Asian Case Reports in Oncology
Vol.06 No.01(2017), Article ID:21465,8
pages
10.12677/ACRPO.2017.61001
Analysis of the Efficacy of Apatinib in the Treatment of Brain Metastases of Lung Adenocarcinoma
Jinlong Zhu, Hong Wang*
Department of Pulmonary Oncology, The 307th Hospital of Chinese PLA, Beijing
*通讯作者。

Received: Jul. 4th, 2017; accepted: Jul. 21st, 2017; published: Jul. 24th, 2017

ABSTRACT
Lung cancer is one of the world’s most common and most malignant tumors. The lethality rate of which is the highest in all types of malignant tumors. The five-year survival rate of it is less than 20%, non-small cell lung cancer accounting for more than 70% of all cases and being diagnosed in the locally advanced stage. The 5-year survival rate of NSCLC is less than 6%. Patients can get relief and survival benefit by chemotherapy. Most patients will get PD in 2 or 3 months after the last chemotherapy, especially for those with negative genetic testing showing dismal efficacy to the application of molecular targeted drugs. Study found that neovascularization provide oxygen and nutrition for the growth of the tumor, to promote its growth. Anti-angiogenic drugs can inhibit tumor from progressing and metastasis by inhibiting angiogenesis. And the vascular epidermal growth factor (VEGF) can activate the downstream pathway to stimulate the proliferation of vessel endothelium via binding vascular epidermal growth factor receptor (VEGFR), thus leading to the growth of tumor. Paclitaxel mesylate is a small molecular anti-angiogenesis inhibitor. It can inhibit solid tumor growth factor tyrosine kinase receptor. Acid apatinib shows excellent efficacy and good tolerance for the advanced NSCLC whose multi-line treatment has been failed. This paper reports a case of apatinib mesylate as a fourth-line drug treatment in patients with advanced non-squamous NSCLC brain metastases.
Keywords:Apatinib, Non-Small-Cell Lung Cancer, Advanced Lung Cancer
甲磺酸阿帕替尼治疗一例肺腺癌脑转移患者的疗效分析
