Hans Journal of Biomedicine
Vol.
13
No.
01
(
2023
), Article ID:
60976
,
10
pages
10.12677/HJBM.2023.131010
Hippo/YAP途径在肿瘤细胞信号通路交互调控中的作用
王莹,任欣欣,赵铁军*
浙江师范大学生命科学学院,浙江 金华
收稿日期:2022年12月19日;录用日期:2023年1月20日;发布日期:2023年1月31日

摘要
Hippo/YAP信号通路在维持组织动态平衡和器官再生中发挥重要作用。近几年,越来越多研究表明Hippo/YAP途径与其他肿瘤相关细胞信号通路交互调控,从而影响多种肿瘤的发生和发展过程。本文主要综述了Hippo/YAP信号通路的组成、调控机制,以及Hippo通路关键因子YAP在与其他肿瘤相关细胞信号通路交互调控中的功能及其分子机制。本文将为深入挖掘并揭示Hippo/YAP通路在肿瘤发生中的作用,并以此为开发靶向药物提供思路。
关键词
Hippo信号通路,YAP,信号通路网络,肿瘤

The Role of Hippo/YAP Pathway in the Crosstalk of Tumor Cell Signaling Pathways
Ying Wang, Xinxin Ren, Tiejun Zhao*
College of Life Sciences, Zhejiang Normal University, Jinhua Zhejiang
Received: Dec. 19th, 2022; accepted: Jan. 20th, 2023; published: Jan. 31st, 2023

ABSTRACT
Hippo/YAP signaling pathway plays a decisive role in maintaining tissue homeostasis and organ regeneration. In recent years, more and more studies have shown that Hippo/YAP pathway crosstalk with other tumor-related cell signaling pathways, thus affecting the occurrence and development of various tumors. This paper mainly reviews the composition and regulatory mechanism of Hippo/YAP signaling pathway, as well as the function and molecular mechanism of YAP, a key factor of Hippo pathway, in crosstalk with other tumor-related cell signaling pathways. This paper will provide ideas for further exploring and revealing the role of Hippo/YAP pathway in tumorigenesis and thereby developing targeted drugs.
Keywords:Hippo Signaling Pathway, YAP, Signal Pathway Network, Tumor

Copyright © 2023 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
Hippo信号通路是调节细胞增殖、凋亡及肿瘤发生的重要途径 [1]。其异常失活将导致YAP在胞质中积累,从而使细胞的增殖凋亡失衡、接触性抑制丧失或细胞恶性转化,促进肿瘤干细胞的自我更新、迁移,最终导致肿瘤形成 [2]。Hippo通路起初被认为是目前阐述较为清楚的、机制较为简单的信号转导通路。然而,近年来多项研究表明,Hippo通路并不是独立在生物体内发挥作用,而是与其他肿瘤相关途径相互关联形成复杂的调控网络,共同调控肿瘤进程 [1] [3]。而YAP作为其重要组成部分和连接点,在多种肿瘤细胞中过表达 [3]。因此,本文重点介绍YAP作为多种重要通路连接点在调控炎症、肿瘤发生中的作用和机制,并讨论其作为治疗靶点的潜力。
2. Hippo/YAP信号通路的组成及功能
Hippo信号通路是由一系列蛋白激酶及转录共激活因子所组成的激酶链,最初通过基因镶嵌筛选调控果蝇组织和器官大小发现 [4]。首先筛选出来相关肿瘤抑制因子有:HPO、SAV、WTS、MATS,这些肿瘤抑制因子构成了一个激酶级联,抑制了下游转录共激活因子Yki的活性 [2]。自从在果蝇中被发现以来,Hippo通路的每个组分的同源物已经在其他物种中被鉴定出来,更成为了哺乳动物细胞中的信号网络中心。在哺乳动物中,Hippo激酶级联由MST1/2、SAV1、LATS1/2和MOBs构成,其下游效应物为YAP,即Yes相关蛋白 [5]。
Hippo激酶级联由TAO激酶(TAOK1/2/3)磷酸化MST1/2后启动。被激活的MST1/2与SAV1结合并招募LATS1/2,使其磷酸化。LATS1/2也可直接与NF2相互作用,并通过MST1/2-SAV1复合体促进自身磷酸化。磷酸化的LATS1/2作用于YAP的Ser127位残基,使其磷酸化并与细胞质内的胞质滞留蛋白14-3-3结合,从而将YAP滞留在细胞质,使其失去生物学活性。相反,当上游激酶级联未启动时,去磷酸化的YAP移位进入细胞核内富集,与TEAD家族结合,最终诱导与细胞增殖和迁移有关的多种基因的表达(图1) [6]。
以上为经典Hippo途径通过YAP进而调控生理活动的机制,而YAP调控的复杂性远不止此,还包括甲基化、乙酰化和去乙酰化和泛素化等 [5]。YAP可通过不依赖于MST1/2和LATS1/2的方式磷酸化或去磷酸化。有研究表明在促炎细胞因子刺激下,YAP是TAK1磷酸化修饰的靶点,其多个位点可被TAK1激酶直接磷酸化并与β-TrCP复合物相互作用,触发蛋白酶体依赖性降解 [7]。越来越多的证据表明Hippo通路可通过YAP与其他肿瘤相关通路相互关联,研究者对Hippo通路的理解也从简单线性通路转变为多个信号通路组成的调控网 [1] [3] [5]。
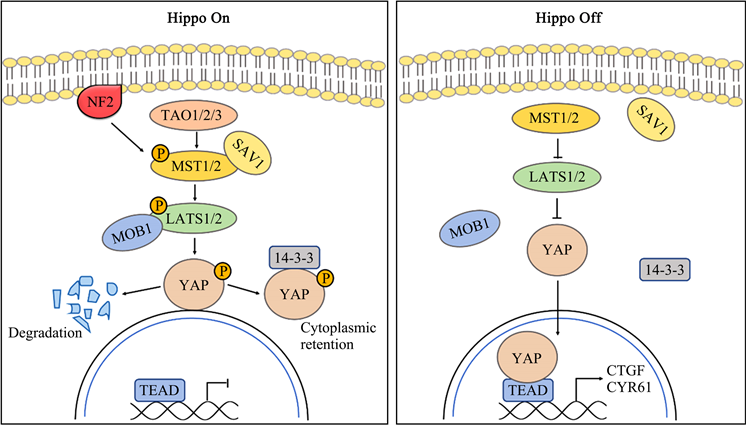
Figure 1. The composition and regulation of Hippo signaling pathway
图1. Hippo信号通路组成及其调控
