Bioprocess
Vol.
11
No.
04
(
2021
), Article ID:
47373
,
9
pages
10.12677/BP.2021.114017
虫草素提高雄性动物繁殖性能及机制研究进展
黄炜乾1,金明昌2,赵颖2,唐谢芳2
1清远一生自然生物研究院有限公司,广东 清远
2广东容大生物股份有限公司,广东 清远
收稿日期:2021年11月11日;录用日期:2021年12月16日;发布日期:2021年12月23日

摘要
虫草素为虫草属所特有的主要生物活性成分。研究表明,虫草素具有多种生理药理作用,如调节免疫、抗病毒、抗氧化、降血脂、抗炎、抗肿瘤、抗菌和促进类固醇激素分泌等。本文综述了虫草素提高雄性动物繁殖性能及机制的研究进展。
关键词
虫草素,雄性动物,繁殖性能,间质细胞,睾酮

Research Progress of Cordycepin on Improving Reproduction and Mechanism in Male Animals
Weiqian Huang1, Mingchang Jin2, Ying Zhao2, Xiefang Tang2
1Qingyuan Yisheng Natural Biology Research Institute Co., Ltd., Qingyuan Guangdong
2Guangdong Rongda Biological Co., Ltd., Qingyuan Guangdong
Received: Nov. 11th, 2021; accepted: Dec. 16th, 2021; published: Dec. 23rd, 2021

ABSTRACT
Cordycepin is the main bioactive component unique to Cordyceps. Studies have shown that cordycepin has a variety of physiological and pharmacological effects, such as regulating immunity, antiviral, antioxidant, hypolipidemic, anti-inflammatory, anti-tumor, anti-bacterial and promoting steroid hormone secretion. This paper reviews the research progress of cordycepin on improving reproduction and mechanism in male animals.
Keywords:Cordycepin, Male Animals, Reproduction, Leydig Cell, Testosterone

Copyright © 2021 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
虫草素(Cordycepin)或称3’-脱氧腺苷,为虫草属如冬虫夏草(Cordyceps sinensis)、蛹虫草(Cordyceps militaris)等所特有的主要生物活性成分(Hawley等,2020) [1],最早从蛹虫草(C.militaris)培养液中分离出来(Cunningham等,1950) [2]。虫草素化学特征与腺苷类似,与腺苷不同的是,其核糖部分的3’位置上没有氧原子(图1) (Chen等,2017) [3]。研究表明,虫草素具有多种生理药理作用,如调节免疫(Zhou等,2008;Xiong等,2013) [4] [5]、抗病毒(Ryu, 2014; Clercq, 2015) [6] [7]、抗氧化(Lei等,2018;Park等,2014) [8] [9]、降血脂(Guo等,2010;Gong等,2021) [10] [11]、抗炎(Tan等,2020;Govindula等,2021) [12] [13]、抗肿瘤(Jeong等,2015;Özenver等,2021) [14] [15]、抗菌(Ahn等,2000;高苏等,2021;Wang等,2021) [16] [17] [18] 和促进类固醇激素分泌(Wang等,1998;Leu等,2011) [19] [20]。本文综述了近年来关于虫草素提高雄性动物繁殖性能及机制的研究进展,旨在为虫草素在动物生产上的应用提供参考依据。
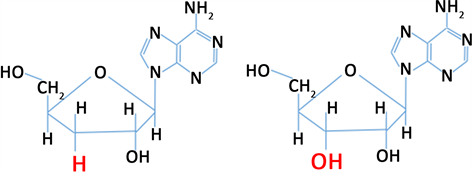 虫草素 腺苷
虫草素 腺苷
Figure 1. The chemical structure of cordycepin and adenosine (Chen et al., 2017) [3]
