Pharmacy Information
Vol.
11
No.
04
(
2022
), Article ID:
54109
,
8
pages
10.12677/PI.2022.114042
款冬花化学成分的研究
秦智彬,张雨晨,曹青,刘生元,陈敏纯,闫抗抗*
西北大学附属医院西安市第三医院药剂科,陕西 西安
收稿日期:2022年6月21日;录用日期:2022年7月20日;发布日期:2022年7月27日

摘要
目的:研究款冬花中的化学成分。方法:综合应用反相色谱、Sephadex LH-20、液相和半制备液相等多种化学色谱方法进行分离纯化,并根据化合物的理化性质和核磁共振波谱进行结构鉴定。结果:从款冬花的95%乙醇提取物中分离得到20个化合物,通过波谱分析等手段鉴定了15个化合物,包括12个倍半萜,2个脂肪酸和1个生物碱。结论:化合物6和15为首次从款冬花中分离得到的。
关键词
款冬花,倍半萜,化学成分

Study on Chemical Constituents of Tussilago farfara L.
Zhibin Qin, Yuchen Zhang, Qing Cao, Shengyuan Liu, Minchun Chen, Kangkang Yan*
Department of Pharmacy, Xi’an No.3 Hospital, The Affiliated Hospital of Northwest University, Xi’an Shaanxi
Received: Jun. 21st, 2022; accepted: Jul. 20th, 2022; published: Jul. 27th, 2022

ABSTRACT
Objective: To study the constituents of Tussilago farfara L. Methods: The compounds were isolated and purified by column chromatography of Sephadex LH-20, HPLC and semi-preparative RP-HPLC. Their structures were elucidated by physicochemical properties and spectral analyses. Results: By the means of chromatography methods and spectroscopic evidence, 20 compounds were isolated from 95% EtOH of T. farfara. We identified 15 compounds by spectroscopic analysis, 12 of which were sesquiterpenoids. Conclusion: Compounds 6 and 15 are identified from Tussilago farfara L. for the first time.
Keywords:Tussilago farfara L., Sesquiterpenoids, Chemical Constituents

Copyright © 2022 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
倍半萜类化合物由三个异戊二烯单位构成,含有15个碳原子。主要分布在植物界和微生物界,多以挥发油形式存在。这些年,对此类化合物的研究较快,每年发现的新型种类数目成倍增长,无论是化合物的数目还是骨架类型都是萜类化合物中最多的一类 [1]。倍半萜类广泛存在于菊科植物中,并表现出有趣的化学多样性和重要的生物学特性,这使它们成为植物化学、药理学,及合成的主要目标 [2]。
款冬花系菊科款冬属植物款冬(Tussilago farfara L.)的花蕾,是一种多年生草本植物,通常在10月下旬至12月下旬花尚未出土时采挖,主要分布于中国,欧洲和北非。款冬花中含有最多的成分是倍半萜,也是化学和药理研究最深入的一部分。根据其中所含有的倍半萜母核,主要以oplopane骨架和bisabolane骨架为主。款冬花中已报道的oplopane型倍半萜共有四十多个,占已报道倍半萜的一半以上。此类倍半萜的不同之处在于其取代基的位置变化,结构变化主要发生在1、7和14位。款冬花中报道的倍半萜中也有少部分是bisabolane型,该类倍半萜的结构特点是在1、5位和8位容易氧化,1位和8位大多酰化成酯。近年来,也有学者从款冬花植物中发现了两个新颖结构的oplopane型倍半萜和一个新颖的bisabolane型倍半萜 [3]。除了oplopane型和bisabolane型倍半萜外,款冬花中还报道了eudesmane型 [4] 倍半萜和guaiane型 [5] 倍半萜。我们对在陕西药材市场采购的款冬花进行了化学成分研究,从中分离得到了20个化合物,通过波谱分析手段最终鉴定了15个化合物(如图1),分别为:6,10-Octadecadienoic acid (1) [6]、Hexadecanoic acid (2) [7]、Senkirkine (3) [8]、(-)-cryptomerion (4) [9]、2,2-dimethyl-6-acetylhromanone (5) [10]、β-oploplenone (6) [11]、7β-(3-ethyl-cis-crotonoyloxy)-14-hydroxy-notonipetranone (7) [12]、14-acetoxy-7β-(3-ethyl-cis-crotonoyloxy)-1α-(2-methylbutyryloxy)-notonipetranone (8) [12]、tussilagone (9) [13]、7β-(3-ethyl-cis-crotonoyloxy)-1α-(2-methylbutyryloxy)-3(14)-dehydro-Z-notonipetrane (10) [13]、(1R,3R,4R,5S,6S)-1,5-diacetoxy-8-angeloyloxy-3,4-epoxybisabola-7(14),10-dien-2-one (11) [14]、(1R,3R,4R,5S,6S)-1-acetoxy-8-angeloyloxy-3,4-epoxy-5-hydroxybisabola-7(14),10-dien-2-one (12) [14]、1β,8-bisangeloyloxy-3α,4α-epoxybisabola-7(14),10-dien-2-one (13) [3]、1β-(3-ethyl-cis-crotonoyloxy)-8-angeloyloxy-3α,4α-epoxybisabola-7(14),10-dien-2-one (14) [3]、(4R,6E)-2-acetoxy-8-angeloyloxy-4-hydroxybisabola-2,6,10-trien-1-one (15) [15]。其中,化合物6和15为首次从款冬花中分离得到的。