3. Hippo/YAP与NF-κB信号通路
NF-κB信号通路由一个转录因子家族组成,在炎症的调节及肿瘤的发生中起着至关重要的作用 [8]。已有大量研究揭示Hippo和NF-κB信号通路存在交互调控,YAP在其中以重要媒介发挥作用。Zhao等研究表明YAP通过激活NF-κB促进破骨细胞生成和骨吸收,进而导致骨质疏松、关节炎和癌症骨转移等疾病的发生 [9],Caire等的研究也证实了这一点 [10]。随后大量研究表明YAP能直接或间接激活NF-κB信号通路进而促进肿瘤的发生及恶性转化。Ye等发现YAP通过抑制NF-κB的关键上游负调节因子——泛素特异性肽酶USP31的表达,从而消除其对NF-κB活性的抑制,最终促进软组织肉瘤的发生 [11] [12]。在子宫内膜癌细胞中,YAP通过上调p65诱导的IL-11转录触发细胞增殖、迁移和侵袭,从而导致子宫内膜癌细胞的恶性化 [13]。Zhao等研究揭示人类T细胞白血病病毒1型(HTLV-1)编码的Tax通过激活NF-κB/p65通路导致人类T细胞白血病(ATL)的发生。Tax激活的p65在一定程度上解除了YAP和LATS1之间的相互作用,从而抑制了YAP的磷酸化、泛素化等降解途径,使YAP移位到细胞核内富集,最终促进ATL细胞的增殖甚至ATL的发生 [14]。此外,Wang等观察到在结肠癌细胞中YAP和NF-κB通路相互激活。NF-κB能够增强人结肠癌细胞和小鼠结肠炎模型中YAP的转录及其活性,同时,YAP可以结合p65启动子来增强NF-κB信号 [15]。也有研究报道Hippo/YAP与NF-κB/p65协同促进乳腺癌细胞迁移 [16]。TNFα在乳腺癌细胞中激活胞质和核内的IKK激酶复合物,IKKβ或IKKε的过表达增强了YAP与TEAD4、YAP与p65之间的相互作用,形成的YAP/TEAD/p65三联体协同增强了己糖激酶2 (HK2)转录,从而促进乳腺癌细胞迁移 [16]。
然而,Rohba等通过对人类基因和等位基因功能的系统形态学分析发现NF-κB通路通过下调YAP对Hippo通路进行负调控 [17]。之后的研究表明,在骨关节炎发病过程中,Hippo/YAP和关节软骨细胞中的NF-κB信号之间存在相互拮抗作用。促炎细胞因子通过TAK1介导的YAP磷酸化和泛素化来促进YAP蛋白酶体降解。反过来,YAP通过与TAK1/IKKs复合物的结合抑制IKKα/β的活性,从而抑制IκBα的磷酸化以及p65活化和核转位,使NF-κB活性降低以维持关节软骨的完整性,减轻骨关节炎进展 [18]。内皮细胞中,YAP通过抑制NF-κB通路调节内皮细胞的激活并抑制血管炎症。一方面,YAP通过激活K48泛素化从而诱导TRAF6的降解,另一方面通过下调K63泛素化以抑制下游TAK1复合物的形成,从而抑制NF-κB通路以抑制血管炎症 [19]。肺泡上皮II型细胞中,YAP通过诱导IκBα表达抑制NF-κB依赖性炎症基因转录,使炎症消退并促进肺泡上皮再生 [20]。此外,在低密度细胞中,YAP易位到细胞核中,形成YAP/TEADs复合物,YAP/TEADs复合物将组蛋白去乙酰化酶7 (HDAC7)招募到NF-κB靶向基因COX-2的启动子区域,抑制COX-2转录,从而减少炎性细胞因子的产生并抑制炎症诱导的癌细胞迁移和转移而发挥肿瘤抑制作用 [21]。另外有研究发现,在钛离子处理下,YAP通过激活NF-κB通路加剧了炎症反应,然而,NF-κB通路的持续激活反过来可能抑制巨噬细胞中YAP的表达,从而协调炎症反应 [22]。在HBV暴露的原代人和小鼠肝细胞(PMH)中,NF-κB的激活伴随着YAP核易位,YAP核易位后与TEAD4结合形成YAP/TEAD4复合物,该复合物直接结合Nfκbia启动子区域的近端结合位点以促进IκBα表达,从而抑制NF-κB信号通路以平衡先天免疫的过度激活,减少组织损伤 [23]。
上述研究表明,YAP在Hippo通路与NF-κB通路的交互调控中发挥着复杂的作用。一方面,Hippo通路通过YAP对NF-κB通路的激活促进炎症反应及肿瘤的迁移、恶化。另一方面,YAP通过抑制NF-κB的活性减轻炎症并发挥肿瘤抑制作用 [24]。NF-κB通路同样可以通过对YAP的正向或负向调节调控Hippo信号通路。从以上研究来看,Hippo与NF-κB通路间的调控存在相互矛盾的地方,还需更深入的研究证明其调控机制。Caire等曾提出猜测,其可能与YAP的亚细胞定位有关:YAP在细胞质中抑制NF-κB途径,而在细胞核中增加NF-κB基因的转录,激活NF-κB信号通路 [25]。
4. Hippo/YAP与Wnt/β-Catenin信号通路
Wnt信号通路在胚胎发育过程中的多个发育事件以及肿瘤发生进程中至关重要,其异常激活与恶性肿瘤的进展、不良预后,甚至癌症相关死亡率的上升密切相关 [26]。Wnt通路包括三个分支:Wnt/β-catenin通路、Wnt/PCP通路和Wnt/Ca2+通路 [27]。Wnt/β-catenin和Hippo/YAP信号通路的广泛联系已经成为一些癌细胞中重要的致瘤信号网络 [28]。
首先,YAP促进β-catenin活性。三阴性乳腺癌中,受体酪氨酸激酶Met能诱导YAP的表达,YAP/TEAD与β-catenin结合,YAP/TEAD4/β-catenin复合物又结合到Wnt靶基因的增强区,促进Wnt靶基因转录,从而调控三阴性乳腺癌细胞的致癌活性 [29]。人类胶质瘤中,YAP通过抑制GSK3β的活性,从而提高β-catenin的蛋白水平和活性,促进人类胶质瘤的生长 [30]。喉癌细胞和胃癌细胞中,YAP同样被发现通过上调β-catenin通路活性增强肿瘤细胞的增殖和侵袭 [31] [32]。此外,YAP通过激活WNT/β-catenin信号通路驱动的肠上皮细胞增殖,调节炎症后的上皮再生,然而过度活化的YAP将导致WNT/β-catenin的过度激活,最终导致结肠癌的发生 [33]。另外有研究通过用药物抑制YAP的表达来抑制与β-catenin通路异常激活密切相关的结肠癌:Celastrol通过上调HSF1表达增强LKB1转录活性,LKB1能加强YAP磷酸化、蛋白酶体降解,抑制YAP活性,从而促进β-catenin降解,并以此抑制结直肠癌细胞的生长 [34],这也间接表明YAP通过激活Wnt/β-catenin通路从而促进肿瘤进展。β-catenin对于YAP也发挥类似的调控作用。Liu等发现β-catenin通过促进YAP的核易位促进黑色素瘤相关成纤维细胞的生物学功能,从而促进肿瘤细胞生长和转移 [35]。Bisso等报道β-catenin通过激活YAP,协同MYC原癌基因驱动成纤维细胞增殖或肝脏肿瘤发生 [36]。在直肠癌中,β-catenin/TCF4复合物与YAP基因第一个内含子内的DNA增强子元件结合,从而驱动YAP的表达,促进肿瘤的发生 [37]。另外有研究通过对85种β-catenin驱动的癌症分类筛选发现:YAP的表达是β-catenin驱动的致癌转化所必需的。YAP与β-catenin与转录因子TBX5形成三元复合物,该复合物定位于抗凋亡基因BCL2L1和BIRC5的启动子并诱导其转录,促进癌症的恶性转化和存活 [38]。这表明β-catenin/YAP复合物在肿瘤进展中起关键作用。相反,也有研究报道Hippo/YAP与Wnt/β-catenin通路之间存在负调控。Physalin F通过促进YAP与β-TrCP/β-catenin破坏复合物结合,加速β-catenin的泛素化和降解,从而抑制Wnt/β-catenin信号通路,显示出对结直肠癌显示出潜在的抗肿瘤疗效 [39]。