图1. 虫草素和腺苷的化学结构(Chen等,2017) [3]
2. 虫草素对雄性动物繁殖性能的影响
2.1. 虫草素对精子发生和精子质量的影响
多项研究表明,虫草素通过促进公猪和雄性大鼠的生精作用,进而提高精子的产生和改善精子的质量。Lin等(2007) [21] 报道,蛹虫草菌丝体(CM)显著提高低繁殖力公猪精子的产量(p < 0.05),显著改善精子活力和形态(p < 0.05);并认为CM的效果是通过CM中虫草素的作用而产生生精作用所致,因为血清虫草素浓度的增加与精子数量的增加规律一致。Chang等(2008) [22] 用7周龄雄性Sprague-Dawley (SD)大鼠,研究了CM的生精作用。结果表明,添加CM后第6周,5%CM组和1%CM组的SD大鼠附睾精子数分别比对照组增加了37%和53% (p < 0.05);添加CM后第2周时,5%CM组和1%CM组大鼠精子活力显著增加34% (p < 0.05) (分别为85% ± 8.5%和85% ± 9.1%,对照组为63% ± 8.7%)。试验组的精子活力出现第二个高峰在第6周,分别比对照组提高19%和31% (p < 0.05)。Sohn等(2012) [23] 用2月龄(YC)和12月龄SD雄性大鼠(MC)经4个月的试验发现,蛹虫草中的虫草素显著提高了MC组大鼠的精子活力和精子运动参数,各虫草素组(5 mg/kg、10 mg/kg和20 mg/kg)之间差异不显著(p > 0.05),见表1。Kopalli等(2019) [24] 采用与Sohn等(2012) [23] 类似的试验设计,经6个月的试验,也得出了同样的结论。
Table 1. Effect of cordycepin on sperm motility and kinematics parameters in middle-age rats (Sohn et al., 2012) [23]
表1. 虫草素对12月龄大鼠精子活力和运动参数的影响(Sohn等,2012) [23]
注:12月龄大鼠添加虫草素与12月龄大鼠比较,同一行数据中标注不同符号的数据之间,差异显著(p < 0.05);标注相同或未标注符号的数据之间,差异不显著(p > 0.05)。
2.2. 虫草素对睾酮分泌的影响
多项研究发现,虫草素能够促进睾丸间质细胞睾酮的合成,进而提高血清睾酮浓度。Chang等(2008) [22] 研究表明,CM显著提高7周龄SD雄性大鼠血清中睾酮水平(p < 0.05)。Hong等(2011) [25] 用6周龄SD雄性大鼠试验表明,与对照组相比,CM显著刺激睾酮的产生(p < 0.05)。Leu等(2011) [20] 报道,用未发育成熟的B6小鼠,腹腔注射虫草素(40 mg/kg),经连续7 d处理后,与生理盐水对照组比较,虫草素组血浆睾酮浓度极显著提高(p < 0.01)。进一步用不同浓度的虫草素(10 uM至5 mM)处理纯化的正常小鼠睾丸间质细胞3 hr。结果发现,随着虫草素浓度的增加,睾酮的产量逐渐增加,1 mM虫草素处理下睾酮产量增加3倍(p < 0.05) [20]。Sohn等(2012) [23] 研究也发现,蛹虫草中的虫草素以剂量依赖性的方式,增加MC组大鼠血清睾酮水平。
2.3. 虫草素对睾丸组织形态学的影响
上述的一些研究还发现,虫草素还能改善睾丸组织形态,从而改善睾丸功能。Sohn等(2012) [23] 报道,从睾丸组织形态学检查显示,与YC组相比,MC组的睾丸发生了各种变化,即生精小管中的细胞排列松散且不规则,细胞物质从生精上皮脱落;生发上皮基底层退化,导致精原细胞(产生精母细胞的干细胞)数量减少)。相反,虫草素处理组表现出密集排列的精原细胞和支持细胞位于基底膜上,被同心的肌成纤维细胞层包围。生精小管间质间隙的间质细胞正常(图2)。Kopalli等(2019) [24] 的试验也得到了类似的结果。

Figure 2. Histomorphological analyses of seminiferous tubules in the testes (stained with hematoxylin and eosin, ×200 magnification) (Sohn et al., 2012) [23]
图2. 睾丸生精小管的组织形态学(苏木精一伊红染色,200×) (Sohn等,2012) [23]