2. 仪器与试剂
1D和2D NMR在Bruker AM-400、DRX-500或Bruker AM-600核磁共振仪上测定,TMS作为内标,δ为ppm,J为Hz;ESI-MS在Burker HCT或Esquire质谱仪上测定;高分辨质谱在Auto-Spec Premier P776质谱仪上测定;拌样及层析用硅胶(100~200,200~300目),均为青岛海洋化工厂生产;反相填充材料RP-18为40~60 μm,Merk公司生产;MCI填充材料为MCI-gel CHP-20P;HPLC分析仪器为Agilent 1260型高效液相色谱仪,色谱柱为Agilent公司的ZORBAX SB-C18反相柱。凝胶为Sephadex LH-20;显色剂非碱为10% H2SO4的乙醇溶液,喷洒后适当加热;生物碱常用显色剂:Dragendorff's,沾湿后显色。
3. 提取与分离
干燥款冬花10 kg,粉碎后用95%的乙醇提取三次(25 L/次),合并提取液,减压蒸馏除去有机溶剂,用乙酸乙酯进行萃取,得到粗提物143.7 g,以聚酰胺拌样,以甲醇水系统(30%~100%)在MCI柱上进行梯度洗脱,以TLC进行检测,合并相同组分得到三个馏分:I-III。对萃取后的水层进行酸碱处理,酸层用乙酸乙酯萃取三次,得水层进行碱处理,并用氯仿萃取三次,得到生物碱5.3 g。
馏分I段有部分结晶,得到化合物2 (1.5 g)。
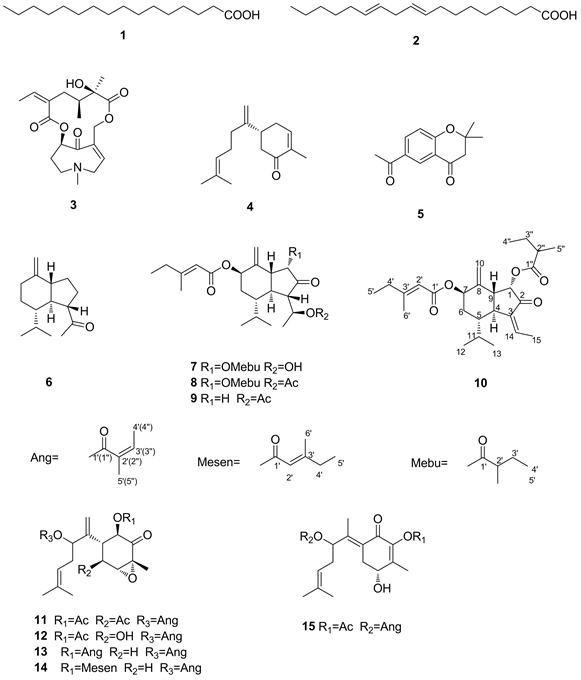
Figure 1. Obtained structural expressions of 15 compounds
图1. 分离得到15个化合物的结构式
馏分II段有小部分结晶,得到化合物9 (2.4 g)。将未结晶的部分,以聚酰胺拌样,以甲醇–水系统(75%~100%)在RP-18柱上进行梯度洗脱,以TLC进行检测,合并相同组分得到I-a、I-b、I-c、I-d和I-e。I-a经过多次硅胶柱层析后得到化合物10 (2.3 g),4 (4.1 mg)和1 (28.9 mg)。以甲醇-水系统(82:28,流速3 mL/min)在分析型HPLC对I-b段进行半制备后得到化合物14 (20.0 mg, tR = 12.6 min),13 (20.0 mg, tR = 11.2 min),和8 (3.8 mg, tR = 15.1 min)。I-c以氯仿为洗脱剂进行柱层析后得到化合物5 (65.2 mg)和混合物,以甲醇-水系统(70:30,流速4 mL/min)在分析型HPLC对混合物进行半制备后得到化合物11 (10.6 mg, tR = 11.9 min)。I-c经过多次硅胶柱层析和半制备后得到化合物6 (27.3 mg)和7 (43.2 mg, tR = 11.8 min)。对I-e段进行半制备(甲醇-水75:25,流速4 mL/min)得到化合物12 (22.2 mg, tR = 10.8 min)和15 (3.4 mg, 15.3 min)
生物碱部分经过多次硅胶柱层析后得到化合物3 (1.1 g)。
4. 结构鉴定
6,10-Octadecadienoic acid (1):无色油状物,C18H32O2∙1H NMR (400 MHz, CDCl3) δH:6~5.35 (4H, m, H-9, 10, 12, 13),2.77 (2H, t, J = 6.1 Hz, H-11),2.34 (2H, t, J = 7.4 Hz, H-2),2.05 (4H, m, H-8, 14),1.63 (2H, m, H-3),1.31 (14H, brs, H-4~7, H-15~17),0.89 (3H, t, J = 6.7 Hz, H-18);13C NMR (100 MHz, CDCl3) δC:14.0 (C-18, CH3),22.6 (CH2),24.6 (CH2),25.6 (CH2),27.1 (CH2),27.2 (CH2),29.0 (CH2),29.0 (CH2),29.1 (CH2),29.3(CH2),29.6 (CH2),31.5 (CH2),34.1 (CH2),127.8 (CH),128.0 (CH),129.9 (CH),130.1 (CH),180.6 (C = 0)。该化合物为不饱和脂肪酸。
Hexadecanoic acid (2):无色针状结晶,C16H32O2∙1H NMR ( CDCl3, 400 MHz) δH:2. 34 (2H, t, J = 7. 5 Hz, H2-2),1.62 ( 2H, brt, J = 7. 3 Hz, H2-3),1.30~1.25 (24H, brs, 12 × -CH2),0.88 (3H, t, J = 6. 5 Hz, Me-16);13C NMR (CDCl3, 100 MHz) δC:180.4 (C-1),34.1 (C-2),24.9 (C-3),29.7~29.0 (10 × -CH2),31.9 (C-14),22.7 (C-15),14.1 (C-16)。由波谱数据推出该化合物为不饱和脂肪酸。
