Journal of Advances in Physical Chemistry
Vol.
08
No.
04
(
2019
), Article ID:
32409
,
9
pages
10.12677/JAPC.2019.84008
Research Progress of Stimuli-Sensitive Polymer Carriers for Anti-Tumor Drug Delivery
Jingguo Li1,2*, Huayang Feng1,2
1Henan Province People’s Hospital & Zhengzhou University People’s Hospital, Zhengzhou Henan
2Henan School of Materials Science and Engineering, Zhengzhou University, Zhengzhou Henan

Received: Sep. 4th, 2019; accepted: Sep. 22nd, 2019; published: Sep. 30th, 2019

ABSTRACT
Stimuli-sensitive polymer nanocarriers have attracted much attention from researchers because of their good application prospects in drug delivery and intelligent controlled release of drugs. They consist of pH sensitive polymer carriers, redox sensitive polymer carriers, hypoxic sensitive polymeric carriers, light sensitive polymeric carriers and ultrasonically sensitive polymeric carriers. This article reviews the research progress of different stimuli-sensitive polymer carriers and multiple stimuli-responsive polymer carriers, and encourages future researchers to design and synthesize novel stimuli-responsive polymer carriers for more efficient drug delivery and intelligent controlled release.
Keywords:Stimulating Sensitive Polymer Nanocarriers, pH Sensitive, Redox Sensitive, Hypoxic Sensitive, Light Sensitive, Ultrasonic Sensitive, Multiple Stimuli Response
用于抗肿瘤药物递送的刺激敏感型聚合物载体的研究进展
李景果1,2*,冯华阳1,2
1河南省人民医院和郑州大学人民医院,河南 郑州
2郑州大学材料科学与工程学院,河南 郑州

收稿日期:2019年9月4日;录用日期:2019年9月22日;发布日期:2019年9月30日

摘 要
刺激敏感型聚合物纳米载体由于在药物递送和药物的智能控释方面具有良好的应用前景,受到了广大研究人员的广泛关注。其种类包括响应内源刺激的pH敏感聚合物载体、氧化还原敏感聚合物载体和低氧敏感聚合物载体,响应外源刺激的光敏感聚合物载体和超声波敏感聚合物载体。本文主要综述不同刺激敏感型聚合物载体和多重刺激响应聚合物载体的研究进展,激励未来的研究人员设计和合成新型刺激响应聚合物载体,以便实现更加高效的药物递送和智能控释的效果。
关键词 :刺激敏感型聚合物纳米载体,pH敏感,氧化还原敏感,低氧敏感,光敏感,超声波敏感,多重刺激响应

Copyright © 2019 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
刺激敏感型聚合物载体是指可以响应外部环境的刺激,在外部环境刺激的条件下发生解体,进而快速释放药物的聚合物载体。由于其具有智能控释化疗药物的能力,它不仅可以在肿瘤组织加速释放药物,达到杀灭肿瘤的效果,同时在正常组织释放药物速率缓慢,从而降低药物的毒副作用,所以受到了越来越多研究人员的广泛关注 [1] [2] [3] [4] [5] 。目前研究的较多的刺激敏感型聚合物载体主要分为响应内源刺激的聚合物载体和响应外源刺激的聚合物载体。其中,内源刺激主要是指不同于正常组织的肿瘤组织的微环境的刺激,如低的pH和缺氧等刺激。而外源刺激主要是指外加的条件的刺激,如光照和超声波的刺激 [5] (图1)。当聚合物载体受到这些刺激时,它就会发生解体,从而快速释放药物,达到智能控释药物的效果。本文主要综述可以响应不同条件刺激和同时响应多种条件刺激的聚合物载体的研究成果,并对其未来的研究方向进行展望。