已有研究对上述情况做出解释。Wnt-off的情况下,YAP通过与Axin1结合而作为WNT破坏复合体的组成部分,在复合物中,YAP招募β-TrCP降解β-Catenin,抑制Wnt信号通路,从而达到抑制肿瘤的作用。Wnt-on时,LRP6通过取代YAP与Axin1结合,从破坏复合物中释放YAP,激活Wnt信号通路以促进肿瘤进展。YAP的这两种不同作用取决于不同的细胞状态(即Wnt-on或Wnt-off)和不同的YAP亚细胞定位(即定位于细胞核或细胞质) (图2) [40]。
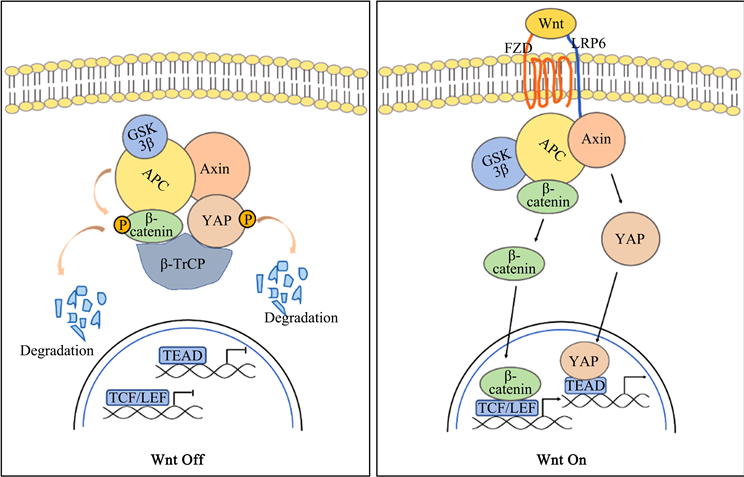
Figure 2. Hippo/YAP crosstalk with Wnt/β-catenin signaling
图2. Hippo/YAP与Wnt/β-catenin信号交互调控
5. Hippo/YAP与TGF-β信号通路
TGF-β是一种多功能细胞因子,能调节多种发育和稳态过程,与组织纤维化、癌症等的发病机制息息相关 [41]。自Ferrigno等对人类胎盘cDNA表达库与Smad7全长进行酵母双杂交筛选鉴定出YAP为Smad7的互作蛋白以来 [42],越来越多研究报道Hippo信号通路通过YAP与TGF-β通路交互调控,促进了多种疾病的进展。
Hiemer等通过全基因组表达分析发现YAP通过结合SMAD2/3并诱导其在细胞核中积累,直接增强乳腺癌细胞中NEGR1和UCA1的转录,促进非致瘤乳腺癌细胞的表型变化及迁移、侵袭 [43]。三阴性乳腺癌细胞中,E3泛素连接酶RAD18过表达并介导YAP的激活,活性YAP诱导了肿瘤相关巨噬细胞极化,从而促进促肿瘤细胞因子TGF-β的释放,激活TGF-β信号通路,极化的巨噬细胞又反过来调控RAD18的表达,进而维持三阴性乳腺癌细胞的致癌表型 [44]。在喉癌细胞中,YAP处于异常激活状态,过表达的YAP与STAT3蛋白相互作用促进血管内皮生长因子(VEGF)的分泌,VEGF通过激活TGF-β信号通路增强巨噬细胞中PD-L1的表达,最终促进喉癌细胞的生长、迁移以及免疫逃逸 [45]。Sun等的研究表明新生儿缺乏25-OH-VD使TGF-β信号通路异常激活,诱导YAP的核转位,从而促进下游促炎指标的分泌,导致新生儿感染性肺炎的发生 [46]。肉瘤细胞中,TGF-β通过磷酸化Smad3来促进YAP的活性,磷酸化的Smad3与YAP/TEAD1-4复合物相互作用并启动转录,增强了透明质酸介导的运动受体(HMMR/RHAMM)的表达,促进了肉瘤和纤维肉瘤的发生和转移进展 [47]。牙龈卟啉单胞菌通过上调GARP增强TGF-β的分泌和生物活性,介导YAP的激活,并进一步加强YAP、TEAD1和Smad2/3与CTGF和CYR61启动子的结合,增强其转录活性,从而促进食管鳞状细胞癌的发生 [48]。Fujii等报道活性YAP驱动Smad2/3在细胞核内积累,协同正向调节结缔组织生长因子CTGF表达,并进一步促进人类恶性间皮瘤生长 [49]。Zhuang等人发现沉默lncRNA MIR497HG将增强YAP和Smad3的相互作用,从而加强CTGF启动子转录活性,促进膀胱癌进展 [50]。Lüönd发现TGF-β诱导Smad2/3的激活,并通过Smad2/3与YAP互作,共同诱导了黑色素瘤细胞从增殖到侵袭型的表型转换 [51]。以上报道中,Hippo/YAP与TGF-β/Smads均协同促进了各种疾病甚至癌症的发生。然而,也有报道表明Hippo/YAP对TGF-β/Smads进行负调控加快肿瘤进展。原发性乳腺肿瘤中,YAP通过抑制Smad3活性从而促进乳腺肿瘤起始细胞的存活和自我更新,从而增加乳腺癌复发转移的风险 [52]。在肝癌细胞中,YAP促进Smad3的胞质保留,诱导肿瘤起始细胞的发生 [53]。这些看似相互矛盾的报道实则反映了Hippo通路与TGF-β通路交互调控构成的调控网络的复杂性。总所周知,TGF-β通路在肿瘤发生早期抑制肿瘤生长 [54],在晚期促进肿瘤细胞增殖和转移 [55]。而YAP在不同组织发育过程中、不同肿瘤细胞中以及同种细胞的不同时期对TGF-β通路的调控均不同。
此外,Labibi等通过数学建模探究了YAP对TGF-β/Smads的调控机制。敲除YAP后Smad2/3核积累到峰值的速度没有明显改变,然而,在这个峰值之后,Smad2/3的磷酸化水平下降更快,Smad4也有类似的情况,这表明YAP不参与Smad2/3-Smad4复合物的入核磷酸化。因此,Labibi等建立的RAADRF模型预测可能存在某些能稳定Smads复合物的保留因子,YAP通过调节保留因子来调控磷酸化Smads的积累 [56]。其具体机制还待更深入研究。
6. Hippo/YAP与其他信号通路
Hippo通路作为调控细胞增殖与凋亡的信号通路,与多种肿瘤发生相关信号通路存在交互调控。研究报道YAP在Akt信号通路的致癌过程中发挥一定的作用。机制上,YAP通过诱导胰岛素样生长因子2 (IGF2)的表达来激活Akt信号通路,从而使细胞进入有丝分裂状态,细胞周期检查点取消,最终促进髓母细胞瘤的致瘤性和放射抗性 [57]。另外,YAP/TEAD复合物直接诱导胰岛素受体底物2 (IRS2)的转录,从而促进了PI3K的激活,并进一步增强了Akt信号通路的活性,加速非酒精性脂肪肝和肝癌的发展 [58]。也有证据表明AP-1通路与Hippo信号级联相互作用,增加了癌症转化的可能性。Koo等人报道YAP驱动的细胞生长也高度依赖于AP-1。YAP通过激活AP-1信号通路促进细胞生长,导致肝脏肿大和肿瘤发生 [59]。He等人发现YAP被AP-1家族成员JUNB和STAT3招募,通过WW结构域直接与JUNB和STAT3相互作用,并作为其AP-1和STAT3的转录激活因子,导致三阴性乳腺癌的低存活率 [60]。此外,在胚胎横纹肌中,Notch信号直接上调YAP活性,YAP又反过来上调Notch配体基因JAG1、DLL1和核心Notch转录因子RBPJ,两者协同促进胚胎横纹肌肉瘤的发生 [61]。Hippo与Hedgehog信号通路的交互调控也与肿瘤的发生相关。Hedgehog通路的异常激活将诱导YAP过表达,YAP又使长链非编码RNA H19异常表达,这是成骨细胞性骨肉瘤的发病机制和恶性转化的原因 [62]。此外,YAP能直接与Hedgehog通路下游靶基因Gli1相互作用并负调控Gli1,从而抑制Hedgehog通路。尽管存在这种负调控,Hedgehog信号能通过上调YAP蛋白水平增强YAP的转录后活性,从而形成负反馈循环 [63]。细胞内信号通路相互交互调控构成的网络错综复杂,随着研究的深入,Hippo通路被发现与多条肿瘤发生发展相关的信号都存在一定的交互调控。因次,进一步阐明Hippo与其它信号交互调控的机制和影响将为炎症、肿瘤等的治疗提供新思路。