Sohn等(2012) [23] 进一步使用光学显微镜评估了大鼠生精功能的相关指标。结果表明,虫草素处理,均可使MC组大鼠生精功能的相关指标得到改善,尤其是在高剂量(20 mg/kg)情况下,除含精子小管指标外,达到显著差异水平(p < 0.05);虫草素5 mg/kg和虫草素10 mg/kg组之间,除生殖细胞数/管、每个支持细胞的生殖细胞数和小管尺寸3个指标外,差异不显著(p > 0.05);虫草素5 mg/kg和虫草素20 mg/kg组之间,精子数/管、支持细胞计数/管和Johnsen评分指标,差异显著(p < 0.05);而虫草素10 mg/kg和虫草素20 mg/kg组之间,除含精子小管指标外,其它指标差异显著(p < 0.05),见表2。Kopalli等(2019) [24] 的研究结果也类似。
Table 2. Effect of cordycepin on spermatogenesis-related values (Sohn et al., 2012) [23]
表2. 虫草素对大鼠生精功能相关指标的影响(Sohn等,2012) [23]
注:1、Johnsen评分定义为精子发生的程度(1~10),并根据睾丸组织活检标本计算。2、12月龄大鼠添加虫草素与12月龄大鼠比较,同一行数据中标注不同符号的数据之间,差异显著(p < 0.05);标注相同或未标注符号的数据之间,差异不显著(p > 0.05)。
3. 虫草素提高雄性动物繁殖性能的机制
睾酮对雄性动物性器官的发育成熟具有重要作用,是雄性动物维持繁殖功能所必需的类固醇激素(张阳海,2018) [26]。在雄性动物中,约95%的睾酮由睾丸间质细胞分泌(Saez, 1994; 刘建中等,2006) [27] [28]。研究表明,虫草素通过提高睾酮水平、降低氧化应激,进而有效改善了动物的繁殖性能(Nguyen, 2021) [29]。
3.1. 促进动物类固醇激素的分泌
睾丸间质细胞对睾酮的合成受下丘脑—垂体—睾丸轴调控,其过程:来自下丘脑分泌的促性腺激素释放激素(GnRH)刺激垂体前叶释放促黄体生成素(LH) (Saez, 1994) [27],LH被输送到睾丸,与睾丸间质细胞表面的促黄体生成素受体(LHR)结合(Oyola等,2017) [30],激活G蛋白,在G蛋白作用下激活腺苷酸环化酶(AC),在AC的作用下促进细胞内环磷酸腺苷(cAMP)的形成(Richards等,2001) [31]。然后,cAMP激活蛋白激酶A(PKA),PKA促进cAMP反应原件结合蛋白(CREB)磷酸化,进一步诱导类固醇生成急性调节蛋白(StAR)的合成(Stocco等,1996) [32]。StAR蛋白通过与线粒体外膜上的转运蛋白(TSPO)结合,将游离胆固醇从线粒体外膜转移到线粒体内膜(Strauss等,2003) [33],在线粒体膜上胆固醇通过P450侧链裂解酶(P450scc,又称CYP11A1)转化为孕烯醇酮(Stocco, 2001) [34]。然后,孕烯醇酮被转运到光滑的内质网,在3β-羟基类固醇脱氢酶(3β-HSD)的作用下酶解为孕酮,孕酮被催化为雄烯二酮,最终雄烯二酮在17β-羟基类固醇脱氢酶(17β-HSD)的作用下生成睾酮(Zirkin等,2018) [35],见图3 (朱清玉等,2021) [36]。
虫草素为腺苷的类似物(Chen等,2017) [3],目前,从动物不同组织细胞中已分离出四种不同的腺苷受体,包括A1、A2a、A2b和A3腺苷受体(Londos等,1980;Jacobson等,2006) [37] [38]。Leu等(2011) [20] 用小鼠原代睾丸间质细胞,分别选择不同腺苷受体拮抗剂、cAMP拮抗剂、PKA抑制剂H89、MAPK抑制剂和PKC抑制剂,与虫草素(15 mM和5 mM)共同处理3 h,考察虫草素对小鼠睾丸间质StAR蛋白及mRNA表达及睾酮生成的影响。结果表明,虫草素通过与腺苷受体A1、A2a和A3结合,激活cAMP-PKA信号转导途径,诱导原代小鼠睾丸间质细胞StAR蛋白的表达,从而促进睾丸间质细胞睾酮的生成。
3.2. 改善动物生殖器官和细胞的氧化应激状态
据报道,活性氧(ROS)如羟基(∙OH)、一氧化氮(NO)和过氧化氢(H2O2)的过量产生可诱导氧化应激,造成细胞损伤(He等,2019) [39]。研究表明,氧化应激是睾丸结构损伤和功能障碍的重要因素之一(Ryan等,2008) [40]。随着年龄的增长,哺乳动物睾丸中积累了大量的多不饱和脂肪酸,这些脂肪酸容易受到自由基的攻击,造成氧化失衡,从而导致睾丸功能受损(Koeberle等,2012) [41]。活性氧增加会造成生殖细胞凋亡,影响精子发生和使精子形态发生改变,造成质膜受损和精子DNA损伤,导致生殖功能低下(Asadi等,2017;Suresh等,2013) [42] [43]。研究发现,虫草素能够有效清除自由基,提高抗氧化酶活
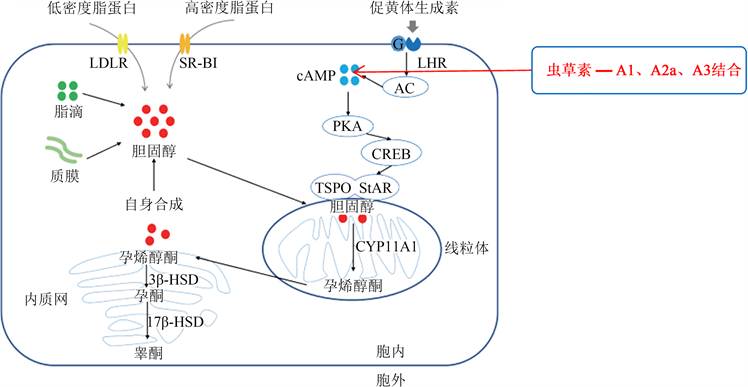