Figure 1. Schematic diagram of polymer carrier releasing drug in response to various internal and external conditions
图1. 聚合物载体响应各种内外条件刺激释放药物的示意图
2. 响应内源刺激释放药物的聚合物载体
响应内源刺激加速释放药物的聚合物载体包括pH敏感型聚合物纳米载体、氧化还原敏感型聚合物载体和低氧敏感型聚合物载体,下面将分别介绍这几类刺激敏感型聚合物载体的研究进展。
2.1. pH敏感型聚合物纳米载体
pH敏感型聚合物载体是指可以响应pH的变化,当pH变化到一定值后,能发生解体,释放药物的载药体系。由于肿瘤组织和细胞内溶酶体的pH值(分别为6.75和5.5)低于血液的pH值(7.23),所以pH敏感型聚合物纳米载体成为了抗肿瘤药物载体研究的热点 [6] [7] [8] [9] 。一般pH敏感型纳米载体的结构中会含有一些pH敏感的化学键,如肼键,缩醛键,苯甲酸亚胺键和腙键等,这些化学键对酸敏感,当pH下降时,他们会发生断裂,从而使聚合物载体发生解体,释放药物 [10] [11] [12] 。Li研究组做了大量的pH敏感载药胶束的工作,他们使用苯甲酸亚胺键将DOX连接到聚合物胶束的结构上构建了pH敏感的药物释放体系 [13] (图2)。苯甲酰亚胺键是独特的酸敏共价键,它在中性和碱性pH环境下相当稳定,但是在弱的酸性条件下,如肿瘤组织液和细胞内溶酶体中就会发生断裂。体外研究显示pH敏感的载药胶束可以有效地进入癌细胞,然后通过响应溶酶体低的pH值快速释放DOX以发挥抗癌活性。之后的研究者通过在聚合物胶束上连接靶向基团等手段赋予了载药体系更多的功能。

Figure 2. Schematic diagram of polymer micelle self-assembly and pH-sensitive drug release
图2. 聚合物胶束自组装和pH敏感药物释放示意图 [13]
2.2. 氧化还原敏感型聚合物载体
氧化还原敏感型聚合物载体是可以响应外部氧化还原环境变化的载体,它的结构中通常含有二硫键或巯基,它们可以在氧化或还原的环境中相互转换 [14] 。由于细胞内富含谷胱甘肽(GSH),它是一种分子量较低的天然还原剂,而细胞外的GSH浓度不到细胞内的百分之一,所以细胞内被认为是处于还原的环境而细胞外是氧化环境 [15] [16] 。所以含有二硫键或巯基的氧化还原敏感型聚合物载体也受到了越来越多研究者的关注 [17] [18] [19] [20] [21] 。当载体处于细胞外时,结构中含有大量的二硫键,可以对载体进行捆绑作用,防止药物泄漏,而当载体进入细胞内后,二硫键断裂,转变为巯基,促进药物释放。Shuai课题组使用含有二硫键的聚合物载体与siRNA复合,制备成了具有还原敏感性的纳米聚合物载体 [22] (图3)。该载体进入细胞内以后,受到细胞内还原剂GSH的影响,二硫键迅速断裂,从而促进了基因药物的释放。该聚合物胶束具有细胞内快速释放药物的优点,是一种非常有应用前景的载体材料。

Figure 3. Schematic diagram of polymer nanocarrier and intracellular drug release
图3. 聚合物纳米载体的制备和细胞内还原敏感释放药物的示意图 [22]
2.3. 低氧敏感聚合物纳米载体
我们知道,肿瘤组织由于生长旺盛,而血液供应相对不足,与正常组相比,其内部往往处于一种缺氧状态。低氧敏感纳米载体因为可以响应缺氧的环境,在缺氧的环境中解体,进而快速释放药物,近年来受到了研究人员的广泛关注 [23] [24] [25] 。一般低氧敏感聚合物纳米载体中都含有硝基、醌基或偶氮键等低氧敏感基团 [26] 。Park等人合成了羧甲基葡聚糖接枝2-硝基咪唑衍生物的低氧敏感聚合物 [27] 。由于含有亲水的羧甲基葡聚糖和疏水的2-硝基咪唑衍生物,该聚合物在常氧的条件下,可以自组装成纳米胶束负载化疗药物。而进入缺氧的肿瘤细胞时,在还原酶的催化下疏水的硝基咪唑转变成了亲水的氨基咪唑,胶束因为疏水嵌段的缺失发生解体(图4)。细胞毒性实验表明载药胶束对缺氧细胞毒性高于常氧细胞。体内生物分布研究表明载药胶束可以在缺氧肿瘤组织中选择性地积累。除了用来做低氧敏感药物载体,这些低氧敏感的化学基团还可以用作氧气探针。Hanaoka等人设计了含有偶氮键的缺氧敏感性荧光探针,他将罗丹明荧光剂与偶氮键结合,利用偶氮键的超快速构相变化将光能转换成动能,来抑制罗丹明发光,当探针进入缺氧的肿瘤部位时,偶氮键发生断裂,罗丹明恢复发光。根据发光的强弱,可以检测出氧气浓度的高低。通过对罗丹明结构的调整,他们有望制备出一系列检测不同氧气浓度水平的缺氧敏感探针 [28] 。
3. 响应外源刺激释放药物的聚合物载体
响应外源刺激加速释放药物的聚合物载体包括光敏感型聚合物纳米载体和超声波敏感型聚合物载体等,下面将分别介绍这两类刺激敏感型聚合物载体的研究进展。
3.1. 光敏感型聚合物纳米载体