7. 总结与展望
Hippo信号通路在细胞生长、维持组织动态平衡和肿瘤发生发展中发挥重要作用。NF-κB、TGF-β、Wnt/β-catenin等信号领域的进展提示:Hippo通路在疾病的发生发展过程中并不是单独发挥作用的,而是通过其效应因子YAP与这些通路交互调控,共同影响细胞生长及肿瘤发生等过程。然而,Hippo与这些信号通路在肿瘤发生发展中的相互作用还存在许多看似矛盾的地方,如YAP既能通过抑制USP31的表达来激活NF-κB通路 [11] [12],又能通过与TAK1/IKKs复合物的结合使NF-κB活性降低 [19]。这一现象在一定程度上体现了多信号通路交互调控后在细胞内发挥的不同功能,也解释了在肿瘤的治疗中,为什么抑制单个分子靶点往往不能达到预期的治疗效果。因此,充分了解Hippo信号通路与其他肿瘤相关通路交互调控的机制及各通路组分在肿瘤发生过程中的功能至关重要。本文主要综述了Hippo通路的组成及其核心组分YAP在信号通路网络中的调控作用,这将有助于加强研究者对Hippo通路作为信号通路调控网络中心的理解,以及为肿瘤的个性化治疗、新靶点的开发提供有益资料。综上所述,对Hippo信号通路持续进行分子探索很可能是一个令人兴奋而有意义的话题。
致谢
我们感谢为这篇文章做出贡献的每一个人。
作者贡献声明
王莹:采集并分析数据,撰写文章;任欣欣:审阅;赵铁军:撰写文章,科研经费支持。
基金项目
本研究得到了浙江省自然科学基金项目(LY21C010001)的支持。
文章引用
王 莹,任欣欣,赵铁军. Hippo/YAP途径在肿瘤细胞信号通路交互调控中的作用
The Role of Hippo/YAP Pathway in the Crosstalk of Tumor Cell Signaling Pathways[J]. 生物医学, 2023, 13(01): 93-102. https://doi.org/10.12677/HJBM.2023.131010
参考文献
- 1. Meng, Z., Moroishi, T. and Guan, K.L. (2016) Mechanisms of Hippo Pathway Regulation. Genes & Development, 30, 1-17. https://doi.org/10.1101/gad.274027.115
- 2. Harvey, K.F., Zhang, X. and Thomas, D.M. (2013) The Hippo Pathway and Human Cancer. Nature Reviews Cancer, 13, 246-257. https://doi.org/10.1038/nrc3458
- 3. Hansen, C.G., Moroishi, T. and Guan, K.L. (2015) YAP and TAZ: A Nexus for Hippo Signaling and Beyond. Trends in Cell Biology, 25, 499-513. https://doi.org/10.1016/j.tcb.2015.05.002
- 4. Misra, J.R. and Irvine, K.D. (2018) The Hippo Signaling Network and Its Biological Functions. Annual Review of Genetics, 52, 65-87. https://doi.org/10.1146/annurev-genet-120417-031621
- 5. Zhang, K., et al. (2015) YAP and TAZ Take Center Stage in Cancer. Biochemistry, 54, 6555-6566. https://doi.org/10.1021/acs.biochem.5b01014
- 6. Totaro, A., Panciera, T. and Piccolo, S. (2018) YAP/TAZ Upstream Signals and Downstream Responses. Nature Cell Biology, 20, 888-899. https://doi.org/10.1038/s41556-018-0142-z
- 7. Santoro, R., et al. (2020) Modulating TAK1 Expression Inhibits YAP and TAZ Oncogenic Functions in Pancreatic Cancer. Molecular Cancer Therapeutics, 19, 247-257. https://doi.org/10.1158/1535-7163.MCT-19-0270
- 8. Dolcet, X., et al. (2005) NF-κB in Development and Progression of Human Cancer. Virchows Archiv, 446, 475-482. https://doi.org/10.1007/s00428-005-1264-9
- 9. Zhao, L., et al. (2018) YAP1 Is Essential for Osteoclastogenesis through a TEADs-Dependent Mechanism. Bone, 110, 177-186. https://doi.org/10.1016/j.bone.2018.01.035
