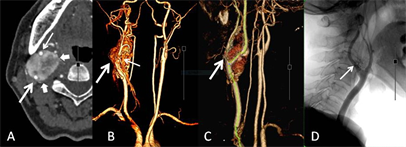
Figure 5. 3D VR and tumoral display. A: Arterial phase; B: coronal VR; C: oblique-coronal VR; D: DSA
图5. 3D VR与瘤体显现。A:动脉期;B:冠状位VR;C:斜矢状VR;D:DSA
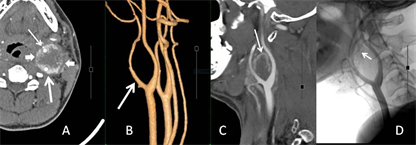
Figure 6. 3D VR and MPR. A: Arterial phase; B: oblique-coronal VR; C: MPR; D: DSA
图6. 3D VR与MPR。A:动脉期;B:斜矢状VR;C:MPR;D:DSA
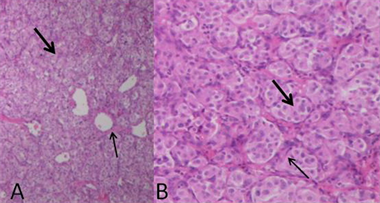
Figure 7. Microscopic finding of region I. A: HE (×40); B: HE (×400)
图7. I区镜下表现。A:HE (×40);B:HE (×400)
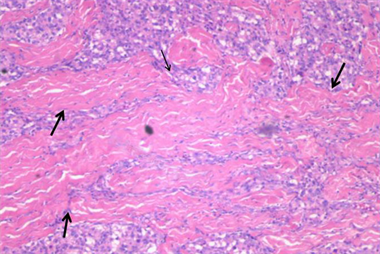
Figure 8. Microscopic finding of region II, HE (×40)
图8. II区镜下表现,HE (×40)
4. 讨论
4.1. CBT的临床病理特征
CBT,发生于颈动脉体的副神经节瘤,属于神经内分泌肿瘤,约占全部副神经节瘤的40% [3] 。CBT可发生于任何年龄,发病高峰为45~50岁,无性别差异。儿童病例有见报道 [17] ,本组年龄最小患者仅10岁。典型临床表现是隐匿增大的无痛性颈部包块,可伴有搏动 [3] [4] ,本组38%肿块触及搏动感。极少数病例可表现为肿块快速进展 [18] 。CBT富血供,主要由咽升动脉、颈升动脉供血。术前栓塞供血动脉可收缩CBT血管系统以减少术中出血。手术切除应在栓塞术1~2天后、2周内进行,一方面消除栓塞诱发的局部水肿,同时又防止血管再通 [3] [5] [6] 。本文58%的手术病例术前做了颈动脉DSA肿瘤动脉栓塞,明显减少了术中出血。大体上,CBT为带纤维假包膜的边缘清晰的分叶状肿块,大小介于1 cm~8.5 cm,质地较均质,瘤内坏死、硬化及囊性变少见。镜下可见主细胞、支持细胞和纤维血管间质。主细胞和血管丰富,支持细胞稀少,位于细胞巢边缘,梭形改变,具有长胞浆突起,类似血管周细胞。纤维组织多寡不一,自散在纤维灶至弥漫胶原纤维增生不等 [3] 。
4.2. CBT的MDCTA强化特征
常规CT上的瘤体明显强化,是扫描速度慢、所采集图像属于静脉期或均衡期的结果 [3] [4] [11] 。有学者报道MDCTA上瘤体动脉期显著强化,密度与颈动脉相当 [7] 。本组资料显示,CBT尽管动脉期强化明显,但MDCTA动脉期和静脉期瘤体强化程度和密度均明显低于颈动脉。
MDCTA反映了CBT内部组织结构的异质性。CT-病理对照分析揭示,I区代表密集的瘤细胞巢、血管间质与少纤维组织,在动脉期显著强化;II区则以纤维和胶原成分为主,瘤细胞巢稀疏,动脉期弱强化。这种内部组织结构的差异,在MDCTA动脉期图上被直观、明确展示。一过性的强化效应,又使这种强烈密度对比在静脉期迅速消退。因此,捕捉、观察MDCTA动脉期影像,在确定CBT性质中有着决定性意义。I区、II区二者的构成比例因瘤而异,从而导致强化表现的巨大差异,I区为主的全瘤显著强化,而以II区为主的则可表现为全瘤的轻微强化,容易与神经鞘瘤等弱强化肿瘤混淆,本组中有2例出现此类情形。基于强化形式的复杂性,为全面认识CBT的MDCTA表现,根据I区、II区结构在瘤体内的分布特点,本文尝试了CBT的MDCTA分型。3型中A型最常见,I区环绕II区;C型最少,全瘤显著强化。B型的强化形式最具多样性,当整体强化较弱时应注意观察那些强化显著的I区,以避免误为弱强化肿瘤。CBT的MDCTA分型,整合了CT表现谱与组织病理学之间的内在联系,有助于系统了解MDCTA对CBT的诊断意义。
4.3. CBT的空间侵犯特征
颈动脉体位于CCB的外膜层或其外侧,因此,CBT的特征性生长方式是推压颈内、颈外动脉并撑大CCB角,被称为Lyre征 [3] [18] [19] 。以CCB为中心向周围侵犯,是CBT的肿瘤学行为特点,本组26个CBT,除了局限于CCB角内那个,包括5个瘤体中心偏离CCB的,均见瘤体包埋颈总动脉远端及颈内外动脉近端,呈纵向和轴向的发展。26个CBT瘤体纵向长径均超过轴位长径,说明沿颈总动脉、颈内外动脉纵轴生长,是CBT的最显著生长方向。文献报道CBT可向上侵犯至颅底,导致手术完整切除困难 [3] [4] 。因此,对于CBT的CTA检查,应注意完全显露其上界及比邻。MDCTA上CCB角膨大与瘤体显现,被视为CBT的特征性表现。本文26个CBT,MIP/VR上出现瘤体显现的仅占73%,有7个未见展示,A、B、C型各2、4、1个,其共同特点是瘤体动脉期强化较弱,密度远低于同步的颈动脉,同时供血动脉未见显示或供血动脉细小。此7例MPR上瘤体清晰可见,因此,无瘤体显现情况下,MIP/VR结合MPR是很有必要的 [1] [2] [12] 。
