Advances in Clinical Medicine
Vol.
10
No.
04
(
2020
), Article ID:
35176
,
7
pages
10.12677/ACM.2020.104087
Correlation between Erythropoietin and Tumor Necrosis Factor Alpha in Children with Malignant Tumor Anemia
Junnan Hao1, Wufeng Xu1, Min Wei1, Lingzhen Wang1, Qianwen Xu2, Lirong Sun1*
1Department of Hematology Pediatrics, The Affiliated Hospital of Qingdao University, Qingdao Shandong
2Department of Pediatrics, Qingdao Branch of Qilu Hospital, Qingdao Shandong

Received: Apr. 1st, 2020; accepted: Apr. 15th, 2020; published: Apr. 22nd, 2020

ABSTRACT
Objective: This article aims to investigate the levels of erythropoietin (EPO) and tumor necrosis factor (TNF)-α in children with tumor-associated anemia (CRA), and to investigate the relationship between them and their correlation with hemoglobin (Hb) levels. Method: Patients (n = 108) with CRA or non-neoplastic hematological anemia and healthy control are selected. The chemiluminescence method was used to detect the serum level of EPO and TNF-α. The correlation between EPO and Hb, TNF-α and Hb, EPO and TNF-α is analyzed. Results: The levels of serum EPO and TNF-α in children with CRA were significantly higher than those in patients with non-anemia and healthy control (p < 0.05). But there was no significant difference compared with non-neoplastic hematological children (p > 0.05). Furthermore, the expected inverse linear relation between serum EPO and Hb levels was found in CRA, and so did the relation between serum TNF-α and Hb levels (r = −0.41, −0.48, p < 0.05). But there was no correlation between TNF-α and EPO or O/P (r = −0.16, −0.24, p > 0.05). Conclusion: The feedback hyperplasia of endogenous EPO to anemia in children’s CRA is sufficient. TNF-α is involved in CRA as a negative regulator, but its mechanism of causing anemia is not related to the inhibition of EPO production.