- 10. Caire, R., et al. (2021) YAP/TAZ: Key Players for Rheumatoid Arthritis Severity by Driving Fibroblast like Synoviocytes Phenotype and Fibro-Inflammatory Response. Frontiers in Immunology, 12, Article ID: 791907. https://doi.org/10.3389/fimmu.2021.791907
- 11. Ye, S., et al. (2018) YAP1-Mediated Suppression of USP31 Enhances NFκB Activity to Promote Sarcomagenesis. Cancer Research, 78, 2705-2720. https://doi.org/10.1158/0008-5472.CAN-17-4052
- 12. Rivera-Reyes, A., et al. (2018) YAP1 Enhances NF-κB-Dependent and Independent Effects on Clock-Mediated Unfolded Protein Responses and Autophagy in Sarcoma. Cell Death & Disease, 9, 1108. https://doi.org/10.1038/s41419-018-1142-4
- 13. Wang, J., et al. (2019) YAP Promotes the Malignancy of Endometrial Cancer Cells via Regulation of IL-6 and IL-11. Molecular Medicine, 25, 32. https://doi.org/10.1186/s10020-019-0103-4
- 14. Zhao, T. and Wang, Z. (2022) HTLV-1 Activates YAP via NF-κB/p65 to Promote Oncogenesis. Proceedings of the National Academy of Sciences of the United States of America, 119, e2115316119. https://doi.org/10.1073/pnas.2115316119
- 15. Wang, Q., et al. (2018) REGγ Controls Hippo Signaling and Reciprocal NF-κB-YAP Regulation to Promote Colon Cancer. Clinical Cancer Research, 24, 2015-2025. https://doi.org/10.1158/1078-0432.CCR-17-2986
- 16. Gao, Y., et al. (2017) TNFα-YAP/p65-HK2 Axis Mediates Breast Cancer Cell Migration. Oncogenesis, 6, e383. https://doi.org/10.1038/oncsis.2017.83
- 17. Rohban, M.H. and Singh, S. (2017) Systematic Morphological Profiling of Human Gene and Allele Function via Cell Painting. eLife, 6, e24060. https://doi.org/10.7554/eLife.24060
- 18. Yang, B., et al. (2020) YAP1 Inhibits the Induction of TNF-α-Stimulated Bone-Resorbing Mediators by Suppressing the NF-κB Signaling Pathway in MC3T3-E1 Cells. Journal of Cellular Physiology, 235, 4698-4708. https://doi.org/10.1002/jcp.29348
- 19. Lv, Y., et al. (2018) YAP Controls Endothelial Activation and Vascular Inflammation through TRAF6. Circulation Research, 123, 43-56. https://doi.org/10.1161/CIRCRESAHA.118.313143
- 20. LaCanna, R., et al. (2019) Yap/Taz Regulate Alveolar Regeneration and Resolution of Lung Inflammation. Journal of Clinical Investigation, 129, 2107-2122. https://doi.org/10.1172/JCI125014
- 21. Zhang, Q., et al. (2018) Yes-Associated Protein (YAP) and Transcriptional Coactivator with PDZ-Binding Motif (TAZ) Mediate Cell Density-Dependent Proinflammatory Responses. Journal of Biological Chemistry, 293, 18071-18085. https://doi.org/10.1074/jbc.RA118.004251
- 22. Tang, K.M., et al. (2021) Role of the Hippo-YAP/NF-κB Signaling Pathway Crosstalk in Regulating Biological Behaviors of Macrophages under Titanium Ion Exposure. Journal of Applied Toxicology, 41, 561-571. https://doi.org/10.1002/jat.4065
- 23. Luo, X., et al. (2021) Hippo Pathway Counter-Regulates Innate Immunity in Hepatitis B Virus Infection. Frontiers in Immunology, 12, Article ID: 684424. https://doi.org/10.3389/fimmu.2021.684424