Senkirkine (3):无色油状,C19H27NO6∙1H NMR (CDCl3, 400 Hz):δH 6.09 (1H, t, J = 2.3 Hz, H-2),3.32 (1H, br d, J = 18, H-3a),3.16 (1H, ddd, J = 18, 2.6, 2.3 Hz, H-3b),2.76 (1H, ddd, J = 12.7, 5.7, 4.2 Hz, H-5a),2.65 (1H, ddd, J = 12.7, 12.0, 4.2 Hz, H-5b),2.46 (1H, dddd, J = 11.4, 12.0, 5.7, 3.2 Hz, H-6a),2.28(1H, dq, J = 14.4, 4.2 Hz, H-6b),4.92 (1H, dd, J = 4.4, 3.2 Hz, H-7),5.32 (1H, d, J = 11.4, Hz, H-9a),4.38 (1H, br d, J = 11.4, H-9b),1.76 (1H, ddq, J = 11.0, 2.5, 7.1 Hz, H-13),2.12 (1H, dd, J = 14.0, 11.0 Hz, H-14a),1.27 (s, H-18),0.84 (3H, d, J = 7.1, H-19),6.54 (1H, t, J = 6.8, H-20),4.71 (lH, dd, J = 14.5, 6.8 Hz, Ha-2l),4.60 (lH, dd, J = 14.5, 6.8 Hz, Hb-2l),2.04 (3H, s, H-22);13C NMR (CDCl3, 100 MHz) δC:134.5 (s, C-1),137.0 (d, C-2),58.9 (t, C-3),53.5 (t, C-5),36.2 (t, C-6),78.0 (d, C-7),64.0 (t, C-9),178.0 (s, C-11),76.6 (s, C-12),38.5 (t, C-13),37.6(t, C-14),134.3 (s, C-15),166.4 (s, C-16),24.5 (q, C-18),10.9 (q, C-19),137.0 (d, C-20),58.9 (t, C-21),40.6 (q, C-22)。由波谱数据推出,该化合物为吡咯烷生物碱。
(-)-cryptomerion (4):无色油状,C15H22O,ESI-MS m/z 218 [M]+;1H NMR(600 MHz, CDCl3) δH:6.75 (1H, m, H-3),2.59 (1H, ddd, J = 1.5, 3.7, 15.9 Hz, Ha-4),2.35 (1H, dd, J = 13.2, 15.9 Hz, Ha-4),2.69 (1H, ddd, J = 4.0, 10.2, 13.6 Hz, H-5),2.46 (1H, dtm, J = 4.4, 18.3 Hz, Ha-6),2.27 (1H, ddm, J = 11.0, 18.3 Hz, Hb-6),2.05 (2H, m, H-8),2.11 (2H, m , H-9),5.09 (1H, tq, J = 1.5, 6.8 Hz, H-10),1.61 (3H, s, H3-12),1.68 (3H, d, J = 1.0 Hz H3-13),4.85 (1H, s, Ha-14),4.82 (1H, s, Hb-14),1.79 (3H, dt, J = 1.4, 2.4 Hz, H3-15);13C NMR (150 MHz, CDCl3) δC:199.8 (s, C-1),135.3 (s, C-2),144.6 (d, C-3),31.4 (t, C-4),41.1 (d, C-5),43.5 (t, C-6),150.7 (s, C-7),34.2 (t, C-8),26.6 (t, C-9),123.7 (d, C-10),131.9 (s, C-11),25.6 (q, C-12),17.7 (q, C-13),109.1 (t, C-14),15.7 (q, C-15)。
2,2-dimethyl-6-acetylhromanone (5):无色油状,C13H14O3,ESI-MS m/z 218 [M]+;1H NMR (400 MHz, methanol-d4) δH:1.49 (6H, s, CH3 × 2),2.59 (3H, s, -COCH3),2.77 (2H, s, H-2),7.00 (1H, d, J = 8.8 Hz, H-8),8.13 (1H, dd, J = 8.8, 2.2 Hz, H-7),8.45(1H, d, J = 2.2 Hz, H-5);13C NMR (100 MHz, methanol-d4) δC:80.3 (s, C-1),48.5 (t, C-2),191.7 (s, C-3),130.2 (s, C-4),135.4 (d, C-5),119.1 (d, C-6),128.1 (d, C-7),119.0(d, C-8),163.4 (s, C-9),26.6 (CH3 × 2),26.4 (-COCH3),196.3 (-COCH3)。
β-oploplenone (6):无色油状;C15H24O,ESI-MS m/z 220 [M]+;1H NMR (400 MHz, methanol-d4) δH:1.34 (2H, m, H-1),1.62 (1H, dtd, J = 20.0, 7.4, 12.8 Hz, H-2a),1.81 (1H, m, H-2b),1.77 (1H, m, H-3),1.25 (1H, m, H-4),1.34 (1H, m, H-5),1.10 (1H, dq, J = 4.4, 13.0 Hz, H-6a),1.75 (1H, m, H-6b),1.95 (1H, m, H-7a),2.35 (1H, ddd, J = 2.4, 4.3, 13.4 Hz, H-7b),1.83 (1H, m, H-9),4.54 (1H, q, J = 1.7 Hz, H-10a),4.62 (1H, q, J = 1.7, H-10b),1.95 (1H, m, H-11),0.95 (3H, d, J = 6.9 Hz, H3-12),0.78 (3H, d, J = 6.9 Hz, H3-13),1.18 (3H, q, H3-15);13C NMR (100 MHz, methanol-d4) δC:28.5 (t, C-1),27.7 (t, C-2),50.4 (d, C-3),52.9 (d, C-4),53.5 (d, C-5),29.7 (t, C-6),36.3 (t, C-7),152.2 (s, C-8),56.8 (d, C-9),103.9 (t, C-10),30.9 (d, C-11),22.3 (q, C-12),16.1 (q, C-13),214.7 (s, C-14),29.7 (q, C-15)。