Keywords:Tumor-Associated Anemia, Erythropoietin, Tumor Necrosis Factor-Alpha, Children

肿瘤相关性贫血儿童促红细胞生成素和肿瘤坏死因子α相关性研究
郝俊楠1,徐武凤1,魏敏1,王玲珍1,许倩文2,孙立荣1*
1青岛大学附属医院血液儿科,山东 青岛
2齐鲁医院青岛分院儿科,山东 青岛

收稿日期:2020年4月1日;录用日期:2020年4月15日;发布日期:2020年4月22日

摘 要
目的:本文旨在检测肿瘤相关性贫血(Cancer-Related Anemia, CRA)儿童促红细胞生成素(EPO)和肿瘤坏死因子(TNF)-α水平,以探讨两者及分别与血红蛋白(Hemoglobin, Hb)水平的相关性。方法:对恶性肿瘤(包括肿瘤贫血42例和肿瘤非贫血33例)及非肿瘤性贫血18例、健康查体儿童15组例采用化学发光法测定血清中EPO、TNF-α水平,分析EPO与Hb、TNF-α与Hb及EPO与TNF-α的相关性。结果:肿瘤贫血患儿血清EPO、TNF-α水平明显高于肿瘤非贫血及健康患儿(p < 0.05),肿瘤贫血患儿血清EPO与非肿瘤贫血比较差异无统计学意义(p > 0.05);肿瘤贫血组患儿血清EPO、TNF-α与Hb均呈负相关关系(r = −0.41、−0.48,p均<0.05),TNF-α与EPO及O/P相关性均无统计学意义(r = −0.16、−0.24,p均>0.05)。结论:儿童CRA中内源性EPO对贫血的反馈性增生是充分的,TNF-α作为负向调控因子参与CRA,但其引起贫血的机制与抑制EPO产生无关。
关键词 :肿瘤相关性贫血,促红细胞生成素,肿瘤坏死因子肿瘤α,儿童

Copyright © 2020 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
不论肿瘤患者有无接受治疗,贫血都是常见的,被命名为肿瘤相关性贫血(CRA)。目前最大的调查研究之一,欧洲癌症贫血调查(ECAS) [1] 中发现,15,367名接受化疗的患者中有39%在随后6个月被发现贫血(Hb < 10.0 g/dL)。CRA的发病机理很复杂,通常很难识别,在大多数情况下是多因素的,并且与非CRA患者相比,有CRA病史的患者有可能会导致更加严重程度的贫血 [2]。
CRA患者中有一部分没有明确病因,这种情况下的贫血多归为慢性病贫血(ACD) [3]。潜在机制目前尚未完全清楚,但认为涉及肿瘤坏死因子(TNF)-α、白介素(IL)-1等炎性细胞因子 [4]。这些细胞因子可能抑制内源性EPO生成,损害造血过程中铁利用并减少红细胞前体的增殖 [5]。这种慢性炎症状态还会引起机体营养和代谢的变化,如体重减轻、低白蛋白血症、活性氧(ROS)增加等 [6]。这些因素交互作用,也使机体产生对EPO的抵抗。因此,分析所有导致CRA的因素至关重要,尤其在开始可能加剧贫血的任何治疗之前。
本研究旨在明确儿童CRA血清EPO水平变化,有无EPO绝对或相对缺乏,以及EPO与TNF-α水平的相关性,明确有无以TNF-α为代表的炎症细胞因子的增高导致机体对EPO抵抗和钝化,分析CRA的发病机制。
2. 对象与方法
2.1. 对象
收集2018年05月至2019年12月青岛大学附属医院医院血液儿科收治患儿为研究对象。恶性肿瘤组:75例,原发疾病为急性淋巴细胞白血病(ALL)、急性髓细胞白血病(AML)、淋巴瘤、肾母细胞瘤、神经母细胞瘤等,均符合相关疾病诊断标准,其中贫血组42(男26女16)例,年龄1~14 (7.2 ± 4.8)岁;未贫血组33 (男17女16)例,年龄1~13 (5.3 ± 2.7)岁;非肿瘤性贫血组:18 (男8女10)例,年龄1~15 (6.5 ± 4.2)岁,原发疾病为缺铁性贫血(IDA)、巨幼细胞性贫血等有非肿瘤性血液病;健康对照组:15 (男8女7)例,年龄1~10 (4.2 ± 3.8)岁,因健康查体于门诊就诊,无贫血且既往无慢性疾病及血液病史。以上所有患儿肾功能都正常。贫血的定义:6~59个月:<11.0 g/dL;5~11岁:<11.5 g/dL;12~16岁:<12.0 g/dL [7]。排除标准为:1) 病例资料极度不完整者;2) 伴有明显低氧血症;3) 有溶血及急、慢性失血,有贫血家族史;4) 合并其他慢性炎症性疾病如支气管哮喘、类风湿关节炎、炎症性肠病等;5) 目前有铁剂、维生素B12或叶酸治疗;6) 2周内有放化疗史,3月内有输血、ESAs及铁剂治疗史,近半年有手术史。分析EPO时采用的对照组:非肿瘤性贫血组18例(研究表明IDA患者EPO的反应增生能力正常)、健康对照组15例;分析TNF-α时对照组:健康对照组15例。本研究通过青岛大学附属医院医学伦理委员会批准。