Figure 3. Testosterone synthesis pathway in Leydig cells (Zhu et al., 2021) [36] and its regulatory mechanism by cordycepin
图3. 睾丸间质细胞睾酮合成途径(朱清玉等,2021) [36] 及虫草素对其调控机制
性,降低氧化应激对细胞的损伤,从而对细胞的功能起到保护作用(孟雪莲等,2014;Lei等,2018;Han等,2020) [8] [44] [45]。Ramesh等(2012) [46] 研究表明,虫草素显著提高12月龄大鼠肝脏、肾脏、心脏和肺中超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、谷胱甘肽过氧化物酶(GPx)、谷胱甘肽还原酶(GR)、谷胱甘肽-S-转移酶(GST)活性以及还原型谷胱甘肽(GSH)、维生素C和维生素E水平,并显著降低丙二醛(MDA)水平(p < 0.05);显著降低血清中天冬氨酸转氨酶(AST)、丙氨酸转氨酶(ALT)、尿素和肌酐水平(p < 0.05)。这些结果说明,虫草素可有效恢复老龄大鼠的抗氧化状态,降低脂质过氧化。过氧化物酶4 (PRx4)是一种防止氧化应激诱导细胞损伤的抗氧化酶,广泛分布于线粒体基质中,在生殖器官中高水平表达(Iuchi等,2009) [47]。谷胱甘肽S-转移酶(GST)及其谷胱甘肽S-转移酶mu5 (GSTm5)发生在纤维鞘中,促进生殖细胞的增殖和分化,在氧化应激时,可以抵抗自由基对睾丸和生精细胞的攻击,其水平会发生改变(Rao等,2000;Hemachand等,2002) [48] [49]。谷胱甘肽过氧化物酶4 (GPx4)是一种抗氧化剂,可防止自由基介导的膜脂、蛋白质和核酸损伤,也被认为是精子成熟过程中的重要结构分子(Ursini等,1999) [50]。
Kopalli等(2019) [24] 用12月龄雄性SD大鼠试验表明,虫草素(10 mg/kg和20 mg/kg)显著改善大鼠精子运动参数如活率、进行性、VAP、VSL、LIN和WOB值(p < 0.05和p < 0.01)以及睾丸组织形态;同时发现,虫草素(20 mg/kg)显著提高老龄大鼠睾丸雄激素受体(AR)、促卵泡激素受体(FSHR)和促黄体生成激素受体(LHR)蛋白和mRNA水平;虫草素(20 mg/kg)显著改善老龄大鼠睾丸抗氧化酶如谷胱甘肽过氧化物酶(GPx4)、谷胱甘肽S-转移酶mu5 (GSTm5)和过氧化物酶(PRx4)的表达。提示20 mg/kg虫草素可以保护大鼠睾丸间质细胞和支持细胞免受衰老诱导的睾丸功能障碍。氧化应激是糖尿病患者生殖损伤的重要原因(Shrilatha等,2007) [51]。研究表明,糖尿病引起睾丸和附睾精子脂质过氧化增加,刺激ROS的产生(Aguirre-Arias等,2017;Karimi等,2011;Shrilatha等,2007) [52] [53] [54],造成氧化应激。Nguyen等(2021) [29] 报道,CM中虫草素显著提高链脲佐菌素(STZ)诱导的糖尿病雄性Wistar大鼠血清中睾酮及GSH、CAT水平(p < 0.05),显著降低血清中MDA水平(p < 0.05)。试验表明,虫草素改善了糖尿病大鼠氧化应激所致的睾丸损伤,提高了生殖功能(Nguyen等,2021) [29]。
4. 结语
虫草素是虫草属中所特有的主要生物活性物质,通过调节下丘脑—垂体—睾丸轴及降低氧化应激对睾丸组织、细胞的损伤,促进睾酮的分泌,从而改善动物的繁殖功能。但目前关于虫草素提高动物繁殖性能的研究主要集中在雄性实验动物上,未来需要进一步加强虫草素在雌性动物上以及在提高畜禽繁殖性能方面的研究,为虫草素在畜禽生产上的应用提供理论依据。
文章引用
黄炜乾,金明昌,赵 颖,唐谢芳. 虫草素提高雄性动物繁殖性能及机制研究进展
Research Progress of Cordycepin on Improving Reproduction and Mechanism in Male Animals[J]. 生物过程, 2021, 11(04): 146-154. https://doi.org/10.12677/BP.2021.114017
参考文献
- 1. Hawley, S.A., Ross, F.A., Russell, F.M., et al. (2020) Mechanism of Activation of AMPK by Cordycepin. Cell Chemi-cal Biology, 27, 214-222.E4. https://doi.org/10.1016/j.chembiol.2020.01.004