4.4. 诊断与鉴别诊断
发生于CCB区的结节,推压、撑宽CCB角,MDCTA三期图上显示I区、II区表现,基本可做出诊断。辨识I区、II区是诊断CBT的关键,需在MDCTA动脉期各方位图上寻找显著强化的I区,尤其是对于强化较弱的B型。颈动脉间隙的肿块均需与CBT鉴别,其中以神经源性肿瘤为主 [3] [8] [9] [10] 。神经鞘瘤推压颈动脉前内侧移位,颈内静脉受压后移 [3] ;神经节细胞瘤常常推压颈动脉和颈内静脉向前外侧移位 [10] 。二者CTA动脉期强化较弱瘤内多见散在迂曲血管强化,瘤实质强化弱,静脉期强化略增强。但部分起源于迷走神经或颈交感干的富血管神经鞘瘤在动脉期显著均质强化 [8] [9] ,类似本文的C型。神经源性肿瘤对颈动脉呈推压改变,不同于CBT对颈动脉的包绕。血管瘤平扫可有静脉石或钙化,在CTA动脉期可表现出多种强化形式,容易与CBT混淆,但静脉期强化持续增强,瘤内及瘤周常可见多发粗大血管影。少见情况包括淋巴结转移和脓肿,病灶边缘不清,均有特定的病史,多见环形强化。
4.5. 小结
多排探测器CTA的快速成像,揭示了CBT的动脉期强化特征,提出了分区和分型的概念,并籍此拓展了不同于常规CT的诊断新认识,在CBT的诊断及术前综合评估、预测中有着重要意义。
文章引用
潘碧涛,胡美玉,潘希敏,赖英荣,江 波. 颈动脉体瘤多排探测器CTA诊断
Multi-Detector Computed Tomography Angiography Diagnosis of Carotid Body Tumor[J]. 医学诊断, 2024, 14(01): 1-10. https://doi.org/10.12677/MD.2024.141001
参考文献
- 1. Amin, M.F. and Ameen, N.F.E. (2013) Diagnostic Efficiency of Multidetector Computed Tomography versus Magnetic Reso-nance Imaging in Differentiation of Head and Neck Paragangliomas from Other Mimicking Vascular Lesions: Comparison with Histopathologic Examination. European Archives of Oto-Rhino-Laryngology, 270, 1045-1053. https://doi.org/10.1007/s00405-012-2084-6
- 2. Shahbandari, M., Arefinejad, M.S. and Hajiahmadi, S. (2023) The Role of CT Angiography to Predict the Shamblin Group in Carotid Body Tumors. Indian Journal of Otolaryngology and Head & Neck Surgery, 75, 1767-1773. https://doi.org/10.1007/s12070-023-03719-z
- 3. Rao, A.B., Koeller, K.K. and Adair, C.F. (1999) From the Archives of the AFIP. Paragangliomas of the Head and Neck: Radiologic-Pathologic Correlation. Armed Forces Institute of Pathology. Ra-diographics, 19, 1605-1632. https://doi.org/10.1148/radiographics.19.6.g99no251605
- 4. ADEF, G.B., Łukasiewicz, A. and Grinievych, V., et al. (2020) Carotid Body Tumor—Radiological Imaging and Genetic Assessment. Polish Journal of Surgery, 92, 39-44.