光敏感型聚合物载体是指可以响应不同条件光照的刺激而发生解体的聚合物载体,它的结构中通常含有光敏感的组份,这些组份在光照刺激下,会发生光致异构化,光二聚化或光裂解等变化,从而使载体结构遭到破坏进而释放药物。通过选择和设计光敏组份,聚合物纳米载体可以响应从红外波段到紫外波段的光的刺激,而红外光由于具有组织穿透性好,生物破坏性弱的优点,受到研究人员的广泛研究 [29] [30] 。Zhao等人将聚合物载体上连接上光敏基团,当其暴露于不同波长的光时,载体的亲疏水平衡遭到破坏,从而可以用于智能控释药物。还有研究人员将可光裂解的基团引入到疏水侧链和主链之间或疏水和亲水链段之间,通过光照使亲疏水嵌段断裂来破坏胶束结构,从而构建光敏型聚合物载体 [31] (图5)。

Figure 4. Schematic diagram of passive targeting of drug-loaded micelles and release of hypoxic sensitive drugs
图4. 载药胶束体内被动靶向以及低氧敏感药物释放示意图 [27]

Figure 5. Schematic illustration of various types of light-responsive block copolymer micelles (a)~(d)
图5. 各种类型的光响应性嵌段共聚物胶束(a)~(d)的示意图 [31]
3.2. 超声波敏感型聚合物纳米载体
超声波敏感型聚合物纳米载体是指可以响应超声波的刺激而发生解体加速释放药物的聚合物载体,他通常是通过在聚合物载体中引入可以响应超声波刺激的微泡得到的。由于微泡在超声波的刺激下会迅速坍塌,而这种坍塌产生的能量可以增强药物向肿瘤组织深部渗透的能力,所以受到了越来越多研究人员的关注 [32] [33] 。Wang等人通过异构组装策略将装载基因药物的聚合物胶束和脂质体微泡结合,制备出超声波敏感的纳米聚合物载体,研究其在药物递送到肿瘤部位后响应间歇性低频超声波额能力。结果表明,超声波响应的纳米载体可以有效地穿透到肿瘤的深部位置 [34] 。
4. 响应多重刺激释放药物的聚合物纳米载体
随着刺激响应聚合物研究的不断深入,响应多重刺激而更加快速释放药物的聚合物载体也被开发了出来。研究人员结合不同刺激敏感聚合物的特点,开发出了如pH和氧化还原双重敏感聚合物载体、温度和pH双重敏感聚合物载体以及温度和氧化还原双重敏感聚合物载体 [35] - [41] 。由于pH和氧化还原双重敏感载体在抗肿瘤药物的智能控释方面研究的更加深入,本章主要介绍pH和氧化还原双重敏感聚合物载体。由于肿瘤细胞存在着低pH和还原性的特点,同时响应pH和氧化还原环境的双重敏感聚合物载体受到了深入的研究。Shuai课题组使用含有巯基和聚乙烯亚胺(PEI)的阳离子聚合物载体与siRNA复合,通过在氧化环境下形成二硫键将药物捆绑起来,制备成了体内长循环时间的稳定的聚合物载体 [42] (图6)。该载体进入pH较低的肿瘤组织部位时,载体中的PEI首先响应pH变化,使载体的表面电位从负电位反转正电位,从而促进载体进入肿瘤细胞。而当载体进入肿瘤细胞以后,受到细胞内还原剂GSH的影响,二硫键迅速断裂成巯基,捆绑作用消失,从而促进了基因药物的释放。该聚合物胶束具有体内稳定性强和细胞内快速释放药物等优点,是一种非常有应用前景的载体材料。
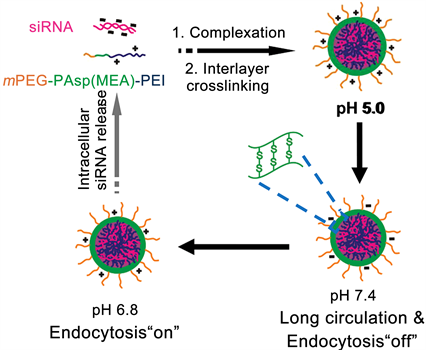
Figure 6. Schematic diagram of polymer nanocarrier loaded gene drug and the dually sensitized release of siRNA inside cell
图6. 双重响应聚合物纳米载体负载基因药物及胞内药物释放示意图 [42]
5. 结论与展望
多年来,研究人员一直都在探索合成更加安全高效的刺激敏感型聚合物载体,以提高抗肿瘤药物的治疗效果,并且降低其毒副作用。本文主要介绍了不同刺激响应聚合物载体的研究现状,以及多重刺激响应聚合物载体的研究进展。虽然刺激响应聚合物载体取得了很大的进展,但是,还存在着很多的问题影响其在临床上的进一步应用,如它们生物相容性、生物体内的可降解性和稳定性依然有限。因此,开发出生物相容性和可降解性优异,体内稳定性高以及响应刺激灵敏性更强的聚合物载体将成为未来刺激敏感型聚合物载体材料的研究重点。
基金项目
本研究获得国家自然科学基金项目(21504082)资助。
文章引用
李景果,冯华阳. 用于抗肿瘤药物递送的刺激敏感型聚合物载体的研究进展
Research Progress of Stimuli-Sensitive Polymer Carriers for Anti-Tumor Drug Delivery[J]. 物理化学进展, 2019, 08(04): 65-73. https://doi.org/10.12677/JAPC.2019.84008
参考文献
- 1. Yang, H.Y., Jang, M.-S., Gao, G.H., Lee, J.H. and Lee, D.S. (2016) Construction of Redox/pH Dual Stimuli-Responsive Pegylated Polymeric Micelles for Intracellular Doxorubicin Delivery in Liver Cancer. Polymer Chemistry, 7, 1813-1825.