7β-(3-ethyl-cis-crotonoyloxy)-14-hydroxy-notonipetranone (7):无色油状;C26H46O6,ESI-MS m/z 448 [M]+;1H NMR (400 MHz, methanol-d4) δH:5.46 (1H, d, J = 4.0 Hz, H-1β),5.54 (1H, br s H-7α),4.78 (1H, s, Ha-10),5.18 (1H, s, Hb-10),2.37 (1H, m, H-11),1.00 (3H, d, J = 6.6 Hz, H3-12),0.82 (3H, t, J = 7.0 Hz, H3-13),1.23 (3H, d, J = 6.6 Hz, H3-15),5.61 (1H, s, H-2'),2.16 (1H, d, J = 7.7 Hz, H-4'),1.07 (3H, t, J = 7.3 Hz, H3-5'),2.10 (3H, s, H3-6'),2.37 (1H, m, H-2''),0.89 (3H, t, J = 7.5 Hz, H3-4''),1.15 (3H, d, J = 6.6 Hz, H3-5'');13C NMR(100 MHz, methanol-d4) δC:73.2 (d, C-1),206.2 (s, C-2),60.4 (d, C-3),47.3 (d, C-4),45.2 (d, C-5),30.2 (t, C-6),74.2 (d, C-7),142.6 (s, C-8),41.7 (d, C-9),113.0 (t, C-10),28.8 (d, C-11),21.7 (q, C-12),17.0 (q, C-13),68.5 (d, C-14),15.7 (q, C-15),175.3 (s, C-1'),115.4 (d, C-2'),162.3 (s, C-3'),34.1 (t, C-4'),12.2 (q, C-5'),17.0 (q, C-6'),165.8 (s, C-1''),45.2 (d, C-2''),27.3 (t, C-3''),11.8(q, C-4''),17.0 (q, C-5'')。
14-acetoxy-7β-(3-ethyl-cis-crotonoyloxy)-1α-(2-methylbutyryloxy)-notonipetranone (8):无色油状;C28H42O7,ESI-MSm/z 490 [M]+;1H NMR (400 MHz, methanol-d4) δH:5.46 (1H, d, J = 4.0 Hz, H-1β),5.54 (1H, br s H-7α),4.78 (1H, s, Ha-10),5.18 (1H, s, Hb-10),2.37 (1H, m, H-11),1.00 (3H, d, J = 6.6 Hz, H3-12),0.82 (3H, t, J = 7.0 Hz, H3-13),1.23 (3H, d, J = 6.6 Hz, H3-15),5.61 (1H, s, H-2'),2.16 (1H, d, J = 7.7 Hz, H-4'),1.07 (3H, t, J = 7.3 Hz, H3-5'),2.10 (3H, s, H3-6'),2.37 (1H, m, H-2''),0.89 (3H, t, J = 7.5 Hz, H3-4''),1.15 (3H, d, J = 6.6 Hz, H3-5'');13C NMR(100 MHz, methanol-d4) δC:74.0 (d, C-1),209.8 (s, C-2),57.5 (d, C-3),47.4 (d, C-4),45.2 (d, C-5),30.3 (t, C-6),75.0 (d, C-7),142.7 (s, C-8),42.5 (d, C-9),113.7 (t, C-10),28.7 (d, C-11),21.8 (q, C-12),17.3 (q, C-13),70.7 (d, C-14),15.7 (q, C-15),172.4 (s, C-1'),115.5 (d, C-2'),163.6 (s, C-3'),34.6 (t, C-4'),12.0 (q, C-5'),17.3 (q, C-6'),176.6 (s, C-1''),45.5 (d, C-2''),27.8 (t, C-3''),12.4(q, C-4''),16.3 (q, C-5'')。
tussilagone (9):无色结晶;C23H34O5,ESI-MS m/z 390 [M]+;1H NMR (400 MHz, CDCl3) δH:2.15 (1H, dd, J = 16.9, 13.9 Hz, H-1α),2.40 (1H, ddd, J = 16.9, 1.0, 5.5 Hz, H-1β),2.50 (1H, dd, J = 3.0, 11.0 Hz, H-3),1.47 (1H, m, H-4),1.97 (1H, dddd, J = 2.0, 2.0, 11.0, 14.0 Hz, H-5),1.45 (1H, ddd, J = 2.0, 11.0, 14.0 Hz, H-6α),2.08 (1H, dt, J = 2.0, 14.0 Hz, H-6β),5.58 (1H, t, J = 3.0 Hz, H-7),2.60 (1H, ddddd, J = 2.0, 2.0, 5.9 11.5, 13.9 Hz, H-9),5.15 (1H, s-like, H-10Z),4.79 (1H, d, J = 1.0 Hz, H-10E),2.30 (1H, dqq, J = 3.0, 6.8, 6.9 Hz, H-11),0.99 (3H, d, J = 6.5 Hz, H3-12),0.78 (3H, d, J = 7.0 Hz, H3-13),5.10 (1H, dq, J = 3.2, 6.6 Hz, H-14),1.22 (3H, d, J = 6.5 Hz, H3-15),5.63 (1H, qt, J = 1.3, 1.3 Hz, H-2'),2.17 (2H, dq, J = 