2.2. 方法
2.2.1. 标本采集与处理
抽取晨醒后患儿外周静脉血3~4 ml放置于添加了分离胶–促凝剂的试管中混匀,4℃,3000 r/min离心5 min,取上层血清置于−80℃冰箱中冷冻保存。
2.2.2. 血清EPO、TNF-α检测
采用化学发光免疫分析法检测样本血清EPO、TNF-α浓度,试剂盒均购于德国Siemens公司。
2.2.3. 血清EPO分析
取血清EPO的对数log10EPO (lgEPO)进行数值转换,对Hb和EPO有相关性的组别进行Hb与lgEPO的简单线性回归分析,得到回归方程(lgEPO = 截距 + 斜率*Hb)。应用回归方程计算相应Hb水平下lgEPO预计值,并计算实测lgEPO/预计lgEPO(简写:O/P)。贫血患者的O/P < 0.80被视为EPO生成减弱 [8],正常对照为1.01 ± 0.11 [9]。
2.2.4. 统计学分析
计量资料的统计描述采用均数 ± 标准差进行表示。多组间比较先经方差分析后采用SNK-q检验,不满足方差齐性假设是应用校正的Welch方差分析后采用Games-Howell检验。计量资料之间相关关系的研究采用Pearson相关分析。p < 0.05表示差异有统计学意义。应用SPSS24.0对数据进行统计分析。
3. 结果
3.1. 不同组别血清EPO水平变化
各组间血清EPO水平差异有统计学意义(F = 13.25, p < 0.05)。肿瘤贫血组血清EPO水平[(341.73 ± 314.00) IU/L]明显高于肿瘤非贫血组EPO水平[(86.00 ± 14.62) IU/L]及健康对照组EPO水平[(32.78 ± 45.12) IU/L] (p均<0.05),但与非肿瘤贫血组[(235.26 ± 312.23) IU/L]比较差异无统计学意义。详见表1。
3.2. 不同组别血清EPO水平与Hb的相关性分析
肿瘤贫血组血清EPO与Hb呈负相关关系(r = −0.41, p < 0.01);肿瘤非贫血组EPO与Hb相关性无统计学意义(r = −0.26, p > 0.05);非肿瘤贫血组EPO与 Hb相关性无统计学意义(r = −0.21, p > 0.05);健康对照组EPO与 Hb相关性无统计学意义(r = −0.14, p > 0.05)。
Table 1. Serum EPO and TNF-α levels in different groups ( χ ¯ ± s )
表1. 不同组别血清EPO、TNF-α水平( )
注:与健康对照组、肿瘤非贫血组比较,*p < 0.05。
建立肿瘤品贫血组lgEPO与Hb的回归方程:lgEPO = 3.85 − 0.02Hb (R2 = 0.62, p < 0.05) (见图1),lgEPO O/P 0.95 ± 0.28,有19.05% (8/42) O/P < 0.8。
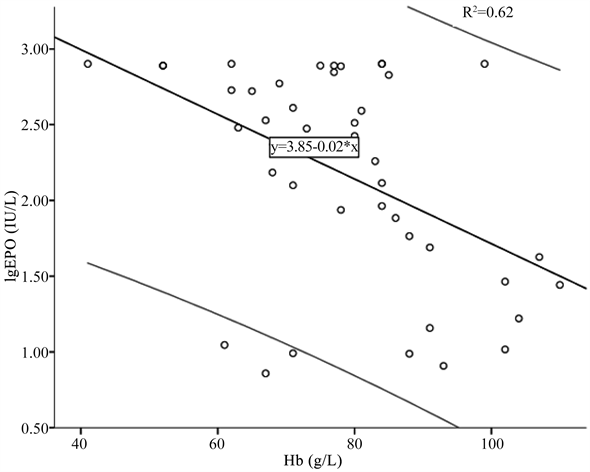
Figure 1. lgEPO and Hb linear regression lines (black lines) in tumor anemia group and 90% forecast period (grey line) (R2 = 0.62, p < 0.05)
图1. 肿瘤贫血组lgEPO与Hb的线性回归线(黑色线)和90%预测期间(灰色线) (R2 = 0.62, p < 0.05)
3.3. 不同组别血清TNF-α水平变化