https://doi.org/10.1039/C5PY01808K - 2. Du, J., Lane, L.A. and Nie, S. (2015) Stimuli-Responsive Nanoparticles for Targeting the Tumor Microenvironment. Journal of Controlled Release, 219, 205-214.
https://doi.org/10.1016/j.jconrel.2015.08.050 - 3. Wang, S., Huang, P. and Chen, X. (2016) Stimuli-Responsive Programmed Specific Targeting in Nanomedicine. ACS Nano, 10, 2991-2994.
https://doi.org/10.1021/acsnano.6b00870 - 4. Shim, M.S. and Kwon, Y.J. (2012) Stimuli-Responsive Polymers and Nanomaterials for Gene Delivery and Imaging Applications. Advanced Drug Delivery Reviews, 64, 1046-1059.
https://doi.org/10.1016/j.addr.2012.01.018 - 5. Tayo, L.L. (2017) Stimuli-Responsive Na-nocarriers for Intracellular Delivery. Biophysical Reviews, 9, 931-940.
https://doi.org/10.1007/s12551-017-0341-z - 6. Engin, K., Leeper, D., Cater, J., et al. (1995) Extracellular pH Distribution in Human Tumours. International Journal of Hyperthermia, 11, 211-216.
https://doi.org/10.3109/02656739509022457 - 7. Yu, P., Yu, H., Guo, C., et al. (2015) Reversal of Doxorubicin Resistance in Breast Cancer by Mitochondria-Targeted pH-Responsive Micelles. Acta Biomaterialia, 14, 115-124.
https://doi.org/10.1016/j.actbio.2014.12.001 - 8. Kocak, G., Tuncer, C. and Bütün, V. (2017) pH-Responsive Polymers. Polymer Chemistry, 8, 144-176.
https://doi.org/10.1039/C6PY01872F - 9. Mao, J., Li, Y., Wu, T., et al. (2016) A Simple Dual-PH Responsive Prodrug-Based Polymeric Micelles for Drug Delivery. ACS Applied Materials & Interfaces, 8, 17109-17117.
https://doi.org/10.1021/acsami.6b04247 - 10. Aryal, S., Hu, C. and Zhang, L. (2009) Polymer-Cisplatin Conjugate Nanoparticles for Acid-Responsive Drug Delivery. ACS Nano, 4, 251-258.
https://doi.org/10.1021/nn9014032 - 11. Shen, Y., Jin, E., Zhang, B., et al. (2010) Prodrugs Forming High Drug Loading Multifunctional Nanocapsules for Intracellular Cancer Drug Delivery. Journal of the American Chemical Society, 132, 4259-4265.
https://doi.org/10.1021/ja909475m - 12. Mackay, J.A., Chen, M., McDaniel, J.R., et al. (2009) Self-Assembling Chimeric Po-lypeptide-Doxorubicin Conjugate Nanoparticles that Abolish Tumours after a Single Injection. Nature Materials, 8, 993-999.
https://doi.org/10.1038/nmat2569 - 13. Li, J., Zhang, L., Lin, Y., et al. (2016) A pH-Sensitive Prodrug Micelle Self-Assembled from Multi-Doxorubicin-Tailed Polyethylene Glycol for Cancer Therapy. RSC Advances, 6, 9160-9163.
https://doi.org/10.1039/C5RA27293A - 14. Tian, H., Tang, Z., Zhuang, X., Chen, X. and Jing, X. (2012) Biodegradable Syn-thetic Polymers: Preparation, Functionalization and Biomedical Application. Progress in Polymer Science, 37, 237-280.
https://doi.org/10.1016/j.progpolymsci.2011.06.004 - 15. Wu, G., Fang, Y., Yang, S., Lupton, J.R. and Turner, N.D. (2004) Glutathione Metabolism and Its Implications for Health. The Journal of Nutrition, 134, 489-492.
https://doi.org/10.1093/jn/134.3.489 - 16. Aluri, S., Janib, S.M. and Mackay, J.A. (2009) Environmentally Responsive Peptides as Anticancer Drug Carriers. Advnced Drug Delivery Reviews, 61, 940-952.
https://doi.org/10.1016/j.addr.2009.07.002 - 17. Xia, J., Du, Y., Huang, L., et al. (2018) Redox-Responsive Micelles from Disulfide Bond-Bridged Hyaluronic Acid-Tocopherol Succinate for the Treatment of Melanoma. Nanomedicine: Nanotechnology, Biology, and Medicine, 14, 713-723.
https://doi.org/10.1016/j.nano.2017.12.017 - 18. Zhang, Y., Guo, Z., Cao, Z., et al. (2018) Endogenous Albumin-Mediated Delivery of Redox-Responsive Paclitaxel-Loaded Micelles for Targeted Cancer Therapy. Biomaterials, 183, 243-257.
https://doi.org/10.1016/j.biomaterials.2018.06.002 - 19. Sun, C., Li, X., Du, X. and Wang, T. (2018) Redox-Responsive Micelles for Triggered Drug Delivery and Effective Laryngopharyngeal Cancer Therapy. International Journal of Biological Macromolecules, 112, 65-73.
