Advances in Clinical Medicine
Vol.
13
No.
10
(
2023
), Article ID:
73962
,
7
pages
10.12677/ACM.2023.13102293
胃泌酸腺肿瘤的临床、内镜及组织学特点
刘婉婷1,谷海燕2,魏良洲3*
1青岛大学医学部,山东 青岛
2青岛大学附属医院病理科,山东 青岛
3青岛大学附属医院消化内科,山东 青岛
收稿日期:2023年9月19日;录用日期:2023年10月13日;发布日期:2023年10月19日

摘要
目的:探讨胃泌酸腺肿瘤的临床、内镜及病理特征,以提高对该疾病的认识、诊断及治疗水平。方法:收集2020年11月至2022年9月青岛大学附属医院收治的3例泌酸腺腺瘤和4例胃底腺型胃癌的临床病理资料,分析肿瘤内镜下表现、病理特征及免疫组织化学结果,并结合文献进行探讨。结果:7例患者年龄55~71岁,平均年龄及中位年龄均为63岁,其中女性5例,男性2例,主要因腹部不适及查体发现,均无HP感染,接受内镜下黏膜剥离术(ESD)者5例,接受内镜下黏膜切除术(EMR)者2例,1例GA-FG浸润深度达1000 um,术后存在黏膜下层增生。病灶最大直径为0.3~1.5 cm。GOGA及GA-FG均以主细胞成分为主,肿瘤表面被小凹上皮所覆盖,细胞异型性小,排列成不规则的腺管状,均未见明显核分裂象,肿瘤周围黏膜均未见HP感染、萎缩及肠化。免疫表型显示MUC6和Pepsinogen呈弥漫强阳性表达,其中2例患者H+/K+-ATPase细胞阳性,MUC5AC只在覆盖在肿瘤表面的小凹上皮中表达,肿瘤细胞未见表达,CD10、MUC2、CDX-2均为阴性,热点区域Ki-67增殖指数为1%~10%,未见P53高表达和β-catenin核阳性表达。结论:胃泌酸腺肿瘤是非常罕见且具有独特病理学特征的肿瘤,预后较好,内镜下病变切除术可基本治愈,但目前对其认识不足,容易漏诊及误诊,因此熟练掌握其内镜下特点和组织学形态对提高消化内科医师的诊断及治疗能力具有重大的意义。
关键词
胃泌酸腺肿瘤,泌酸腺腺瘤,胃底腺型胃癌

Clinical, Endoscopic and Histological Features of Gastric Acid Gland Tumors
Wanting Liu1, Haiyan Gu2, Liangzhou Wei3*
1Medical Science Center, Qingdao University, Qingdao Shandong
2Department of Pathology, The Affiliated Hospital of Qingdao University, Qingdao Shandong
3Department of Gastroenterology, The Affiliated Hospital of Qingdao University, Qingdao Shandong
Received: Sep. 19th, 2023; accepted: Oct. 13th, 2023; published: Oct. 19th, 2023

ABSTRACT
Objective: To explore the clinical, endoscopic and pathological features of gastric urinary gland tumours in order to improve the understanding, diagnosis and treatment of this disease. Methods: Clinicopathological data of 3 cases of urinary gland adenoma and 4 cases of gastric carcinoma of fundic gland type adenocarcinoma admitted to Affiliated Hospital of Qingdao University from November 2020 to September 2022 were collected, and endoscopic manifestations of the tumours, pathological features and immunohistochemical results were analysed and discussed in conjunction with the literature. Results: Seven patients were aged 55~71 years, with a mean and median age of 63 years, including five females and two males, mainly due to abdominal discomfort and findings on physical examination, none of them had HP infection, five underwent endoscopic mucosal dissection (ESD), and two underwent endoscopic mucosal resection (EMR), and in one case, the depth of infiltration of GA-FG was up to 1,000 um, and there existed submucosal hyperplasia after surgery. The maximum diameter of the lesion was 0.3~1.5 cm. Both GOGA and GA-FG were dominated by the principal cellular component, and the surface of the tumour was covered by a small concave epithelium, with small cellular isoforms arranged in irregular glandular tubular shapes, and none of them showed obvious nuclear schizophrenia, and none of the peripheral mucosae of the tumour was HP infected, atrophied or enteric. Immunophenotyping showed that MUC6 and Pepsinogen were diffusely and strongly positively expressed, of which two patients were positive for H+/K+-ATPase cells, MUC5AC was only expressed in the small concave epithelium covering the surface of the tumour, and the tumour cells did not express it, CD10, MUC2 and CDX-2 were negative, and the Ki-67 proliferation index in hotspots was between 1% and 10%, and the high expression of P53 and β-cellulose nuclear positive expression were not seen. Conclusions: Gastric urinary gland tumours are very rare tumours with unique pathological features, with good prognosis, and can be basically cured by endoscopic resection of the lesions, but they are currently under-recognised and easily missed and misdiagnosed, so proficiency in their endoscopic features and histological morphology is of great significance in improving the diagnostic and therapeutic ability of gastroenterologists.