祝金龙,王红*
解放军第307医院肺部肿瘤科,北京

收稿日期:2017年7月4日;录用日期:2017年7月21日;发布日期:2017年7月24日

摘 要
肺癌是世界上最常见也是恶性程度最高的恶性肿瘤之一,其致死率高居各类恶性肿瘤之首,五年生存率不足20%,其中非小细胞肺癌占其中70%以上,发现时多为晚期,非小细胞肺癌的5年生存率不足6%,患者可以通过选择放化疗得到疾病缓解和生存获益,仍有大部分患者会在末次化疗后的2到3个月内再次出现疾病的进展,特别是对于基因检测阴性的患者,对针对应用此类基因的靶向药物疗效不佳,研究发现新生血管为肿瘤生长提供氧和营养,促进肿瘤的生长,而血管内皮生长因子(VEGF)能激活下游通路刺激血管通过结合血管内皮细胞增生的表皮生长因子受体(VEGFR),从而导致肿瘤的生长,抗血管生成药物通过抑制抑制血管生成能够抑制肿瘤发生发展和转移,甲磺酸阿帕替尼属于小分子抗血管生成抑制剂,作用于血管表皮生长因子酪氨酸激酶受体,通过抗血管生成来抑制实体肿瘤生长,甲磺酸阿帕替尼对于多线治疗失败的的非小细胞肺癌晚期治疗优确切的疗效,具有较好的耐受性。本文报道1例甲磺酸阿帕替尼作为四线药物治疗晚期非鳞NSCLC脑转移患者。
关键词 :甲磺酸阿帕替尼,非鳞非小细胞肺癌,晚期肺癌

Copyright © 2017 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 病例概况
患者刘xx,男,55岁,吸烟40余年,每天20支以上。患者2014年1月因“头晕、头痛2个月”入院,于天坛医院完善相关检查发现左枕叶占位性病变、左肺上叶占位,而后行“左枕叶占位切除术”,术后病理示:肺腺癌脑转移。EGFR基因检测:未见突变;EML4-ALK融合基因为阴性;KRAS为野生型。2014年3月14日、2014年4月4日行一线培美曲塞(900 mg d1) + 顺铂(130 mg 分d1-3)化疗2周期。2014年3月25日至4月29日行全脑放疗20次,Dt40Gy/20f。局部加量5次,Dt10Gy/5f。此后患者分别于2014年3月在我院,2014年5月在304医院行细胞免疫治疗2疗程。2014年7月疗效评价PR。