各组间血清TNF-α水平差异有统计学意义(F = 9.16, p < 0.05)。肿瘤贫血组血清TNF-α水平[(11.64 ± 9.19) pg/mL]明显高于肿瘤非贫血组TNF-α水平[(4.22 ± 2.52) pg/mL]及健康对照组TNF-α水平[(3.16 ± 0.75) pg/ml] (p均<0.05) (血清TNF-α正常参考范围:0~8.10 pg/mL)。
3.4. 不同组别血清TNF-α水平与Hb的相关性分析
肿瘤贫血组血清TNF-α与Hb呈负相关关系(r = −0.48, p < 0.05);肿瘤非贫血组血清TNF-α与Hb相关性无统计学意义(r = −0.25, p > 0.05);健康对照组血清TNF-α与Hb相关性无统计学意义(r = −0.19, p > 0.05)。
3.5. 不同组别血清TNF-α水平与EPO的相关性分析
肿瘤贫血组血清TNF-α与EPO及O/P相关性均无统计学意义(r = −0.16, p > 0.05; r = −0.24, p > 0.05);肿瘤非贫血组血清TNF-α与EPO相关性均无统计学意义(r = −0.23, p > 0.05);健康对照组血清TNF-α与EPO相关性均无统计学意义(r = −0.21, p > 0.05)。
4. 讨论
贫血,其表现是外周血RBC数减少或Hb浓度降低,是影响肿瘤患者预后的独立危险因素 [10]。恶性肿瘤患儿贫血严重程度可能与多种变量相关联,包括患儿的年龄、疾病的程度、性质和持续治疗的时间等。还有学者提出特定方面的营养缺乏、骨髓的肿瘤浸润及并发感染等因素也参与了贫血的发病过程 [11]。这些因素对于贫血是单独还是协同发挥作用目前尚不清楚。
EPO是一种调控红细胞生成的糖蛋白。据文献报道,肾功能正常的贫血患者EPO合成机制尚完整,血清EPO水平增高,其贫血机制与EPO水平无关 [12]。将单纯IDA患者做正常参照,成人CRA患者的EPO水平相对于前者是低的,说明CRA患者的EPO对贫血的反应是钝化的 [13]。故在本研究中,纳入包括IDA患者在内的其他非肿瘤性贫血病例。与EPO水平正常的健康对照组相比,CRA患者和非肿瘤性贫血患者EPO均是明显升高的,但两者比较无差异,不存在EPO的钝化,与Corazza等 [14] 的研究结果相似。但非肿瘤性贫血病例除5例IDA外,还有其他类型的贫血,其贫血对EPO的反应是否一致有待进一步研究。
有研究表明ALL贫血患儿血清EPO水平和Hb水平之间存在反比关系,并且可以通过反馈方式调节Hb水平 [15]。本研究中在肿瘤贫血组中发现了这种反比关系,并对EPO和Hb进行线性回归分析得到O/P,超过80%的病例O/P > 0.8,再次证明患儿EPO对贫血反应无钝化 [16]。本研究纳入对象均为肾功能正常者,其中有原发部位在肾脏的肿瘤如肾母细胞瘤患者5例,在贫血状态下有4例血清EPO水平是升高的,另有1例为一侧肾切除术后,其EPO无升高,O/P < 0.8,其贫血原因不能简单的归为EPO生成的缺乏,肾切除术可能与其发病相关。虽然CRA中EPO反馈性升高,但贫血无纠正。慢性炎症及营养代谢障碍对红细胞生成的抑制作用,可能与之有关。
ACD又名炎症性贫血,是由慢性感染、恶性肿瘤和自身免疫性疾病等因素引起,缓解期贫血可能是ACD的一种表现形式 [17]。这种炎症状态表现为CRP升高、体重减轻、低白蛋白血症和EPO抵抗性贫血,并涉及免疫、营养和代谢多种发病机制 [18]。炎症本身也可干扰营养状态,继而使贫血加重。炎症细胞因子如IL-1和TNF-α等,既可以抑制红系祖细胞的增殖,也钝化了血清EPO对Hb下降的正常反应,参与贫血的发生 [19]。TNF-α与炎症和肿瘤密切相关,在多种肿瘤微环境中均有表达,并被归类为肿瘤促进剂 [20]。本研究发现CRA中TNF-α与Hb两者呈负相关,说明TNF-α抑制了红细胞的生成。同时,TNF-α也可直接抑制EPO的生成 [21]。但也有研究表明EPO可通过巨噬细胞诱导一氧化氮合成酶抑制TNF-α、IL-6等炎性因子的形成 [22]。但Tsopra等 [23] 在慢性淋巴细胞白血病中的证实,贫血的发生独立于EPO生成和刺激,可能是TNF-α对红细胞生成早期红系发育的直接抑制作用导致。本研究对CRA中EPO及O/P与TNF-α进行分析,均未发现相关性,与以上研究结果一致。