- 5. Selim, M., Aljehani, S.H., Aljuhani, A.B., et al. (2021) Preoperative Super-Selective Embolization of Carotid Body Tumor and Multi-disciplinary Approach. Cureus, 13, e12879. https://doi.org/10.7759/cureus.12879
- 6. Faragò, G., Castellani, G., Ponzi, S., et al. (2013) Preoperative Embolization of Carotid Chemodectoma: A Technical Challenge that Can Be Customized Accord-ing to Angioarchitecture. Illustrative Cases. The Neuroradiology Journal, 26, 678-682. https://doi.org/10.1177/197140091302600611
- 7. Suthiphosuwan, S., Bai, H.D., Yu, E, et al. (2020) Computed To-mography Angiography Lightbulb Sign: Characteristic Enhancement Pattern on Neck Computed Tomography Angiography in Differentiating Paraganglioma from Schwannoma of the Carotid Space. The Neuroradiology Journal, 33, 437-442. https://doi.org/10.1177/1971400920924318
- 8. Xu, T., Liu, Y., Li, S., et al. (2022) Pre-Operative Embolization and Ex-cision of Vagal Schwannoma with Rich Vascular Supply: A Case Report and Literature Review. Medicine (Baltimore), 28, e28760. https://doi.org/10.1097/MD.0000000000028760
- 9. Najeeb, T., Khan, M. (2016) Sympathetic Chain Schwannoma Re-sembling Carotid Body Tumour. Journal of College of Physicians and Surgeons Pakist, 26, S68-70.
- 10. Britten, A.G., Ente-zami, P. and Chang, B.A. (2020) Cervical Ganglioneuroma Mimicking a Carotid Body Tumour. BMJ Case Reports, 13, e238469. https://doi.org/10.1136/bcr-2020-238469
- 11. Nashnoush, M., Lad, M., Masood, I., et al. (2023) Multiparamet-ric Analysis of Carotid Body Tumours: A Pictorial Essay. Journal of Ultrasound in Medicine, 26, 553-561. https://doi.org/10.1007/s40477-022-00711-1
- 12. Jin, Z., He W., Wu D., et al. (2016) Color Doppler Ultrasound in Di-agnosis and Assessment of Carotid Body Tumors: Comparison with Computed Tomography Angiography. Ultrasound in Medicine & Biology, 42, 2106-2113. https://doi.org/10.1016/j.ultrasmedbio.2016.04.007
- 13. Pacini1, P., Polti1, G., Faggiano, A., et al. (2021) Multiparametric Ultrasound Evaluation of a Case of Bilateral Carotid Body Tumor. Journal of Ultrasound, 24, 311-315. https://doi.org/10.1007/s40477-021-00581-z
- 14. Lozano-Corona, R., Anaya-Ayala, J.E., Martínez-Martínez, R., et al. (2018) Usefulness of Preoperative Three-Dimen- sional Volumetric Analysis of Carotid Body Tumors. Neuroradiology, 60, 1281-1286. https://doi.org/10.1007/s00234-018-2095-0
- 15. Hoffmann-Wieker, C.M., Rebelo, A., Moll, M., et al. (2023) Association of Tumor Volumetry with Postoperative Outcomes for Cervical Paraganglioma. Diagnostics, 13, 744. https://doi.org/10.3390/diagnostics13040744
- 16. Jasper, A., Mammen, S., Gowri, M.S., et al. (2021) Imaging Criteria to Predict Shamblin Group in Carotid Body Tumors—Revisited. Diagnostic and Interventional Radiology, 27, 354-359. https://doi.org/10.5152/dir.2021.20028
- 17. Hogana, A.R., Solaa, J.E., Jerniganb, S.C., et al. (2018) A Pediatric Carotid Body Tumor. Journal of Pediatric Surgery, 53, 1432-1436. https://doi.org/10.1016/j.jpedsurg.2018.04.004
- 18. Kihara, C., Patel, S., Moss, R., et al. (2023) A Rapidly Progressing Carotid Body Tumor: A Case Report. Cureus, 15, e43654. https://doi.org/10.7759/cureus.43654
- 19. Venkatanarasimha, N., Olubaniyi, B., Freeman, S.J., et al. (2011) Usual and Unusual Causes of Splaying of the Carotid Artery Bifurcation: The Lyre Sign—A Pictorial Review. Emergency Radiology, 18, 75-79. https://doi.org/10.1007/s10140-010-0907-6
NOTES
*通讯作者。