Keywords:Gastric Oxyntic Gland Tumour, Gastric Oxyntic Gland Adenoma, Gastric Adenocarcinoma of the Fundic Gland

Copyright © 2023 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
来源于胃分泌腺的肿瘤统称为胃泌酸腺肿瘤(gastric oxyntic gland tumour, GOGT),是近年来新发现的肿瘤类型。其中据2019年世界卫生组织(WHO)消化系统肿瘤分类将局限于黏膜内的泌酸腺肿瘤分类为泌酸腺腺瘤(gastric oxyntic gland adenoma, GOGA),将伴有黏膜下浸润的泌酸腺肿瘤分类为胃底腺型腺癌(gastric adenocarcinoma of the fundic gland, GA-FG) [1] 。GOGT是目前较为罕见的肿瘤类型,但随着内镜诊断技术的提高,检出率也随之升高,在日本的检出率高达0.36%,但美国报告的病例却不到25例 [2] 。本文中,我们通过7个病例结合文献复习进一步总结其临床病理特征并探讨其发病机制,为下一步诊疗提供更多思路。
2. 资料与方法
2.1. 临床资料
收集2020年11月至2022年9月青岛大学附属医院收治的3例GOGA和4例GA-FG患者资料,均已签署胃镜检查知情同意书且给予病理处理权限。7例患者年龄55~71岁,平均年龄及中位年龄均为63岁,其中女性5例,男性2例,主要因腹部不适及查体发现,均无HP感染,接受内镜下黏膜剥离术(ESD)者5例,接受内镜下黏膜切除术(EMR)者2例,1例GA-FG浸润深度达1000 um,术后复查黏膜下层增厚,与固有肌层分界清,考虑为黏膜下层增生(见表1)。
Table 1. Endoscopic clinical features of gastric acid gland adenoma and fundus adenocarcinoma
表1. 胃泌酸腺腺瘤和胃底腺型腺癌的内镜临床特征
2.2. 方法
利用普通白光内镜和窄带成像结合放大内镜(narrowband imaging combined with magnifying endoscopy, NBI-ME)技术分析GOGA和GA-FG内镜下特点。肿瘤位置根据胃体的分段可分为上段、中段和下段,根据胃壁的四等分方法分为胃前壁、胃后壁,胃大弯和胃小弯。术后标本通过HE染色后显微镜下观察并分析GOGA和GA-FG的病理组织特征,判断有无黏膜下浸润以及浸润深度。并采用免疫组化检查进行抗MUC5AC、MUC6、Pepsinogen、H+/K+-ATPase、Ki67、CD10、MUC2、CDX-2及p53标记。
3. 结果
3.1. 内镜表现
内镜下表现为肿瘤扁平隆起、表面黏膜发红,长径0.3~1.5 cm不等(图1(a))。NBI-ME示病变局部微结构紊乱,腺体开口不规则扭曲,窝间部(intervening part, IP)增宽,微血管略扩张,表面覆白色不透明物质(WOS) (图1(b)),靛胭脂染色后边界尚清晰(图1(c))。
3.2. 病理检查
7例患者中,肿瘤均位于黏膜固有层,未见脉管侵犯。其中3例GOGA无黏膜下浸润,3例GA-FG出现黏膜下浸润现象,1例GA-FG因胃镜下活检组织较少无法判断是否存在黏膜下浸润。GA-FG与GOGA在细胞形态及组织结构上表现相似,黏膜深层的肿瘤组织由类似主细胞及壁细胞的细胞组成,以主细胞为主,细胞呈柱状,细胞质轻度嗜碱性,细胞核位于基底部,可见轻度异型性;壁细胞呈卵圆形或圆锥形,细胞质细颗粒状,呈典型的嗜酸性,核居中深染。肿瘤表面被小凹上皮所覆盖,肿瘤细胞排列呈腺管状,伴有不同程度的腺管异常分支、融合,呈一种连续不断的形态,形成所谓的“无尽腺”结构,GOGA及GA-FG均未见明显的核分裂象,肿瘤周围黏膜均未见HP感染、萎缩或肠化(图1(d))。