https://doi.org/10.1016/j.ijbiomac.2018.01.136 - 20. Maiti, C., Parida, S., Kayal, S., et al. (2018) Redox-Responsive Core-Cross-Linked Block Copolymer Micelles for Overcoming Multidrug Resistance in Cancer Cells. ACS Applied Materials & Interfaces, 10, 5318-5330.
https://doi.org/10.1021/acsami.7b18245 - 21. Liu, B., Tan, L., He, C., et al. (2018) Redox-Responsive Micelles Self-Assembled from Multi-Block Copolymer for Co-Delivery of Sirna and Hydrophobic Anticancer Drug. Polymer Bulletin, 76, 4237-4257.
https://doi.org/10.1007/s00289-018-2600-y - 22. Chen, W., Yuan, Y., Cheng, D., et al. (2014) Co-Delivery of Doxorubicin and siRNA with Reduction and pH Dually Sensitive Nanocarrier for Synergistic Cancer Therapy. Small, 10, 2678-2687.
https://doi.org/10.1002/smll.201303951 - 23. Qian, C., Yu, J., Chen, Y., et al. (2016) Light-Activated Hypoxia-Responsive Nanocarriers for Enhanced Anticancer Therapy. Advanced Materials, 28, 3313-3320.
https://doi.org/10.1002/adma.201505869 - 24. Zeng, Y., Ma, J., Zhan, Y., et al. (2018) Hypoxia-Activated Prodrugs and Re-dox-Responsive Nanocarriers. International Journal of Nanomedicine, 13, 6551-6574.
https://doi.org/10.2147/IJN.S173431 - 25. Kizaka-Kondoh, S., Inoue, M., Harada, H. and Hiraoka, M. (2003) Tumor Hypoxia: A Target for Selective Cancer Therapy. Cancer Science, 94, 1021-1028.
https://doi.org/10.1111/j.1349-7006.2003.tb01395.x - 26. Liu, J.N., Bu, W. and Shi, J. (2017) Chemical Design and Synthesis of Functionalized Probes for Imaging and Treating Tumor Hypoxia. Chemical Reviews, 117, 6160-6224.
https://doi.org/10.1021/acs.chemrev.6b00525 - 27. Thambi, T., Deepagan, V.G., Yoon, H.Y., et al. (2014) Hypoxia-Responsive Polymeric Nanoparticles for Tumor-Targeted Drug Delivery. Biomaterials, 35, 1735-1743.
https://doi.org/10.1016/j.biomaterials.2013.11.022 - 28. Piao, W., Tsuda, S., Tanaka, Y., et al. (2013) Development of Azo-Based Fluorescent Probes to Detect Different Levels of Hypoxia. Angewandte Chemie International Edition, 52, 13028-13032.
https://doi.org/10.1002/anie.201305784 - 29. Babin, J., Pelletier, M., Lepage, M., et al. (2009) A New Two-Photon-Sensitive Block Copolymer Nanocarrier. Angewandte Chemie International Edition, 48, 3329-3332.
https://doi.org/10.1002/anie.200900255 - 30. Fomina, N., Sankaranarayanan, J. and Almutairi, A. (2012) Photochemical Me-chanisms of Light-Triggered Release from Nanocarriers. Advanced Drug Delivery Reviews, 64, 1005-1020.
https://doi.org/10.1016/j.addr.2012.02.006 - 31. Zhao, Y. (2012) Light-Responsive Block Copolymer Micelles. Macromolecules, 45, 3647-3657.
https://doi.org/10.1021/ma300094t - 32. Baghbani, F. and Moztarzadeh, F. (2017) Bypassing Multidrug Resistant Ovarian Cancer Using Ultrasound Responsive Doxorubicin/Curcumin Co-Deliver Alginate Nanodroplets. Colloids and Surfaces B: Biointer-faces, 153, 132-140.
https://doi.org/10.1016/j.colsurfb.2017.01.051 - 33. Baghbani, F., Chegeni, M., Moztarzadeh, F., Hadian-Ghazvini, S. and Raz, M. (2017) Novel Ultrasound-Responsive Chitosan/Perfluorohexane Nanodroplets for Image-Guided Smart Delivery of an Anticancer Agent: Curcumin. Materials Science and Engineering: C, 74, 186-193.
https://doi.org/10.1016/j.msec.2016.11.107 - 34. Wang, P., Yin, T., Li, J., et al. (2016) Ultrasound-Responsive Microbubbles for Sonography-Guided siRNA Delivery. Nanomedicine: Na-notechnology, Biology and Medicine, 12, 1139-1149.
https://doi.org/10.1016/j.nano.2015.12.361 - 35. Alex, M.R.A., Nehate, C., Veeranarayanan, S., et al. (2017) Self Assembled Dual Responsive Micelles Stabilized with Protein for Co-Delivery of Drug and siRNA in Cancer Therapy. Biomaterials, 133, 94-106.