- 24. Wang, S., et al. (2020) The Crosstalk between Hippo-YAP Pathway and Innate Immunity. Frontiers in Immunology, 11, 323. https://doi.org/10.3389/fimmu.2020.00323
- 25. Caire, R., et al. (2022) YAP Transcriptional Activity Dictates Cell Response to TNF in Vitro. Frontiers in Immunology, 13, Article ID: 856247. https://doi.org/10.3389/fimmu.2022.856247
- 26. Yu, F., et al. (2021) Wnt/β-Catenin Signaling in Cancers and Targeted Therapies. Signal Transduction and Targeted Therapy, 6, 307. https://doi.org/10.1038/s41392-021-00701-5
- 27. Eisenmann, D.M. (2005) Wnt Signaling. WormBook. 1-17. https://doi.org/10.1895/wormbook.1.7.1
- 28. Li, N., Lu, N. and Xie, C. (2019) The Hippo and Wnt Signalling Pathways: Crosstalk during Neoplastic Progression in Gastrointestinal Tissue. FEBS Journal, 286, 3745-3756. https://doi.org/10.1111/febs.15017
- 29. Quinn, H.M., Vogel, R. and Popp, O. (2021) YAP and β-Catenin Cooperate to Drive Oncogenesis in Basal Breast Cancer. Cancer Research, 81, 2116-2127. https://doi.org/10.1158/0008-5472.CAN-20-2801
- 30. Wang, Y., et al. (2017) β-Catenin-Mediated YAP Signaling Promotes Human Glioma Growth. Journal of Experimental & Clinical Cancer Research, 36, 136. https://doi.org/10.1186/s13046-017-0606-1
- 31. Tang, X., et al. (2019) Knockdown of YAP Inhibits Growth in Hep-2 Laryngeal Cancer Cells via Epithelial-Mesenchymal Transition and the Wnt/β-Catenin Pathway. BMC Cancer, 19, Article No. 654. https://doi.org/10.1186/s12885-019-5832-9
- 32. Zeng, M., et al. (2021) CBX2 Depletion Inhibits the Proliferation, Invasion and Migration of Gastric Cancer Cells by Inactivating the YAP/β-Catenin Pathway. Molecular Medicine Reports, 23, 137. https://doi.org/10.3892/mmr.2020.11776
- 33. Deng, F., et al. (2018) YAP Triggers the Wnt/β-Catenin Signalling Pathway and Promotes Enterocyte Self-Renewal, Regeneration and Tumorigenesis after DSS-Induced Injury. Cell Death & Disease, 9, 153. https://doi.org/10.1038/s41419-017-0244-8
- 34. Wang, S., et al. (2019) LKB1 and YAP Phosphorylation Play Important Roles in Celastrol-Induced β-Catenin Degradation in Colorectal Cancer. Therapeutic Advances in Medical Oncology, 11, 1-19. https://doi.org/10.1177/1758835919843736
- 35. Liu, T., et al. (2019) The β-Catenin/YAP Signaling Axis Is a Key Regulator of Melanoma-Associated Fibroblasts. Signal Transduction and Targeted Therapy, 4, 63. https://doi.org/10.1038/s41392-019-0100-7
- 36. Bisso, A., et al. (2020) Cooperation between MYC and β-Catenin in Liver Tumorigenesis Requires Yap/Taz. Hepatology, 72, 1430-1443. https://doi.org/10.1002/hep.31120
- 37. Konsavage, W.M., et al. (2012) Wnt/β-Catenin Signaling Regulates Yes-Associated Protein (YAP) Gene Expression in Colorectal Carcinoma Cells. Journal of Biological Chemistry, 287, 11730-11739. https://doi.org/10.1074/jbc.M111.327767