1.3, 7.5 Hz, H-4'),1.07 (3H, t, J = 7.5 Hz, H3-5'),2.15 (3H, d, J = 1.3 Hz, H3-6'),2.15 (3H, s, OCOCH3);13C NMR (100 MHz, CDCl3) δC:42.5 (t, C-1),214.9 (s, C-2),57.2 (d, C-3),49.0 (d, C-4),43.8 (d, C-5),31.1 (t, C-6),72.9 (d, C-7),146.0(s, C-8),42.2 (d, C-9),110.1 (t, C-10),27.5 (d, C-11),21.5(q, C-12),15.4 (q, C-13),69.5 (d, C-14),15.3 (q, C-15),165.9 (s, C-1'),114.5 (d, C-2'),162.0 (s, C-3'),33.8 (t, C-4'),11.9 (q, C-5'),18.9 (q, C-6'),170.9 (OCOCH3),21.3 (OCOCH3)。
7β-(3-ethyl-cis-crotonoyloxy)-1α-(2-methylbutyryloxy)-3(14)-dehydro-Z-notonipetrane (10):无色油状;C26H38O5,ESI-MS m/z 430 [M]+;1H NMR (500 MHz, methanol-d4) δH:5.56 (1H, d, J = 4.0 Hz, H-1β),(1H, d, J = 3.3 Hz, H-7α),4.82 (1H, s, Ha-10),5.18 (1H, s, Hb-10),2.02 (1H, m, H-11),0.98 (3H, d, J = 6.6 Hz, H3-12),0.90(3H, t, J = 7.0 Hz, H3-13),6.42 (1H, q, J = 7.3 Hz, H-14),2.18 (3H, d, J = 7.3 Hz, H3-15),5.64 (1H, s, H-2'),1.07 (3H, t, J = 7.3 Hz, H3-5'),2.15 (3H, s, H3-6'),2.40 (1H, m, H-2''),0.87 (3H, t, J = 7.3 Hz, H3-4''),1.13 (3H, d, J = 7.0 Hz, H3-5'');13C NMR(125 MHz, methanol-d4) δC:73.2 (d, C-1),200.1 (s, C-2),139.0 (s, C-3),44.6 (d, C-4),40.3 (d, C-5),29.6 (t, C-6),72.3(d, C-7),140.4 (s, C-8),45.7 (d, C-9),112.5 (t, C-10),27.3 (d, C-11),21.2 (q, C-12),15.0 (q, C-13),136.7 (d, C-14),15.5 (q, C-15),165.8 (s, C-1'),114.4 (d, C-2'),162.0 (s, C-3'),33.7 (t, C-4'),11.4 (q, C-5'),18.8 (q, C-6'),175.4 (s, C-1''),40.8 (d, C-2''),26.7 (t, C-3''),11.8 (q, C-4''),16.5 (q, C-5'')。
(1R,3R,4R,5S,6S)-1,5-diacetoxy-8-angeloyloxy-3,4-epoxybisabola-7(14),10-dien-2-one (11):无色油状;C24H32O8,ESI-MS m/z 448 [M]+;1H NMR (400 MHz, CDCl3) δH:5.68 (1H, d, J = 12.7 Hz, H-1),3.40 (1H, s, H-4),5.35 (1H, d, J = 8.8 Hz, H-5),2.87 (1H, d, J = 8.8, 12.7 Hz, H-6),5.23 (1H, dd, J = 3.2, 7.1 Hz, H-8),2.33 (2H, m, H-9),5.04 (1H, tq, J = 1.0, 7.3 Hz, H-10),1.68 (3H, d, J = 1.0Hz, H3-12),1.62 (3H, s, H3-13),5.24 (1H, s, Ha-14),5.33 (1H, d, J = 0.7Hz, Hb-14),1.48 (3H, s, H3-15),6.08 (1H, qq J = 1.5, 7.1 Hz, H-3'),1.97 (3H, dq, J = 1.5, 7.1 Hz, H-4'),1.88 (3H, dq, J = 1.5, 1.5 Hz, H3-5');13C NMR (100 MHz, CDCl3) δC:72.3 (d, C-1),199.1 (s, C-2), 61.0 (d, C-3),65.4 (d, C-4),74.5 (d, C-5),48.1 (d, C-6),145.7 (s, C-7),75.6 (d, C-8),31.5 (t, C-9),119.3 (d, C-10),134.0 (s, C-11),24.4 (q, C-12),18.7 (q, C-13),113.3 (t, C-14),14.6 (q, C-15),166.4 (s, C-1'),127.5 (s, C-2'),138.1 (d, C-3'),16.6 (q, C-4'),19.5 (q, C-5'),19.3 (1-COCH3),169.5 (1-COCH3),19.2 (5-COCH3),170.1 (5-COCH3)。
通过波谱数据推出,化合物6、7、8、9、10和11为oplopane型倍半萜,属于款冬花中一类较为常见的倍半萜。