2014年7月29日、8月20日、9月18日、10月11日行一线第3~6周期培美曲塞(1000 mg d1)联合顺铂(130 mg 分d1-2)化疗;4周期后疗效评价维持PR。2015年9月复查胸部CT提示左肺上叶病灶较前增大,并出现颈部淋巴结转移,考虑疾病进展。于2015年9月24日开始行紫杉醇(330 mg d1)联合卡铂(600 mg d1)方案二线治疗化疗6周期,疗效评价PR。2016年4月复查提示疾病进展,三线治疗2016-04-23开始行吉西他滨(1900 mg,d1、d8)联合洛铂(60 mg,d1)方案2周期,疗效评价PD,并出现多发颅内转移。四线治疗于2016年7月2日开始给予甲磺酸阿帕替尼0.25 g口服2/日、替莫唑胺100 mg 1/日口服治疗。2016年10月18日复查肺部病灶稳定,颅内病灶较前稍增大,疗效评价SD,4线治疗有效目前PFS4个月(图1~13)。期间多次建议靶病灶放疗,患者拒绝。
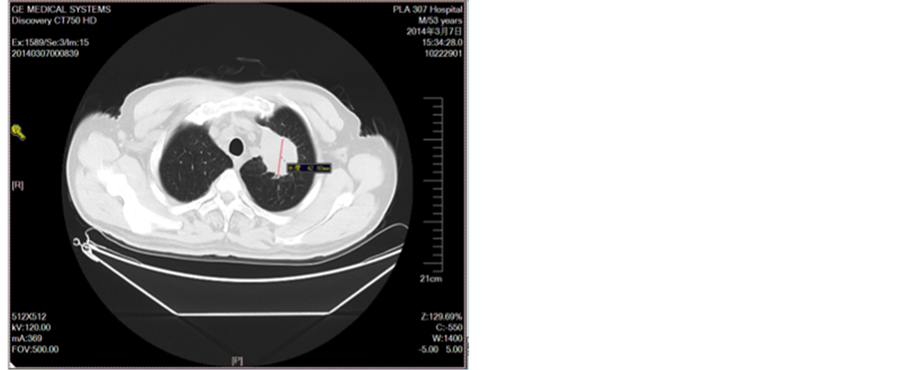
Figure 1. Before treatment
图1. 未治疗前
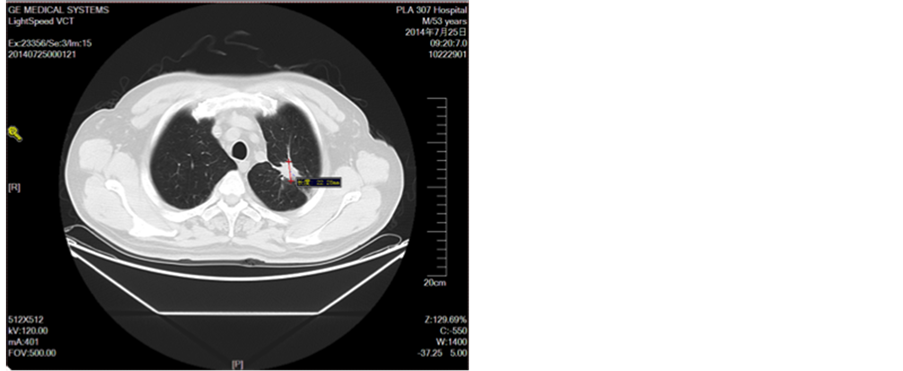
Figure 2. Line 4 cycle
图2. 一线4周期
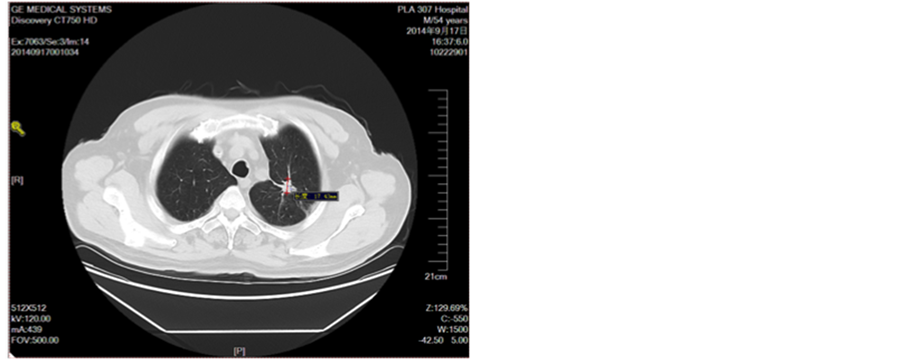
Figure 3. Line 6 cycle
图3. 一线6周期
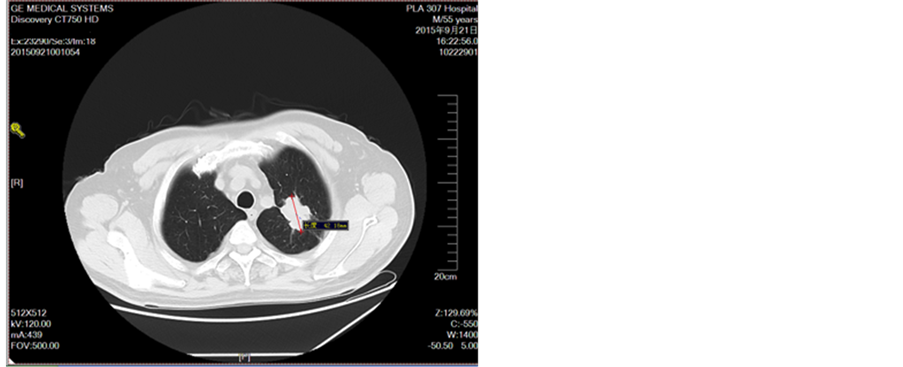
Figure 4. Disease development in September 2015
图4. 2015年9月疾病进展
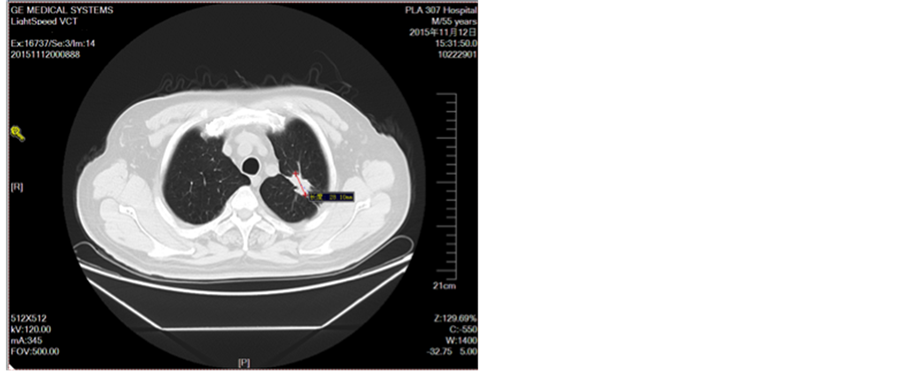
Figure 5. Second-line treatment
图5. 二线治疗2周期图
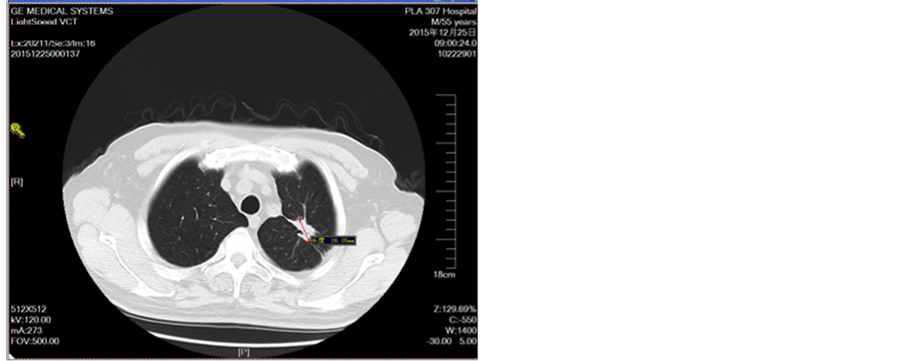
Figure 6. Second-line treatment for 4 cycles
图6. 二线治疗4周期

Figure 7. Review of disease progression in December 2015
图7. 2015年12月复查疾病进展