- 38. Rosenbluh, J., et al. (2012) β-Catenin-Driven Cancers Require a YAP1 Transcriptional Complex for Survival and Tumorigenesis. Cell, 151, 1457-1473. https://doi.org/10.1016/j.cell.2012.11.026
- 39. Chen, C., et al. (2018) YAP-Dependent Ubiquitination and Degradation of β-Catenin Mediates Inhibition of Wnt Signalling Induced by Physalin F in Colorectal Cancer. Cell Death & Disease, 9, 591. https://doi.org/10.1038/s41419-018-0645-3
- 40. Azzolin, L., et al. (2014) YAP/TAZ Incorporation in the β-Catenin Destruction Complex Orchestrates the Wnt Response. Cell, 158, 157-170. https://doi.org/10.1016/j.cell.2014.06.013
- 41. Massagué, J. (2008) TGFbeta in Cancer. Cell, 134, 215-230. https://doi.org/10.1016/j.cell.2008.07.001
- 42. Ferrigno, O., et al. (2002) Yes-Associated Protein (YAP65) Interacts with Smad7 and Potentiates Its Inhibitory Activity against TGF-Beta/Smad Signaling. Oncogene, 21, 4879-4884. https://doi.org/10.1038/sj.onc.1205623
- 43. Hiemer, S.E., Szymaniak, A.D. and Varelas, X. (2014) The Transcriptional Regulators TAZ and YAP Direct Transforming Growth Factor β-Induced Tumorigenic Phenotypes in Breast Cancer Cells. Journal of Biological Chemistry, 289, 13461-13474. https://doi.org/10.1074/jbc.M113.529115
- 44. Yan, X., He, Y. and Yang, S. (2022) A Positive Feedback Loop: RAD18-YAP-TGF-β between Triple-Negative Breast Cancer and Macrophages Regulates Cancer Stemness and Progression. Cell Death Discovery, 8, Article No. 196. https://doi.org/10.1038/s41420-022-00968-9
- 45. Du, X.X., et al. (2021) YAP/STAT3 Promotes the Immune Escape of Larynx Carcinoma by Activating VEGFR1-TGFβ Signaling to Facilitate PD-L1 Expression in M2-Like TAMs. Experimental Cell Research, 405, Article ID: 112655. https://doi.org/10.1016/j.yexcr.2021.112655
- 46. Sun, Q., et al. (2021) 25(OH)-Vitamin D Alleviates Neonatal Infectious Pneumonia via Regulating TGFβ-Mediated Nuclear Translocation Mechanism of YAP/TAZ. Bioengineered, 12, 8931-8942. https://doi.org/10.1080/21655979.2021.1990000
- 47. Ye, S., Liu, Y. and Fuller, A.M. (2020) TGFβ and Hippo Pathways Cooperate to Enhance Sarcomagenesis and Metastasis through the Hyaluronan-Mediated Motility Receptor (HMMR). Molecular Cancer Research, 18, 560-573. https://doi.org/10.1158/1541-7786.MCR-19-0877
- 48. Qi, Y.J., et al. (2020) Porphyromonas gingivalis Promotes Progression of Esophageal Squamous Cell Cancer via TGFβ-Dependent Smad/YAP/TAZ Signaling. PLOS Biology, 18, e3000825. https://doi.org/10.1371/journal.pbio.3000825
- 49. Fujii, M., et al. (2012) TGF-β Synergizes with Defects in the Hippo Pathway to Stimulate Human Malignant Mesothelioma Growth. Journal of Experimental Medicine, 209, 479-494. https://doi.org/10.1084/jem.20111653
- 50. Zhuang, C., et al. (2020) Silencing of lncRNA MIR497HG via CRISPR/Cas13d Induces Bladder Cancer Progression Through Promoting the Crosstalk between Hippo/Yap and TGF-β/Smad Signaling. Frontiers in Molecular Biosciences, 7, Article ID: 616768. https://doi.org/10.3389/fmolb.2020.616768