(1R,3R,4R,5S,6S)-1-acetoxy-8-angeloyloxy-3,4-epoxy-5-hydroxybisabola-7(14),10-dien-2-one (12):无色油状;C22H30O7,ESI-MS m/z 406 [M]+;1H NMR (400 MHz, CDCl3) δH:5.68 (1H, d, J = 13.7 Hz, H-1),3.51 (1H, s, H-4),4.25 (1H, d, J = 8.5 Hz, H-5),2.56 (1H, d, J = 8.5, 13.7 Hz, H-6),4.72 (1H, dd, J = 2.7, 8.8 Hz, H-8),2.18 (1H, m, Ha-9),2.52 (1H, m , Hb-9),5.13 (1H, tq, J = 1.0, 7.1 Hz, H-10),1.70 (3H, d, J = 1.0 Hz, H3-12),1.64 (3H, s, H3-13),5.08 (1H, s, Ha-14),5.20 (1H, d, J = 0.7 Hz, Hb-14),1.48 (3H, s, H3-15),6.20 (1H, qq J = 1.5, 7.3 Hz, H-3'),2.00 (3H, dq, J = 1.5, 7.3 Hz, H-4'),1.92 (3H, dq, J = 1.5, 1.5 Hz, H3-5');13C NMR (100 MHz, CDCl3) δC:72.2 (d, C-1),201.9 (s, C-2),62.5 (d, C-3),70.2 (d, C-4),73.8 (d, C-5),54.5 (d, C-6),148.3 (s, C-7),76.8 (d, C-8),33.3 (t, C-9),120.6 (d, C-10),135.4 (s, C-11),26.0 (q, C-12),18.1 (q, C-13),114.3 (t, C-14),14.7 (q, C-15),168.4 (s, C-1'),129.1 (s, C-2'),140.0 (d, C-3'),16.0 (q, C-4'),20.3 (q, C-5'),20.7 (1-COCH3),171.4 (1-COCH3)。
1β,8-bisangeloyloxy-3α,4α-epoxybisabola-7(14),10-dien-2-one (13):无色油状;C25H34O6,ESI-MS m/z 430 [M]+;1H NMR (600 MHz, methanol-d4) δH:5.74 (1H, d, J = 12.8 Hz, H-1),3.40 (1H, br d, J = 4.4 Hz, H-4),2.21 (2H, brddd, J = 4.4, 8.0, 15.0 Hz, H-5),2.78 (1H, ddd, J = 8.0, 11.6, 12.8 Hz, H-6),5.07 (1H, dd, J = 6.0, 8.0 Hz, H-8),2.35 (2H, m, H-9),5.03 (1H, t, J = 6.3 Hz, H-10),1.64 (3H, brs, H3-12),1.58 (3H, brs, H3-13),5.24 (1H, s, Ha-14),5.09 (1H, s, Hb-14),1.42 (3H, s, H3-15),6.05 (1H, qq J = 1.2, 7.2 Hz, H-3'),1.95 (3H, dd J = 1.2, 7.2 Hz, H-4'),1.86 (3H, d, J = 1.2 Hz, H3-5'),5.60 (1H, d J = 1.2 Hz, H-2''),2.12 (2H, br q, J = 7.4 Hz, H-4''),1.01 (3H, t, J = 7.4 Hz, H3-5''),2.09 (3H, br s, H-6'');13C NMR (150 MHz, methanol-d4) δC:75.6 (d, C-1),203.4 (s, C-2),62.8 (d, C-3),65.8 (d, C-4),33.4 (t, C-5),45.5 (d, C-6),149.4 (s, C-7),76.6 (d, C-8),32.9 (t, C-9),120.6 (d, C-10),135.6 (s, C-11),26.1 (q, C-12),18.3 (q, C-13),114.7 (t, C-14),15.2 (q, C-15),168.3 (s, C-1'),129.3 (s, C-2'),139.7 (d, C-3'),16.2 (q, C-4'),21.3 (q, C-5'),167.0 (s, C-2''),114.3 (d, C-2''),164.7 (s, C-3''),32.9 (t, C-4''),12.4 (q, C-5''),19.1 (q, C-6'')。
1β-(3-ethyl-cis-crotonoyloxy)-8-angeloyloxy-3α,4α-epoxy-bisabola-7(14),10-dien-2-one (14):无色油状;C25H34O6,ESI-MS m/z 430 [M]+;1H NMR (400 MHz, methanol-d4) δH:5.74 (1H, d, J = 12.8 Hz, H-1),3.40 (1H, br d, J = 4.4 Hz, H-4),2.21 (2H, brddd, J = 4.4, 8.0, 15.0 Hz, H-5),2.78 (1H, ddd, J = 8.0, 11.6, 12.8 Hz, H-6),5.07 (1H, dd, J = 6.0, 8.0 Hz, H-8),2.35 (2H, m, H-9),5.03 (1H, t, J = 6.3 Hz, H-10),1.64 (3H, brs, H3-12),1.58 (3H, brs, H3-13),5.24 (1H, s, Ha-14),5.09 (1H, s, Hb-14),1.42 (3H, s, H3-15),6.05 (1H, qq J = 1.2, 7.2 Hz, H-3'),1.95 (3H, dd J = 1.2, 7.2 Hz, H-4'),1.86 (3H, d, J = 1.2 Hz, H3-5'),5.60 (1H, d J = 1.2 Hz, H-2''),2.12 (2H, br q, J = 7.4 Hz, H-4''),1.01 (3H, t, J = 7.4 Hz, H3-5'');13C NMR (100 MHz, methanol-d4) δC:75.4 (d, C-1),202.9 (s, C-2),62.7 (d, C-3),65.7 (d, C-4),33.6 (t, C-5),46.5 (d, C-6),149.3 (s, C-7),75.6 (d, C-8),32.1 (t, C-9),120.5 (d, C-10),135.5 (s, C-11),26.0 (q, C-12),18.1 (q, C-13),113.5 (t, C-14),15.1 (q, C-15),168.1 (s, C-1'),128.6 (s, C-2'),139.3 (d, C-3'),16.1 (q, C-4'),20.7 (q, C-5'),168.2 (s, C-2''),129.0 (s, C-2''),139.5 (d, C-3''),16.1 (q, C-4''),20.8 (q, C-5'')。