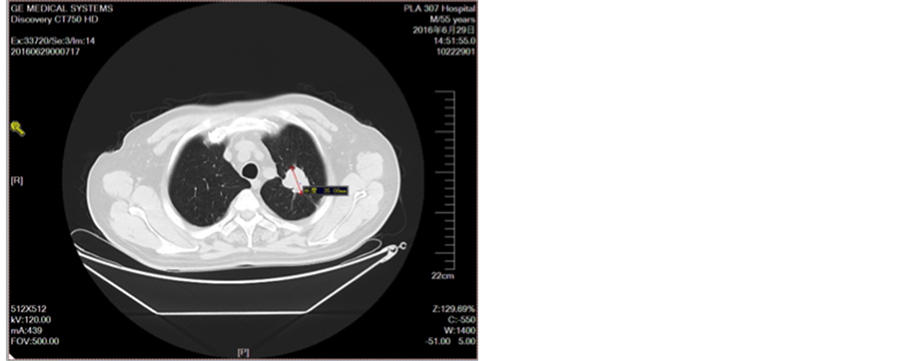
Figure 8. After three cycles of chemotherapy
图8. 三线化疗2周期后
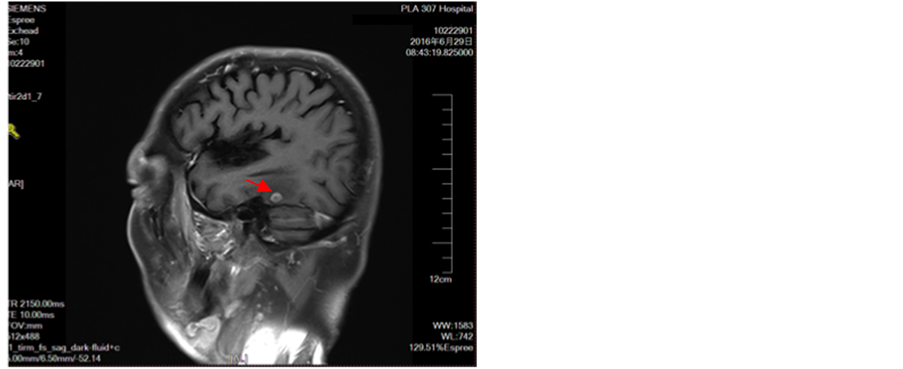
Figure 9. Appears intracranial multiple metastases
图9. 出现颅内多发转移瘤
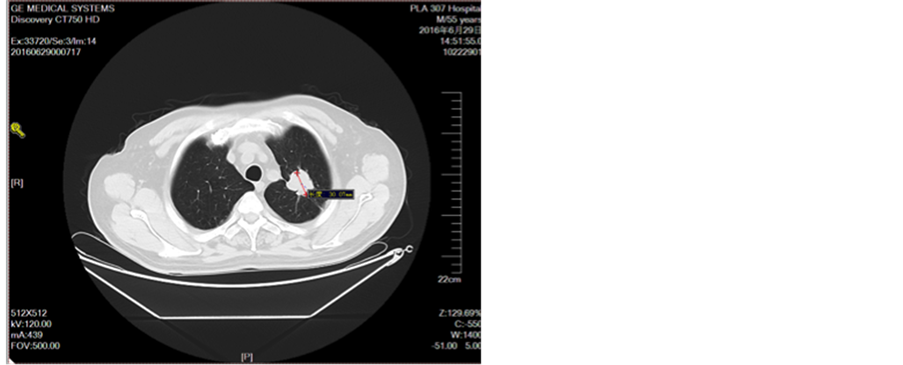
Figure 10. Oral treatment after oral administration
图10. 口服艾坦治疗1月后
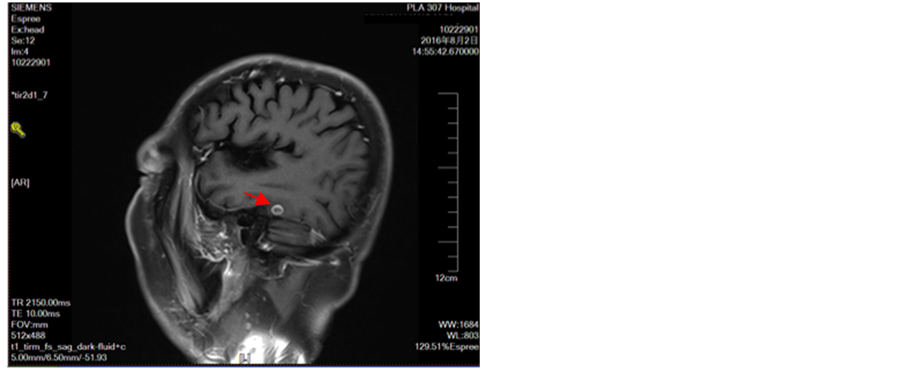
Figure 11. Intracranial multiple metastases
图11. 颅内多发转移瘤
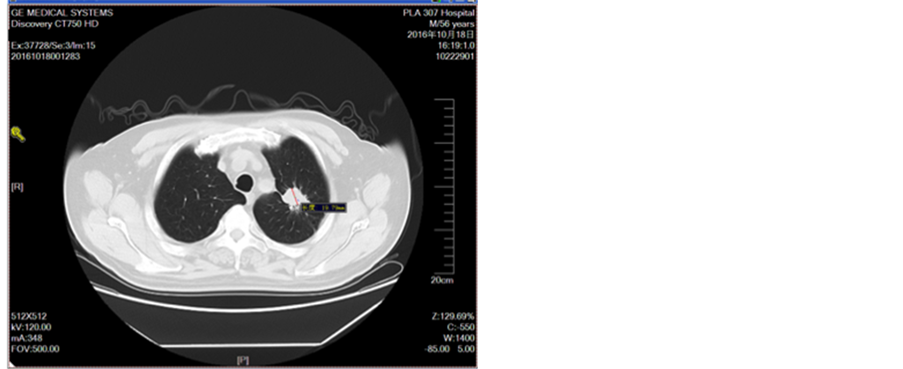
Figure 12. Oral administration of apatinit for 4 months
图12. 口服阿帕替尼治疗4月
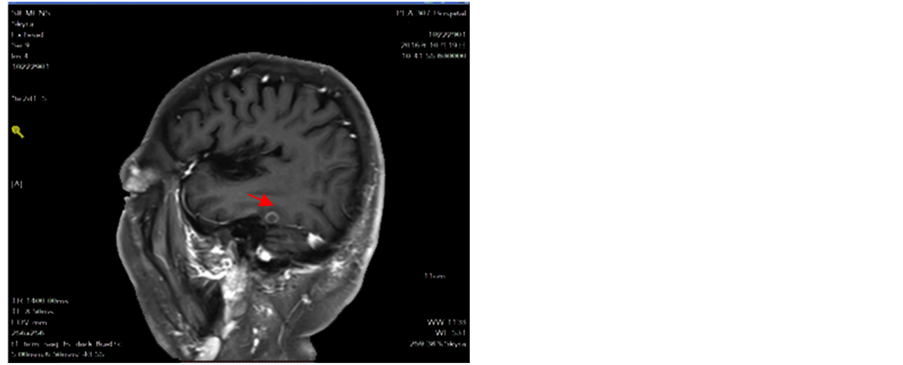
Figure 13. Intracranial tumor slightly increased lung tumor stability
图13. 颅内肿瘤略增大肺部肿瘤稳定
2. 讨论
该患者为三线治疗后疾病进展的非小细胞肺癌,经过四线给予口服甲磺酸阿帕替尼治疗之后,疾病处于稳定状态,左肺上叶靶病灶曾多次建议患者做局部治疗患者及家属表示拒绝,如果适当局部靶病灶治疗介入可能疗效更好。甲磺酸阿帕替尼是我国自主研发生产的多靶点小分子抗血管生成药物,其作用靶点主要为VEGFR-2,也可作用于一类受体酪氨酸激酶,包括c-kit、RET和c-src,VEGF-2通过激活丝裂原活化蛋白激酶(MAPK)信号通路从而促进血管内皮细胞增殖。甲磺酸阿帕替尼阻断VEGFR-2,从而降低MAPK的活化,抑制血管内皮细胞的增殖。