综上所述,在儿童肿瘤贫血患儿中,EPO和TNF-α均参与贫血的发生。内源性EPO对贫血的反馈性增生是充分的,TNF-α作为负向调控因子参与贫血的机制与抑制EPO产生无关,与成人研究结果相反 [24]。有必要进行更多研究了解儿童CRA中EPO和TNF-α之间的分子调节机制,更好地了解CRA的发病机制指导预防和治疗。
文章引用
郝俊楠,徐武凤,魏 敏,王玲珍,许倩文,孙立荣. 肿瘤相关性贫血儿童促红细胞生成素和肿瘤坏死因子α相关性研究
Correlation between Erythropoietin and Tumor Necrosis Factor Alpha in Children with Malignant Tumor Anemia[J]. 临床医学进展, 2020, 10(04): 555-561. https://doi.org/10.12677/ACM.2020.104087
参考文献
- 1. Ludwig, H., Van Belle, S., Barrett-Lee, P., et al. (2004) The European Cancer Anaemia Survey (ECAS): A Large, Multinational, Prospective Survey Defining the Prevalence, Incidence, and Treatment of Anaemia in Cancer Patients. European Journal of Cancer, 40, 2293-2306. https://doi.org/10.1016/j.ejca.2004.06.019
- 2. Rodgers, G.M., Becker, P.S., Blinder, M., et al. (2012) Cancer and Chemotherapy-Induced Anemia. Journal of the National Compre-hensive Cancer Network, 10, 628-653. https://doi.org/10.6004/jnccn.2012.0064
- 3. Cullis, J. (2013) Anaemia of Chronic Disease. Clinical Medicine, 13, 193-196. https://doi.org/10.7861/clinmedicine.13-2-193
- 4. Macciò, A. and Madeddu, C. (2012) Inflammation and Ovarian Cancer. Cytokine, 58, 133-147. https://doi.org/10.1016/j.cyto.2012.01.015
- 5. Bode, J.G., Albrecht, U., Hussinger, D., et al. (2012) Hepatic Acute Phase Proteins—Regulation by IL-6- and IL-1-Type Cytokines Involving STAT3 and Its Crosstalk with NF-κB-Dependent Signaling. European Journal of Cell Biology, 91, 496-505. https://doi.org/10.1016/j.ejcb.2011.09.008
- 6. Kundu, J.K. and Surh, Y.J. (2012) Emerging Avenues Linking Inflammation and Cancer. Free Radical Biology & Medicine, 52, 2013-2037. https://doi.org/10.1016/j.freeradbiomed.2012.02.035
- 7. 王卫国, 孙锟, 等. 儿科学[M]. 第9版. 北京: 人民卫生出版社, 2018: 331-332.
- 8. Cazzola, M. (2003) Erythropoietin Therapy: Need for Rationality and Active Surveil-lance. Haematologica, 88, 601-605.