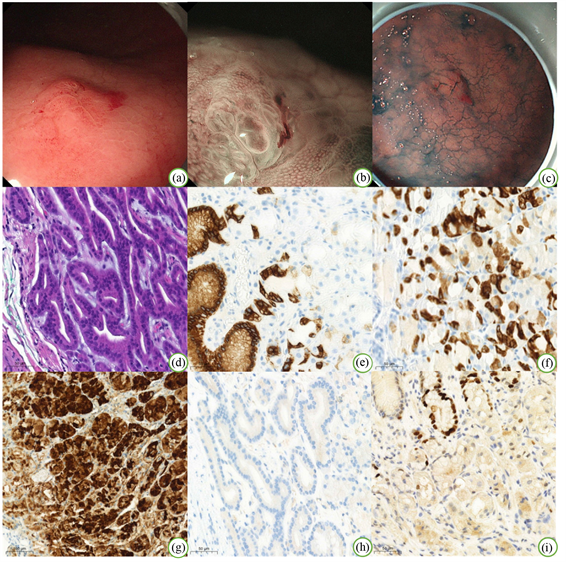 注:(a) 普通胃镜检查;(b) 放大胃镜 + 内镜窄带成像术;(c) 靛胭脂染色;(d) HE 200×;(e) 小凹上皮细胞MUC5AC阳性200×;(f) 肿瘤细胞MUC6弥漫阳性200×;(g) 肿瘤细胞Pepsinogen弥漫阳性200×;(h) 肿瘤细胞H+/K+-ATPase阴性200×;(i) 肿瘤细胞热点区域Ki-67 200×。
注:(a) 普通胃镜检查;(b) 放大胃镜 + 内镜窄带成像术;(c) 靛胭脂染色;(d) HE 200×;(e) 小凹上皮细胞MUC5AC阳性200×;(f) 肿瘤细胞MUC6弥漫阳性200×;(g) 肿瘤细胞Pepsinogen弥漫阳性200×;(h) 肿瘤细胞H+/K+-ATPase阴性200×;(i) 肿瘤细胞热点区域Ki-67 200×。
Figure 1. Endoscopic and pathological images
图1. 内镜下及病理图片
3.3. 免疫组化染色
7例患者免疫组化结果示,MUC6和Pepsinogen呈弥漫强阳性表达(图1(f)、图1(g)),其中2例患者 H+/K+-ATPase细胞阳性(图1(h)),MUC5AC只在覆盖在肿瘤表面的小凹上皮中表达,肿瘤细胞未见表达(图1(e)),CD10、MUC2、CDX-2均为阴性,热点区域Ki-67增殖指数为1%~10% (图1(i)),未见P53高表达和β-catenin核阳性表达。
4. 讨论
GOGA及GA-FG是近几年来新发现并命名的肿瘤类型,该类肿瘤相对罕见,在国内的报道案例亦极少。随着临床内镜及病理医师对该疾病认识的增加、内镜诊断技术的提高以及ESD技术的成熟,该病的检出率及相关报道也相应增多。但目前病因不明,与传统胃腺癌不同的是疾病的发生与幽门螺旋杆菌感染无关,一项研究中的8位患者中的有7位存在抑酸治疗病史,提示疾病的发生可能与抑酸治疗相关,可以推测主细胞增殖的连续性可能与胃酸环境的变化有关,病变的发生发展可能与胃内酸蚀有关 [3] [4] 。研究表明,患者一般为老年人,平均年龄为66岁,有轻微的男性偏好,男:女比例为2.2:1 [5] 。大部分患者无特殊临床表现,或仅因上腹隐痛就诊,常由体检胃镜筛查时发现。
胃黏膜层由小凹上皮及胃固有腺体组成,来源于二者之间的腺体颈部分泌的未分化细胞可分化为小凹上皮细胞、主细胞、壁细胞、颈部粘液细胞及内分泌细胞 [6] 。其中,颈部粘液细胞和主细胞属于同一细胞系,颈部粘液细胞被认为是主细胞的前体 [7] 。由类似胃底腺主细胞和壁细胞的肿瘤细胞构成了GOGT,按照腺体的分化特征,GOGT可分为主细胞为主型、壁细胞为主型及混合型三大类,但大多数报道为主细胞为主型肿瘤。
消化内科医师可根据内镜下表现进行初步判断。GOGT好发于胃体上1/3处(80%),胃体下1/3处的发生率仅为1%,主要发生在非萎缩性泌酸黏膜,且以隆起型肿瘤最常见,肿瘤平均直径为1 cm,报告的最大病例为8.5 cm [8] [9] 。肿瘤起源于胃底腺区的胃黏膜,表面几乎都是由正常的小凹上皮覆盖,黏膜深层的肿瘤组织由类似主细胞及壁细胞的细胞组成,肿瘤腺管异常分支、融合,呈一种连续不断的形态,即所谓的“无尽的腺体”模式 [9] 。通常,邻近的泌酸腺粘膜正常,没有任何肠化生、萎缩 [10] 。认为是一种表面呈SMT样抬高的病变,肿瘤表面有血管扩张或分支血管 [11] 。另外,多数病例在NBI-ME下表现为没有明确的分界线、腺开口部的扩大、隐窝间部的增宽以及缺乏不规则的微血管结构。GA-FG常侵犯黏膜下层,但仅表现出轻微的组织学异型性,采用靛胭脂染色后的内镜检查可显示病变中的黑色色素沉着,有助于早期病例的发现 [12] 。