从而起到抗血管生成的作用,抑制肿瘤的生长,在治疗晚期非鳞非小细胞肺癌的二期临床研究中,甲磺酸阿帕替尼显示出诸多优点,疗效显著,使中位PFS延长2.8个月,优于索拉非尼的1.4个月,ORR和DCR均高于索拉非尼,常见的不良反应为高血压、蛋白尿、手足综合症等,血液学毒性较低,与高度的靶点选择性相关,出血发生率较低(9.9%),主要为咯血及便血;目前,我们期待临床3期的研究结果,总体上,甲磺酸阿帕替尼具有较好的安全性及有效性 [1] - [12] 。
3. 总结
甲磺酸阿帕替尼作为我国具有自主产权的抗血管生成靶向药物,在多种晚期肿瘤治疗中产生了良好的疗效,比如胃癌、乳腺癌、骨肉瘤等疾病,近年来在化疗失败的晚期非鳞非小细胞肺癌的治疗中也是取得了良好的治疗效果。以甲磺酸阿帕替尼为代表的抗血管生成药物在肺癌中的研究虽然取得了一定的进步,但仍旧面临巨大的挑战,仍需通过大量的实验数据研究来明确抗血管生成药物治疗肺癌的机制,探索更有价值的标志物,从而提高患者的疗效,延长患者的生存时间。通过此例病例,根据患者疗效证明,甲磺酸阿帕替尼对于多线治疗失败的非鳞非小细胞肺癌的治疗有较好的疗效,使得我们在以后应用抗血管生成药物治疗肺癌增加了信心,期待甲磺酸阿帕替尼更多的临床治疗新进展,为肿瘤患者的治疗带来新的希望。
文章引用
祝金龙,王 红. 甲磺酸阿帕替尼治疗一例肺腺癌脑转移患者的疗效分析
Analysis of the Efficacy of Apatinib in the Treatment of Brain Metastases of Lung Adenocarcinoma[J]. 亚洲肿瘤科病例研究, 2017, 06(01): 1-8. http://dx.doi.org/10.12677/ACRPO.2017.61001
参考文献 (References)
- 1. Mok, T., et al. (2014) A correlate Ⅳ Biomarker Analysis of the Combination of Bevacizumab and Carboplatin-Based Chemo Therapy for Advanced Non-Squamous Non-Small-Cell Lung Cancer: Results of the Phase 2 Randomized ABIGAIL Study. Journal of Thoracic Oncology, 9, 848-55.