https://doi.org/10.1016/j.biomaterials.2017.04.022 - 36. Teo, J.Y., Chin, W., Ke, X., et al. (2017) pH and Redox Dual-Responsive Biodegradable Polymeric Micelles with High Drug Loading for Effective Anticancer Drug Delivery. Nanomedicine: Nanotechnology, Biology and Medicine, 13, 431-442.
https://doi.org/10.1016/j.nano.2016.09.016 - 37. Zhuang, W., Xu, Y., Li, G., et al. (2018) Redox and pH Dual-Responsive Polymeric Micelles with Aggregation-Induced Emission Feature for Cellular Imaging and Chemotherapy. ACS Applied Materials & Interfaces, 10, 18489-18498.
https://doi.org/10.1021/acsami.8b02890 - 38. Yu, H., Cui, Z., Yu, P., et al. (2015) pH-and NIR Light-Responsive Micelles with Hyperthermia-Triggered Tumor Penetration and Cytoplasm Drug Release to Reverse Doxorubicin Resistance in Breast Cancer. Advanced Functional Materials, 25, 2489-2500.
https://doi.org/10.1002/adfm.201404484 - 39. Xu, X., Li, L., Zhou, Z., Sun, W. and Huang, Y. (2016) Dual-pH Responsive Micelle Platform for Co-Delivery of Axitinib and Doxorubicin. International Journal of Pharmaceutics, 507, 50-60.
https://doi.org/10.1016/j.ijpharm.2016.04.060 - 40. Wang, Y., Luo, Q., Zhu, W., et al. (2016) Reduction/pH Dual-Responsive Nano-Prodrug Micelles for Controlled Drug Delivery. Polymer Chemistry, 7, 2665-2673.
https://doi.org/10.1039/C6PY00168H - 41. Sang, M.M., Liu, F.L., Wang, Y., et al. (2018) A Novel Redox/pH Dual-Responsive and Hyaluronic Acid-Decorated Multifunctional Magnetic Complex Micelle for Targeted Gambogic Acid Delivery for the Treatment of Triple Negative Breast Cancer. Drug Delivery, 25, 1846-1857.
https://doi.org/10.1080/10717544.2018.1486472 - 42. Li, J., Yu, X., Wang, Y., et al. (2014) A Reduction and pH Dual-Sensitive Polymeric Vector for Long-Circulating and Tumor-Targeted Sirna Delivery. Advanced Materials, 26, 8217-8224.
https://doi.org/10.1002/adma.201403877 - 43. Yang, H.Y., Jang, M.-S., Gao, G.H., Lee, J.H. and Lee, D.S. (2016) Construction of Redox/pH Dual Stimuli-Responsive Pegylated Polymeric Micelles for Intracellular Doxorubicin Delivery in Liver Cancer. Polymer Chemistry, 7, 1813-1825.
https://doi.org/10.1039/C5PY01808K - 44. Du, J., Lane, L.A. and Nie, S. (2015) Stimuli-Responsive Nanoparticles for Targeting the Tumor Microenvironment. Journal of Controlled Release, 219, 205-214.
https://doi.org/10.1016/j.jconrel.2015.08.050 - 45. Wang, S., Huang, P. and Chen, X. (2016) Stimuli-Responsive Programmed Specific Targeting in Nanomedicine. ACS Nano, 10, 2991-2994.
https://doi.org/10.1021/acsnano.6b00870 - 46. Shim, M.S. and Kwon, Y.J. (2012) Stimuli-Responsive Polymers and Nanomaterials for Gene Delivery and Imaging Applications. Advanced Drug Delivery Reviews, 64, 1046-1059.
https://doi.org/10.1016/j.addr.2012.01.018 - 47. Tayo, L.L. (2017) Stimuli-Responsive Na-nocarriers for Intracellular Delivery. Biophysical Reviews, 9, 931-940.
https://doi.org/10.1007/s12551-017-0341-z - 48. Engin, K., Leeper, D., Cater, J., et al. (1995) Extracellular pH Distribution in Human Tumours. International Journal of Hyperthermia, 11, 211-216.
https://doi.org/10.3109/02656739509022457 - 49. Yu, P., Yu, H., Guo, C., et al. (2015) Reversal of Doxorubicin Resistance in Breast Cancer by Mitochondria-Targeted pH-Responsive Micelles. Acta Biomaterialia, 14, 115-124.
https://doi.org/10.1016/j.actbio.2014.12.001 - 50. Kocak, G., Tuncer, C. and Bütün, V. (2017) pH-Responsive Polymers. Polymer Chemistry, 8, 144-176.
https://doi.org/10.1039/C6PY01872F - 51. Mao, J., Li, Y., Wu, T., et al. (2016) A Simple Dual-PH Responsive Prodrug-Based Polymeric Micelles for Drug Delivery. ACS Applied Materials & Interfaces, 8, 17109-17117.
https://doi.org/10.1021/acsami.6b04247 - 52. Aryal, S., Hu, C. and Zhang, L. (2009) Polymer-Cisplatin Conjugate Nanoparticles for Acid-Responsive Drug Delivery. ACS Nano, 4, 251-258.