- 2. Cunningham, K.G., Manson, W., Spring, F.S., et al. (1950) Cordycepin, a Metabolic Product Isolated from Cultures of Cordyceps militaris (Linn.) Link. Nature, 166, 949. https://doi.org/10.1038/166949a0
- 3. Chen, Y.C., Chen, Y.H., Pan, B.S., et al. (2017) Func-tional Study of Cordyceps sinensis and Cordycepin in Male Reproduction: A Review. Journal of Food and Drug Analy-sis, 25,197-205. https://doi.org/10.1016/j.jfda.2016.10.020
- 4. Zhou, X.X., Luo, L.P., Dressel, W., et al. (2008) Cordycepin Is an Immunoregulatory Active Ingredient of Cordyceps sinensis. The American Journal of Chinese Medi-cine, 36, 967-980. https://doi.org/10.1142/S0192415X08006387
- 5. Xiong, Y., Zhang, S., Xu, L., et al. (2013) Suppression of T-Cell Activation in Vitro and in Vivo by Cordycepin from Cordyceps militaris. Journal of Surgical Re-search, 185, 912-922. https://doi.org/10.1016/j.jss.2013.06.057
- 6. Ryu, E.H., Son, M.K., Lee, M.J., et al. (2014) Cordycepin Is a Novel Chemical Suppressor of Epstein-Barr Virus Replication. Oncoscience, 1, 866-881. https://doi.org/10.18632/oncoscience.110
- 7. Clercq, E.D. (2015) Curious (Old and New) Antiviral Nucleoside Analogues with Intriguing Therapeutic Potential. Current Medicinal Chemistry, 22, 3866-3880. https://doi.org/10.2174/0929867322666150625094705
- 8. Lei, J., Wei, Y., Song, P., et al. (2018) Cordycepin Inhibits LPS-Induced Acute Lung Injury by Inhibiting Inflammation and Oxidative Stress. European Journal of Phar-macology, 818, 110-114. https://doi.org/10.1016/j.ejphar.2017.10.029
- 9. Park, E.S., Kang, D.H., Yang, M.K., et al. (2014) Cordycepin, 3′-Deoxyadenosine, Prevents Rat Hearts from Ischemia/Reperfusion Injury via Activation of Akt/GSK-3β/p70S6K Sig-naling Pathway and HO-1 Expression. Cardiovascular Toxicology, 14, 1-9. https://doi.org/10.1007/s12012-013-9232-0
- 10. Guo, P., Kai, Q., Gao, J., et al. (2010) Cordycepin Prevents Hy-perlipidemia in Hamsters Fed a High-Fat Diet via Activation of AMP-Activated Protein Kinase. Journal of Pharmaco-logical Sciences, 113, 395-403.