- 2. Zhou, C., et al. (2015) BEYOND: A Randomized, Double-Blind, Placebo-Controlled, Multicenter, Phase 3 Study of First-Line Carboplatin/Paclitaxel Plus Bevacizumab or Placebo in Chinese Patients with Advanced or Recurrent NonSquamous Non-Small-Cell Lung Cancer. Journal of Clinical Oncology, 33, 2197-2204. https://doi.org/10.1200/JCO.2014.59.4424
- 3. Reck, M., et al. (2014) Docetaxel Plus Nintedanib versus Docetaxel Plus Placebo in Patients with Previously Treated Non-Small-Cell Lung Cancer (LUME-Lung 1). A Phase 3, Double-Blind, Randomized Controlled Trial. Lancet Oncology, 15, 143-155. https://doi.org/10.1016/S1470-2045(13)70586-2
- 4. Seto, T., et al. (2014) Erlotinib Alone or with Bevacizumab as First-Line Therapy in Patients with Advanced Non-Squamous Non-Small-Cell-Lung Cancer Harbouring EGFR Mutations (J025567): An Open-Label Randomised, Multicentre, Phase 2 Study. Lancet Oncology, 15, 1236-1244. https://doi.org/10.1016/S1470-2045(14)70381-X
- 5. Sandler, A., et al. (2006) Paclitaxel-Carboplatin Alone or with Bevacizumab for Non-Small-Cell Lung Cancer. The New England Journal of Medicine, 355, 2542-2550. https://doi.org/10.1056/NEJMoa061884
- 6. Reck, M., et al. (2009) Phase 3 Trial of Cisplatin Plus Gemcitabine with Either Placebo or Bevarizumab as First-Line Therapy Fornonsquamous Non-Small-Cell Lung Cancer: AVAil. Journal of Clinical Oncology, 27, 1227-1234. https://doi.org/10.1200/JCO.2007.14.5466
- 7. Patel, J. D., et al. (2013) Point Break: A Randomized Phase 3 Study of Pemetrexed Plus Carboplatin and Bevacizumah Followed by Maintenance Pemetrexed and Bevacizumah versus Paclitaxel Plus Carboplatin and Bevacizumab Followed by Maintenance Bevacizumab in Patients with Stage 3 B or 4 Non-Squamousnon-Small-Cell Lung Cancer. Journal of Clinical Oncology, 31, 4349-4357. https://doi.org/10.1200/JCO.2012.47.9626
- 8. Garon, E.B., et al. (2014) Ramucirumab Plus Docetaxel versus Placebo Plus Docetaxel for Second-Line Treatment of Stage 4 Non-Small-Cell Lung Cancer after Disease Progression on Platinum-Based Therapy (REVEL): Amulti Centre, Double-Blind, Randomized Phase 3 Trial. Lancet, 384, 665-673. https://doi.org/10.1016/S0140-6736(14)60845-X
- 9. Barlesi, F., et al. (2013) Randomized Phase 3 Trial of Maintenance Bevacizumab with or without Pemetrexed after First-Line Induction with Bevacizumab, Cisplatin, and Pemetrexed Inadvanced Nonsquamous Non-Small-Cell Lung Cancer: AVAPERL (M022089). Journal of Clinical Oncology, 31, 3004-3011.
- 10. Lynch, T.J., el al. (2014) Safety and Effect 4 Eness of Bevacizumab-Containing Treatment for Non-Small-Cell Lung Cancer : Final Results of the ARIES Observational Cohort Study. Journal of Thoracic Oncology, 9, 1332-1339.
- 11. Crino, L., et al. (2010) Safety and Efficacy of First-Line Bevacizumab-Based Therapy in Advanced Non-Squamous Non-Small-Cell Lung Cancer (SAiL, M019390): A Phase 4 Study. Lancet Oncology, 11, 733-740. https://doi.org/10.1016/S1470-2045(10)70151-0
- 12. Heist, R.S., et al. (2008) VEGF Polymorphisms and Survival 4 in Early-Stage Non-Small-Cell Lung Cancer. Journal of Clinical Oncology, 26, 856-862. https://doi.org/10.1200/JCO.2007.13.5947