- 51. Lüönd, F. and Pirkl, M. (2022) Hierarchy of TGFβ/SMAD, Hippo/YAP/TAZ, and Wnt/β-Catenin Signaling in Melanoma Phenotype Switching. Life Science Alliance, 5, e202101010. https://doi.org/10.26508/lsa.202101010
- 52. Sun, J.G., et al. (2016) Yap1 Promotes the Survival and Self-Renewal of Breast Tumor Initiating Cells via Inhibiting Smad3 Signaling. Oncotarget, 7, 9692-706. https://doi.org/10.18632/oncotarget.6655
- 53. Chen, C.L., et al. (2013) Reciprocal Regulation by TLR4 and TGF-β in Tumor-Initiating Stem-Like Cells. Journal of Clinical Investigation, 123, 2832-2849. https://doi.org/10.1172/JCI65859
- 54. Zhang, T., et al. (2014) Ellagic Acid Exerts Anti-Proliferation Effects via Modulation of Tgf-β/Smad3 Signaling in MCF-7 Breast Cancer Cells. Asian Pacific Journal of Cancer Prevention, 15, 273-276. https://doi.org/10.7314/APJCP.2014.15.1.273
- 55. Kumar, K.J., et al. (2015) Antrodin C Inhibits Epithelial-to-Mesenchymal Transition and Metastasis of Breast Cancer Cells via Suppression of Smad2/3 and β-Catenin Signaling Pathways. PLOS ONE, 10, e0117111. https://doi.org/10.1371/journal.pone.0117111
- 56. Labibi, B., et al. (2020) Modeling the Control of TGF-β/Smad Nuclear Accumulation by the Hippo Pathway Effectors, Taz/Yap. iScience, 23, Article ID: 101416. https://doi.org/10.1016/j.isci.2020.101416
- 57. Fernandez, L.A., et al. (2012) Oncogenic YAP Promotes Radioresistance and Genomic Instability in Medulloblastoma through IGF2-Mediated Akt Activation. Oncogene, 31, 1923-1937. https://doi.org/10.1038/onc.2011.379
- 58. Jeong, S.H., et al. (2018) Hippo-Mediated Suppression of IRS2/AKT Signaling Prevents Hepatic Steatosis and Liver Cancer. Journal of Clinical Investigation, 128, 1010-1025. https://doi.org/10.1172/JCI95802
- 59. Koo, J.H. and Plouffe, S.W. (2020) Induction of AP-1 by YAP/TAZ Contributes to Cell Proliferation and Organ Growth. Genes & Development, 34, 72-86. https://doi.org/10.1101/gad.331546.119
- 60. He, L., Pratt, H. and Gao, M. (2021) YAP and TAZ Are Transcriptional Co-Activators of AP-1 Proteins and STAT3 during Breast Cellular Transformation. eLife, 10, e67312. https://doi.org/10.1101/2021.02.18.431832
- 61. Slemmons, K.K., et al. (2017) A Novel Notch-YAP Circuit Drives Stemness and Tumorigenesis in Embryonal Rhabdomyosarcoma. Molecular Cancer Research, 15, 1777-1791. https://doi.org/10.1158/1541-7786.MCR-17-0004
- 62. Chan, L.H., et al. (2014) Hedgehog Signaling Induces Osteosarcoma Development through Yap1 and H19 Overexpression. Oncogene, 33, 4857-4866. https://doi.org/10.1038/onc.2013.433
- 63. Tariki, M., et al. (2014) The Yes-Associated Protein Controls the Cell Density Regulation of Hedgehog Signaling. Oncogenesis, 3, e112. https://doi.org/10.1038/oncsis.2014.27
NOTES
*通讯作者。