(4R,6E)-2-acetoxy-8-angeloyloxy-4-hydroxybisabola-2,6,10-trien-1-one (15):无色油状;C22H30O6,ESI-MS m/z 390 [M]+;1H NMR (600 MHz, methanol-d4) δH:3.99 (1H, ddd, J = 8.1, 3.7, 3.7 Hz, H-5),2.52 (1H, brddd, J = 14.7, 3.7 Hz, H-6α),2.96 (1H, dd, J = 14.7, 3.7 Hz, H-6β),5.54 (1H, t, J = 7.3 Hz, H-8),2.12 (2H, m, H-9),4.96 (1H, br t, J = 6.3 Hz, H-10),1.54 (3H, s, H3-12),1.44 (3H, s, H3-13),1.81 (3H, s, H3-14),2.11(3H, d, J = 1.8 Hz, H3-15),5.63 (1H, qq J = 7.0, 1.5 Hz, H-3'),1.82 (1H, dq, J = 7.0, 1.5 Hz, H-4'),1.71 (3H, dq, J = 1.5, 1.5 Hz, H3-5'),1.89 (3H, s, OCOCH3);13C NMR (150 MHz, methanol-d4) δC:184.8 (s, C-1),146.9 (s, C-2),149.6 (s, C-3),69.4 (d, C-4),37.9 (t, C-5),128.9 (s, C-6),144.6 (s, C-7),75.0 (d, C-8),32.7 (t, C-9),119.7 (d, C-10),137.0 (s, C-11),26.2 (q, C-12),18.1 (q, C-13),15.6 (q, C-14),14.1 (q, C-15),168.6 (s, C-1'),128.9 (s, C-2'),140.1 (d, C-3'),16.2 (q, C-4'),20.3 (q, C-5'),20.9 (1-COCH3) 170.4 (1-COCH3)。
通过波谱数据得出,化合物12、13、14和15均为没药烷型倍半萜。
5. 结果与讨论
在对款冬花中化学成分的研究中,发现其含有吡咯烷生物碱 [16]、倍半萜 [17]、酚类化合物 [18]、黄酮 [19] 等化学成分,不管是国外学者,还是国内学者对其成分研究最多的是生物碱、酚类化合物和黄酮。对于款冬花中所含的倍半萜化合物还要追溯于 20 世纪 90 年代末由Yaoita Y等分离得到新的没药烷型倍半萜 [9] [14],近年来,也有学者从款冬花植物中发现了两个新颖结构的oplopane型倍半萜7β-angeloyloxy-14-hydroxy-notonipetranone和1α-hydroxy-7β-(4-methylsenecioyloxy)-oplopa-3(14)Z,8(10)-dien-2-one,一个新颖结构的没药烷型倍半萜1α-(3″-ethyl-cis-crotonoyloxy)-8-angeloyloxy-3β,4β-epoxy-bisabola-7(14),10-diene [3]。本文中共分离得到20个化合物,运用各种波谱手段鉴定得到15个化合物,其中化合物1和2为脂肪酸,化合物3为吡咯烷生物碱,化合物6、7、8、9、10和11为oplopane型倍半萜,化合物12、13、14和15均没药烷型倍半萜。
款冬花中报道的倍半萜具有广泛的活性,包括抗炎、抗过敏、抗癌、神经保护等。本文通过鉴定化学结构,探讨结构特征,从结构特征上看,款冬花中主要以oplopane型倍半萜和没药烷型倍半萜为主,其他骨架类型的倍半萜相对较少。而且,这些结构的化学活性相对较低。此外,有文献报道的款冬花中抗炎活性的结果与构效关系有一定关系。因此,我们下一步将对已分离得到的化合物进行抗炎活性的筛选,从而发现其抗炎活性与结构之间的关系。
基金项目
“科技+”行动计划——医学研究项目(2019114613YX001SF044 (16))。
文章引用
秦智彬,张雨晨,曹 青,刘生元,陈敏纯,闫抗抗. 款冬花化学成分的研究
Study on Chemical Constituents of Tussilago farfara L.[J]. 药物资讯, 2022, 11(04): 327-334. https://doi.org/10.12677/PI.2022.114042
参考文献
- 1. Xue, S.Y., Li, Z.Y., Zhi, H.J., Sun, H.F., Zhang, L.Z., Guo, X.Q. and Qin, X.M. (2012) Metabolic Finger-printing Investigation of Tussilago farfara L. by GC-MS and Multivariate Data Analysis. Biochemical Systematics and Ecology, 41, 6-12. https://doi.org/10.1016/j.bse.2011.11.003
- 2. Akçin, Ö.E. (2007) Morphological and Anatom-ical Characteristics of Cichorium intybus L., Tragopogon latifolius Boiss. and Tussilago farfara L. (Asteraceae). Inter-national Journal of Natural and Engineering Sciences, 1, 81-85.