https://doi.org/10.1021/nn9014032 - 53. Shen, Y., Jin, E., Zhang, B., et al. (2010) Prodrugs Forming High Drug Loading Multifunctional Nanocapsules for Intracellular Cancer Drug Delivery. Journal of the American Chemical Society, 132, 4259-4265.
https://doi.org/10.1021/ja909475m - 54. Mackay, J.A., Chen, M., McDaniel, J.R., et al. (2009) Self-Assembling Chimeric Po-lypeptide-Doxorubicin Conjugate Nanoparticles that Abolish Tumours after a Single Injection. Nature Materials, 8, 993-999.
https://doi.org/10.1038/nmat2569 - 55. Li, J., Zhang, L., Lin, Y., et al. (2016) A pH-Sensitive Prodrug Micelle Self-Assembled from Multi-Doxorubicin-Tailed Polyethylene Glycol for Cancer Therapy. RSC Advances, 6, 9160-9163.
https://doi.org/10.1039/C5RA27293A - 56. Tian, H., Tang, Z., Zhuang, X., Chen, X. and Jing, X. (2012) Biodegradable Syn-thetic Polymers: Preparation, Functionalization and Biomedical Application. Progress in Polymer Science, 37, 237-280.
https://doi.org/10.1016/j.progpolymsci.2011.06.004 - 57. Wu, G., Fang, Y., Yang, S., Lupton, J.R. and Turner, N.D. (2004) Glutathione Metabolism and Its Implications for Health. The Journal of Nutrition, 134, 489-492.
https://doi.org/10.1093/jn/134.3.489 - 58. Aluri, S., Janib, S.M. and Mackay, J.A. (2009) Environmentally Responsive Peptides as Anticancer Drug Carriers. Advnced Drug Delivery Reviews, 61, 940-952.
https://doi.org/10.1016/j.addr.2009.07.002 - 59. Xia, J., Du, Y., Huang, L., et al. (2018) Redox-Responsive Micelles from Disulfide Bond-Bridged Hyaluronic Acid-Tocopherol Succinate for the Treatment of Melanoma. Nanomedicine: Nanotechnology, Biology, and Medicine, 14, 713-723.
https://doi.org/10.1016/j.nano.2017.12.017 - 60. Zhang, Y., Guo, Z., Cao, Z., et al. (2018) Endogenous Albumin-Mediated Delivery of Redox-Responsive Paclitaxel-Loaded Micelles for Targeted Cancer Therapy. Biomaterials, 183, 243-257.
https://doi.org/10.1016/j.biomaterials.2018.06.002 - 61. Sun, C., Li, X., Du, X. and Wang, T. (2018) Redox-Responsive Micelles for Triggered Drug Delivery and Effective Laryngopharyngeal Cancer Therapy. International Journal of Biological Macromolecules, 112, 65-73.
https://doi.org/10.1016/j.ijbiomac.2018.01.136 - 62. Maiti, C., Parida, S., Kayal, S., et al. (2018) Redox-Responsive Core-Cross-Linked Block Copolymer Micelles for Overcoming Multidrug Resistance in Cancer Cells. ACS Applied Materials & Interfaces, 10, 5318-5330.
https://doi.org/10.1021/acsami.7b18245 - 63. Liu, B., Tan, L., He, C., et al. (2018) Redox-Responsive Micelles Self-Assembled from Multi-Block Copolymer for Co-Delivery of Sirna and Hydrophobic Anticancer Drug. Polymer Bulletin, 76, 4237-4257.
https://doi.org/10.1007/s00289-018-2600-y - 64. Chen, W., Yuan, Y., Cheng, D., et al. (2014) Co-Delivery of Doxorubicin and siRNA with Reduction and pH Dually Sensitive Nanocarrier for Synergistic Cancer Therapy. Small, 10, 2678-2687.
https://doi.org/10.1002/smll.201303951 - 65. Qian, C., Yu, J., Chen, Y., et al. (2016) Light-Activated Hypoxia-Responsive Nanocarriers for Enhanced Anticancer Therapy. Advanced Materials, 28, 3313-3320.
https://doi.org/10.1002/adma.201505869 - 66. Zeng, Y., Ma, J., Zhan, Y., et al. (2018) Hypoxia-Activated Prodrugs and Re-dox-Responsive Nanocarriers. International Journal of Nanomedicine, 13, 6551-6574.
https://doi.org/10.2147/IJN.S173431 - 67. Kizaka-Kondoh, S., Inoue, M., Harada, H. and Hiraoka, M. (2003) Tumor Hypoxia: A Target for Selective Cancer Therapy. Cancer Science, 94, 1021-1028.
https://doi.org/10.1111/j.1349-7006.2003.tb01395.x - 68. Liu, J.N., Bu, W. and Shi, J. (2017) Chemical Design and Synthesis of Functionalized Probes for Imaging and Treating Tumor Hypoxia. Chemical Reviews, 117, 6160-6224.
https://doi.org/10.1021/acs.chemrev.6b00525 - 69. Thambi, T., Deepagan, V.G., Yoon, H.Y., et al. (2014) Hypoxia-Responsive Polymeric Nanoparticles for Tumor-Targeted Drug Delivery. Biomaterials, 35, 1735-1743.