- 11. Gong, X.B., Li, T.J., Wan, R.Z., et al. (2021) Cordycepin Attenuates High-Fat Di-et-Induced Non-Alcoholic Fatty Liver Disease via Down-Regulation of Lipid Metabolism and Inflammatory Responses. International Immunopharmacology, 91, Article ID: 107173. https://doi.org/10.1016/j.intimp.2020.107173
- 12. Tan, L., Song, X.M.T., Ren, Y.L., et al. (2020) An-ti-Inflammatory Effects of Cordycepin: A Review. Phytotherapy Research, 35, 1284-1297. https://doi.org/10.1002/ptr.6890
- 13. Govindula, A., Pai, A., Baghel, S., et al. (2021) Molecular Mechanisms of Cordycepin Emphasizing Its Potential against Neuroinflammation: An Update. European Journal of Pharmacology, 908, Article ID: 174364. https://doi.org/10.1016/j.ejphar.2021.174364
- 14. Jeong, J.W. and Choi, Y.H. (2015) Anti-Cancer Properties and Relevant Mechanisms of Cordycepin, an Active Ingredient of the Insect Fungus Cordyceps spp. Journal of Life Science, 25, 607-614. https://doi.org/10.5352/JLS.2015.25.5.607
- 15. Özenver, N., Boulos, J.C. and Efferth, T. (2021) Activity of Cordycepin from Cordyceps sinensis against Drug-Resistant Tumor Cells as Determined by Gene Expression and Drug Sensitivity Profiling. Natural Product Communications, 16, 1-12. https://doi.org/10.1177/1934578X21993350
- 16. Ahn, Y.J., Park, S.J., Lee, S.G., et al. (2000) Cordycepin: Selec-tive Growth Inhibitor Derived from Liquid Culture of Cordyceps militaris against Clostridium spp. Journal of Agricul-tural and Food Chemistry, 48, 2744-2748. https://doi.org/10.1021/jf990862n
- 17. 高苏, 马婕馨, 刘警鞠, 等. 虫草素的抑菌活性及机理研究[J]. 生物技术通报, 2021, 37(8): 137-144.
- 18. Wang, Y., Pei, Z.J., Lou, Z.X., et al. (2021) Evaluation of Anti-Biofilm Capability of Cordycepin against Candida albicans. Infection and Drug Resistance, 14, 435-448.
- 19. Wang, S.M., Lee, L.J., Lin, W.W., et al. (1998) Effects of a Water-Soluble Extract of Cordyceps sinensis on Steroidogenesis and Capsular Mor-phology of Lipid Droplets in Cultured Rat Adrenocortical Cells. Journal of Cellular Biochemistry, 69, 483-489. https://doi.org/10.1002/(SICI)1097-4644(19980615)69:4%3C483::AID-JCB9%3E3.0.CO;2-J
- 20. Leu, S.F., Song, L.P., Pao, H.Y., et al. (2011) The in Vivo and in Vitro Stimulatory Effects of Cordycepin on Mouse Leydig Cell Steroidogenesis. Bioscience Biotechnology and Biochemistry, 75, 723-731. https://doi.org/10.1271/bbb.100853
- 21. Lin, W.H., Tsai, M.T., Chen, Y.S., et al. (2007) Improvement of Sperm Production in Subfertile Boars by Cordyceps militaris Supplement. American Journal of Chinese Medicine, 35, 631-641. https://doi.org/10.1142/S0192415X07005120
- 22. Chang, Y., Jeng, K.C., Huang, K.F., et al. (2008) Effect of Cordyceps Militaris Supplementation on Sperm Production, Sperm Motility and Hormones in Sprague-Dawley Rats. American Journal of Chinese Medicine, 36, 849-859. https://doi.org/10.1142/S0192415X08006296
- 23. Sohn, S.H., Lee, S.C., Hwang, S.Y., et al. (2012) Effect of Long-Term Administration of Cordycepin from Cordyceps militaris on Testicular Function in Middle-Aged Rats. Planta Medica, 78, 1620-1625.