- 9. Manfred, N., et al. (2016) Iron Deficiency or Anemia of Inflammation: Differ-ential Diagnosis and Mechanisms of Anemia of Inflammation. Wiener Medizinische Wochenschrift, 166, 411-423. https://doi.org/10.1007/s10354-016-0505-7
- 10. Victor, P., Paitan, A., Cindy, L., et al. (2018) Anemia as a Prognostic Factor in Cancer Patients. La Revista Peruana de Medicina Experimental y Salud Pública, 35, 250-258. https://doi.org/10.17843/rpmesp.2018.352.3171
- 11. Grotto, H.Z.W. (2008) Anaemia of Cancer: An Overview of Mechanisms Involved in Its Pathogenesis. Medical Oncology, 25, 12-21. https://doi.org/10.1007/s12032-007-9000-8
- 12. Nayak-Rao, S. and Mccormick, B. (2013) Erythropoietin Use in CKD Patients with Cancer: To Tread with Caution? Journal of Nephrology, 26, 829-835. https://doi.org/10.5301/jn.5000316
- 13. Blindar, V., Zubrikhina, G., Davydova, T., et al. (2019) Development of Strategic Approaches to Modern Diagnosis of Anemic Syndrome in Patients with Breast Cancer. Kliniceskaja Laboratornaja Diagnostika, 64, 210-215. https://doi.org/10.18821/0869-2084-2019-64-4-210-215
- 14. Corazza, F., Beguin, Y., Bergmann, P., et al. (1998) Anemia in Children with Cancer Is Associated with Decreased Erythropoietic Activity and Not with Inadequate Erythropoietin Production. Blood, 92, 1793-1798. https://doi.org/10.1182/blood.V92.5.1793
- 15. Steele, M.G. and Narendran, A. (2012) Mechanisms of Defective Erythropoiesis and Anemia in Pediatric Acute Lymphoblastic Leukemia (ALL). Annals of Hematology, 91, 1513-1518. https://doi.org/10.1007/s00277-012-1475-5
- 16. Tisi, M.C., Bozzoli, V., Giachelia, M., et al. (2014) Anemia in Diffuse Large B-Cell Non-Hodgkin Lymphoma: The Role of Interleukin-6, Hepcidin and Erythropoietin. Leukemia & Lymphoma, 55, 270-275. https://doi.org/10.3109/10428194.2013.802314
- 17. Demarmels Biasiutti, F. (2010) Anemia of Chronic Disorder: Pathogenesis, Clinical Presentation and Treatment. Therapeutische Umschau Revue Thérapeutique, 67, 225-228. https://doi.org/10.1024/0040-5930/a000041
- 18. Maccio, A., Madeddu, C., Gramignano, G., et al. (2015) The Role of Inflammation, Iron, and Nutritional Status in Cancer-Related Anemia: Results of a Large, Prospective, Observational Study. Haematologica, 100, 124-132. https://doi.org/10.3324/haematol.2014.112813
- 19. Buck, I., Morceau, F., Grigorakaki, C., et al. (2009) Linking Anemia to Inflammation and Cancer: The Crucial Role of TNFα. Biochemical Pharmacology, 77, 1572-1579. https://doi.org/10.1016/j.bcp.2008.12.018
- 20. Atretkhany, K.N., Gogoleva, V.S. and Drutskaya, M.S. (2020) Distinct Modes of TNF Signaling through Its Two Receptors in Health and Disease. Journal of Leukocyte Biology, 1-13. https://doi.org/10.1002/JLB.2MR0120-510R
- 21. Ek, T., Mellander, L. and Abrahamsson, J. (2005) Interferon γ and Tumour Necrosis Factor α in Relation to Anaemia and Prognosis in Childhood Cancer. Acta Paediatrica, 94, 435-437. https://doi.org/10.1111/j.1651-2227.2005.tb01914.x
- 22. Nairz, M., Sonnweber, T., Schroll, A., et al. (2012) The Pleiotropic Effects of Erythropoietin in Infection and Inflammation. Microbes & Infection, 14, 238-246. https://doi.org/10.1016/j.micinf.2011.10.005
- 23. Tsopra, O.A., Ziros, P.G., Lagadinou, E.D., et al. (2009) Dis-ease-Related Anemia in Chronic Lymphocytic Leukemia Is Not Due to Intrinsic Defects of Erythroid Precursors: A Pos-sible Pathogenetic Role for Tumor Necrosis Factor-Alpha. Acta Haematologica, 121, 187-195. https://doi.org/10.1159/000220331
- 24. 刘东芳, 张成侠, 冯志刚, 等. 铁调素、IFN-γ、TNF-α等负调控因子对肿瘤性贫血患者红系增生的影响[J]. 医学临床研究, 2015(10): 1940-1942.
NOTES
*通讯作者。