https://doi.org/10.1016/j.biomaterials.2013.11.022 - 70. Piao, W., Tsuda, S., Tanaka, Y., et al. (2013) Development of Azo-Based Fluorescent Probes to Detect Different Levels of Hypoxia. Angewandte Chemie International Edition, 52, 13028-13032.
https://doi.org/10.1002/anie.201305784 - 71. Babin, J., Pelletier, M., Lepage, M., et al. (2009) A New Two-Photon-Sensitive Block Copolymer Nanocarrier. Angewandte Chemie International Edition, 48, 3329-3332.
https://doi.org/10.1002/anie.200900255 - 72. Fomina, N., Sankaranarayanan, J. and Almutairi, A. (2012) Photochemical Me-chanisms of Light-Triggered Release from Nanocarriers. Advanced Drug Delivery Reviews, 64, 1005-1020.
https://doi.org/10.1016/j.addr.2012.02.006 - 73. Zhao, Y. (2012) Light-Responsive Block Copolymer Micelles. Macromolecules, 45, 3647-3657.
https://doi.org/10.1021/ma300094t - 74. Baghbani, F. and Moztarzadeh, F. (2017) Bypassing Multidrug Resistant Ovarian Cancer Using Ultrasound Responsive Doxorubicin/Curcumin Co-Deliver Alginate Nanodroplets. Colloids and Surfaces B: Biointer-faces, 153, 132-140.
https://doi.org/10.1016/j.colsurfb.2017.01.051 - 75. Baghbani, F., Chegeni, M., Moztarzadeh, F., Hadian-Ghazvini, S. and Raz, M. (2017) Novel Ultrasound-Responsive Chitosan/Perfluorohexane Nanodroplets for Image-Guided Smart Delivery of an Anticancer Agent: Curcumin. Materials Science and Engineering: C, 74, 186-193.
https://doi.org/10.1016/j.msec.2016.11.107 - 76. Wang, P., Yin, T., Li, J., et al. (2016) Ultrasound-Responsive Microbubbles for Sonography-Guided siRNA Delivery. Nanomedicine: Na-notechnology, Biology and Medicine, 12, 1139-1149.
https://doi.org/10.1016/j.nano.2015.12.361 - 77. Alex, M.R.A., Nehate, C., Veeranarayanan, S., et al. (2017) Self Assembled Dual Responsive Micelles Stabilized with Protein for Co-Delivery of Drug and siRNA in Cancer Therapy. Biomaterials, 133, 94-106.
https://doi.org/10.1016/j.biomaterials.2017.04.022 - 78. Teo, J.Y., Chin, W., Ke, X., et al. (2017) pH and Redox Dual-Responsive Biodegradable Polymeric Micelles with High Drug Loading for Effective Anticancer Drug Delivery. Nanomedicine: Nanotechnology, Biology and Medicine, 13, 431-442.
https://doi.org/10.1016/j.nano.2016.09.016 - 79. Zhuang, W., Xu, Y., Li, G., et al. (2018) Redox and pH Dual-Responsive Polymeric Micelles with Aggregation-Induced Emission Feature for Cellular Imaging and Chemotherapy. ACS Applied Materials & Interfaces, 10, 18489-18498.
https://doi.org/10.1021/acsami.8b02890 - 80. Yu, H., Cui, Z., Yu, P., et al. (2015) pH-and NIR Light-Responsive Micelles with Hyperthermia-Triggered Tumor Penetration and Cytoplasm Drug Release to Reverse Doxorubicin Resistance in Breast Cancer. Advanced Functional Materials, 25, 2489-2500.
https://doi.org/10.1002/adfm.201404484 - 81. Xu, X., Li, L., Zhou, Z., Sun, W. and Huang, Y. (2016) Dual-pH Responsive Micelle Platform for Co-Delivery of Axitinib and Doxorubicin. International Journal of Pharmaceutics, 507, 50-60.
https://doi.org/10.1016/j.ijpharm.2016.04.060 - 82. Wang, Y., Luo, Q., Zhu, W., et al. (2016) Reduction/pH Dual-Responsive Nano-Prodrug Micelles for Controlled Drug Delivery. Polymer Chemistry, 7, 2665-2673.
https://doi.org/10.1039/C6PY00168H - 83. Sang, M.M., Liu, F.L., Wang, Y., et al. (2018) A Novel Redox/pH Dual-Responsive and Hyaluronic Acid-Decorated Multifunctional Magnetic Complex Micelle for Targeted Gambogic Acid Delivery for the Treatment of Triple Negative Breast Cancer. Drug Delivery, 25, 1846-1857.
https://doi.org/10.1080/10717544.2018.1486472 - 84. Li, J., Yu, X., Wang, Y., et al. (2014) A Reduction and pH Dual-Sensitive Polymeric Vector for Long-Circulating and Tumor-Targeted Sirna Delivery. Advanced Materials, 26, 8217-8224.
https://doi.org/10.1002/adma.201403877
NOTES
*通讯作者。