- 24. Kopalli, S.R., Cha, K.M., Lee, S.H., et al. (2019) Cordycepin, an Active Constituent of Nutrient Powerhouse and Potential Medicinal Mushroom Cordyceps militaris Linn., Ameliorates Age-Related Testicular Dysfunction in Rats. Nutrients, 11, Article No. 906. https://doi.org/10.3390/nu11040906
- 25. Hong, I.P., Choi, Y.S., Woo, S.O., et al. (2011) Effect of Cordyceps militaris on Testosterone Production in Sprague-Dawley Rats. Inter-national Journal of Industrial Entomology, 23, 143-146. https://doi.org/10.7852/ijie.2011.23.1.143
- 26. 张阳海, 李永, 曹迪, 等. 睾酮对动物生殖和生长发育影响的研究进展[J]. 家畜生态学报, 2018, 39(1): 1-7.
- 27. Saez, J.M. (1994) Leydig Cells: Endocrine, Paracrine, and Auto-crine Regulation. Endocrine Reviews, 15, 574-626. https://doi.org/10.1210/edrv-15-5-574
- 28. 刘建中, 郭海彬, 邓春华, 等. 大鼠睾丸Leydig细胞的培养和鉴定[J]. 中华男科学杂志, 2006, 12(1): 14-17.
- 29. Nguyen, T.V., Chumnanpuen, P., Parunyakul, K., et al. (2021) A Study of the Aphrodisiac Properties of Cordyceps militaris in Streptozotocin-Induced Diabetic Male Rats. Veterinary World, 14, 537-544. https://doi.org/10.14202/vetworld.2021.537-544
- 30. Oyola, M.G. and Handa, R.J. (2017) Hypothalam-ic-Pituitary-Adrenal and Hypothalamic-Pituitary-Gonadal Axes: Sex Differences in Regulation of Stress Responsivity. Stress, 20, 476-494. https://doi.org/10.1080/10253890.2017.1369523
- 31. Richards, J.S. (2001) New Signaling Pathways for Hormones and Cyclic Adenosine 3’,5’-Monophosphate Action in Endocrine Cells. Molecular Endocrinol-ogy, 15, 209-218.
- 32. Stocco, D.M. and Clark, B.J. (1996) Regulation of the Acute Production of Steroids in Steroidogenic Cells. Endocrine Reviews, 17, 221-244. https://doi.org/10.1210/edrv-17-3-221
- 33. Strauss, J.F., Kishida, T., Christenson, L.K., et al. (2003) START Domain Proteins and the Intracellular Trafficking of Cholesterol in Steroidogenic Cells. Molecular and Cellular Endocrinology, 202, 59-65. https://doi.org/10.1016/S0303-7207(03)00063-7
- 34. Stocco, D.M. (2001) Tracking the Role of a StAR in the Sky of the New Millennium. Molecular Endocrinology, 15, 1245-1254. https://doi.org/10.1210/mend.15.8.0697
- 35. Zirkin, B.R. and Papadopoulos, V. (2018) Leydig Cells: Formation, Function, and Regulation. Biology of Reproduction, 99, 101-111. https://doi.org/10.1093/biolre/ioy059
- 36. 朱清玉, 郭乐薇, 刘红羽, 等. 睾丸间质细胞睾酮合成机制的研究进展[J]. 中国畜牧杂志, 2021, 57(5): 28-33.