- 3. 李静, 秦雪梅, 李震宇. 款冬花中倍半萜类成分的研究进展[J]. 中草药, 2017, 48(14): 2964-2971.
- 4. 钟云青. 款冬花散治疗慢性阻塞性肺疾病急性加重期(痰热郁肺证)临床观察[J]. 中国中医急症, 2017, 26(1): 149-151.
- 5. Li, W., Huang, X. and Yang, X.W. (2012) New Sesquiterpenoids from the Dried Flower Buds of Tussi-lago farfara and Their Inhibition on NO Production in LPS-Induced RAW264.7 Cells. Fitoterapia, 83, 318-322. https://doi.org/10.1016/j.fitote.2011.11.011
- 6. Jang, H., Lee, J.W., Lee, C., Jin, Q., Choi, J.Y., Lee, D., Han, S.B., Kim, Y., Hong, J.T., Lee, M.K. and Hwang, B.Y. (2016) Sesquiterpenoids from Tussilago farfara Inhibit LPS-Induced Nitric Oxide Production in Macrophage RAW 264.7 Cells. Archives of Pharmacal Research, 39, 127-132. https://doi.org/10.1007/s12272-015-0667-7
- 7. Liu, L.L., Yang, J.L. and Shi, Y.P. (2011) Sesquiterpenoids and Other Constituents from the Flower Buds of Tussilago farfara. Journal of Asian Natural Products Research, 13, 920-929. https://doi.org/10.1080/10286020.2011.600251
- 8. 刘世旺, 付宏征, 林文翰. 糙苏的化学成分研究(I) [J]. 中草药, 1999(3): 161-164.
- 9. Shen, Q., Cai, G.M., He, G.X., et al. (2009) Study on the Chemical Constitu-ents of Fruits of Cannabis sativa L. Natural Product Research and Development, 21, 784-786.
- 10. Roxana, L., Andrea, S., Susanne, S., Christine, R., Liselotte, K. and Brigitte, K. (2000) Quantitative Analysis of the Pyrrolizidine Alkaloids Senkirkine and Senecionine in Tussilago farfara L. by Capillary Electrophoresis. Phytochemical Analysis, 11, 366-369. https://doi.org/10.1002/1099-1565(200011/12)11:6<366::AID-PCA538>3.0.CO;2-1
- 11. Yaoit, Y., Suzuki, N. and Kikuchi, M. (2001) Structures of New Sesquiterpenoids from Farfarae Flos. Chemical and Pharmaceutical Bulletin, 49, 645-648. https://doi.org/10.1248/cpb.49.645
- 12. Liu, Y.F., Yang, X.W. and Wu, B. (2007) Chemical Constit-uents of the Flower Buds of Tussilago farfara. Journal of Chinese Pharmaceutical Sciences, 16, 288-293.
- 13. Piers, E. and Gavai, A.V. (1990) A (Z)-Ethylidenecyclopentane Annulation Method. Total Syntheses of (±)-Anhydrooplopanone, (±)-Oplopanone, and (±)-8-epi-Oplopanone. The Journal of Organic Chemistry, 55, 2380-2390. https://doi.org/10.1021/jo00295a028
- 14. Kikuchi, M. and Suzuki, N. (1992) Studies on the Constituents of Tussi-lago farfara L. II. Structures of New Sesquiterpenoids Isolated from the Flower Buds. Chemical and Pharmaceutical Bulletin, 40, 2753-2755. https://doi.org/10.1248/cpb.40.2753
- 15. Park, H.R., Yoo, M.Y., Seo, J.H., Kim, I.S., Kim, N.Y., Kang, J.Y., Cui, L., Lee, C.S., Lee, C.H. and Lee, H.S. (2008) Sesquiterpenoids Isolated from the Flower Buds of Tussilago farfara L. Inhibit Diacylglycerol Acyltransferase. Journal of Agricultural and Food Chemistry, 56, 10493-10497. https://doi.org/10.1021/jf801978r
- 16. Yaoit, Y., Suzuki, N. and Kikuchi, M. (2001) Structures of New Sesquit-erpenoids from Farfarae Flos. Chemical and Pharmaceutical Bulletin, 49, 645-648. https://doi.org/10.1248/cpb.49.645
- 17. Baba, H., Yaoita, Y. and Kikuchi, M. (2007) Sesquiterpenoids from Ligu-laria dentata. Helvetica Chimica Acta, 90, 1302-1312. https://doi.org/10.1002/hlca.200790131
- 18. 王建全, 张亚娜, 林敏. 乙醇/硫酸铵双水相体系分离纯化款冬花总黄酮[J]. 井冈山大学学报(自然科学版), 2016, 37(1): 40-45.
- 19. Editorial Committee of Flora of China. Chinese Academy of Sciences (1999) Flora of China. Vol. 77(1), Science Press, Beijing, 93.
NOTES
*通讯作者。