- 37. Londos, C., Cooper, D. and Wolff, J. (1980) Subclasses of External Adenosine Receptors. Proceedings of the National Academy of Sciences of the United States of America, 77, 2551-2554. https://doi.org/10.1073/pnas.77.5.2551
- 38. Jacobson, K.A. and Gao, Z.G. (2006) Adenosine Receptors as Thera-peutic Targets. Nature Reviews Drug Discovery, 5, 247-264. https://doi.org/10.1038/nrd1983
- 39. He, M.T., Lee, A.Y., Cho, E.J., et al. (2019) Protective Effect of Cordyceps Militaris against Hydrogen Peroxide-Induced Oxidative Stress in Vitro. Nutrition Research and Practice, 13, 279-285. https://doi.org/10.4162/nrp.2019.13.4.279
- 40. Ryan, M.J., Dudash, H.J., Docherty, M., et al. (2008) Ag-ing-Dependent Regulation of Antioxidant Enzymes and Redox Status in Chronically Loaded Rat Dorsiflexor Muscles. Journals of Gerontology, 63, 1015-1026. https://doi.org/10.1093/gerona/63.10.1015
- 41. Koeberle, A., Shindou, H., Harayama, T., et al. (2012) Polyun-saturated fatty acids are incorporated into maturating male mouse germ cells by lysophosphatidic acid acyltransferase 3. The FASEB Journal, 26, 169-180. https://doi.org/10.1096/fj.11-184879
- 42. Asadi, N., Bahmani, M., Kheradmand, A., et al. (2017) The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidant in Improving It: A Review. Journal of Clinical and Diagnostic Research, 11, IE01-IE05. https://doi.org/10.7860/JCDR/2017/23927.9886
- 43. Suresh, S., Prithiviraj, E., Lakshmi, N.V., et al. (2013) Effect of Mucuna pruriens (Linn.) on Mitochondrial Dysfunction and DNA Damage in Epididymal Sperm of Streptozoto-cin-Induced Diabetic Rat. Journal of Ethnopharmacology, 145, 32-41. https://doi.org/10.1016/j.jep.2012.10.030
- 44. 孟雪莲, 陈长兰, 孔维娟, 等. 虫草素抑制脂多糖诱导的小胶质细胞活化及神经保护作用[J]. 食品科学, 2014, 35(19): 224-230.
- 45. Han, F., Dou, M., Wang, Y.X., et al. (2020) Cordycepin Protects Renal Ischemia/Reperfusion Injury through Regulating Inflammation, Apoptosis, and Oxidative Stress. Acta Biochimica et Biophysica Sinica, 52, 125-132. https://doi.org/10.1093/abbs/gmz145
- 46. Ramesh, T., Yoo, S.K., Kim, S.W., et al. (2012) Cordycepin (3′-Deoxyadenosine) Attenuates Age-Related Oxidative Stress and Ameliorates Antioxidant Capacity in Rats. Experi-mental Gerontology, 47, 979-987. https://doi.org/10.1016/j.exger.2012.09.003
- 47. Iuchi, Y., Okada, F., Tsunoda, S., et al. (2009) Peroxiredoxin 4 Knockout Results in Elevated Spermatogenic Cell Death Via Oxidative Stress. Biochemical Journal, 419, 149-158. https://doi.org/10.1042/BJ20081526
- 48. Rao, A.V. and Shaha, C. (2000) Role of Glutathione S-Transferases in Oxidative Stress-Induced Male Germ Cell Apoptosis. Free Radical Biology & Medicine, 29, 1015-1027. https://doi.org/10.1016/S0891-5849(00)00408-1
- 49. Hemachand, T., Gopalakrishnan, B., Salunke, D.M., et al. (2002) Sperm Plasma-Membrane-Associated Glutathione S-Transferases as Gamete Recognition Molecules. Journal of Cell Science, 115, 2053-2065. https://doi.org/10.1242/jcs.115.10.2053
- 50. Ursini, F., Kiess, M., Maiorino, M., et al. (1999) Dual Function of the Selenoprotein PHGPx during Sperm Maturation. Science, 285, 1393-1396. https://doi.org/10.1126/science.285.5432.1393
- 51. Muralidhara, B.S. (2007) Occurrence of Oxidative Impair-ments Response of Antioxidant Defences and Associated Biochemical Perturbations in Male Reproductive Milieu in the Streptozotocin-Diabetic Rat. International Journal of Andrology, 30, 508-518. https://doi.org/10.1111/j.1365-2605.2007.00748.x
- 52. Aguirre-Arias, M.V., Velarde, V. and Moreno, R.D. (2017) Effects of Ascorbic Acid on Spermatogenesis and Sperm Parameters in Diabetic Rats. Cell and Tissue Research, 370, 305-317. https://doi.org/10.1007/s00441-017-2660-6
- 53. Karimi, J., Goodarzi, M.T., Tavilani, H., et al. (2011) Relationship between Advanced Glycation End Products and Increased Lipid Peroxidation in Semen of Diabetic Men. Diabetes Research and Clinical Practice, 91, 61-66. https://doi.org/10.1016/j.diabres.2010.09.024
- 54. Muralidhara, B.S. (2007) Early Oxidative Stress in Testis and Epididymal Sperm in Streptozotocin-Induced Diabetic Mice: Its Progression and Genotoxic Consequences. Reproductive Toxicology, 23, 578-587. https://doi.org/10.1016/j.reprotox.2007.02.001