免疫组化染色可协助判断肿瘤细胞类型。MUC5AC用于小凹上皮细胞的表达;MUC6用于颈粘液细胞或幽门腺细胞的表达;Pepsinogen用于主细胞的表达,H+/K+-ATPase用于壁细胞的表达 [13] [14] 。在GOGA及GA-FG中很少检测到MUC5AC阳性的肿瘤细胞,研究发现MUC5AC仅在体积大的和黏膜下浸润范围大的晚期GA-FG病变中表达,提示细胞分化在疾病进展过程中从胃底腺型转变为小凹上皮型 [8] 。由于肿瘤细胞大多数由主细胞或壁细胞构成,因此壁细胞型肿瘤细胞表达H+/K+-ATPase,主细胞型肿瘤细胞表达Pepsinogen,不成熟主细胞可以同时表达Pepsinogen和MUC6。
该疾病的发病机制可能与常规胃癌不同。先前的一项研究报告发现,GA-FG发生机制为CTNNB1、GNAS、AXIN和APC基因突变,β-连环蛋白核内聚集,提示WNT-β-环蛋白信号通路激活 [4] [15] [16] 。最近的研究表明,GNAS突变可能发生在部分的GA-FG中,作为激活WNT-β-连环蛋白信号通路的替代机制 [9] [17] [18] [19] 。虽然GOGA及GA-FG频繁出现GNAS激活突变,但GNAS突变在常规胃腺瘤和腺癌中却很少发生 [20] [21] 。值得注意的是,GNAS突变似乎与粘膜下侵袭和更大体积的肿瘤有关 [22] 。
GA-FG在胃早癌中约占1% [15] ,较为罕见,发病率低,与其他疾病的鉴别尤为重要。1) 神经内分泌肿瘤:通常呈黄色,外观坚实,肿瘤表面血管较少,炎性背景较轻,GA-FG的组织学特征与神经内分泌肿瘤相似,用常规HE染色进行区分较为困难,但免疫组织化学检查可将两者完全区分,神经内分泌肿瘤特异性的CgA、CD56及突触素阳性。2) 胃底腺息肉伴异型增生:由于胃底腺息肉与正常的胃底腺细胞相似,具有轻度异型性,GA-FG可误诊为胃底腺息肉,但伴有异型增生的胃底腺息肉往往涉及小凹上皮的异型,而不是深层的胃底腺区域。与胃底腺息肉相关的病变与周围粘膜有相对清晰的边缘,在内镜检查中没有SMT样抬高的形状。3) 深在性囊性胃炎:其特点是胃黏膜腺体向黏膜肌层以下生长,伴囊性扩张,周围围绕黏膜固有层的间质,利用超声内镜在粘膜下层检测到低回声病变有助于进行区分,病理组织学上没有细胞及结构的异型有助于排除恶性肿瘤可能性。4) 褪色凹陷型的GA-FG要与MALT淋巴瘤、未分化型胃癌、正常胃黏膜伴局灶性萎缩等签别,发红隆起型的GA-FG要与增生性息肉、树莓样的小凹上皮型腺癌鉴别:根据硬度、色调的不均匀,表面构造的不规则,扩张血管的分布等特征,基本上可以进行鉴别。在实际临床中,虽然存在难以鉴别的病变,但可以通过活检进行确诊。
在治疗方面,由于其肿瘤体积较小和良性的生物学行为,GOGT被认为是内镜切除的指征 [23] 。目前报道的绝大多数GA-FG仅浸润至表浅粘膜下层,不具有传统肠型或弥漫型胃癌的强侵袭性,并且遵循良性过程 [24] 。既往研究中有一例黏膜下浸润的GA-FG,在未行ESD之前随访了12年,肿瘤保持稳定,没有淋巴结或远处转移,切除后随访也未发现转移和复发,证明其生长缓慢 [12] 。然而,根据日本胃癌治疗指南,浸润粘膜下层深度超过500 um时,内镜下切除无法达到治愈标准 [25] 。肿瘤范围比较大、凹陷型、合并小凹型或像肠型粘液表型转化的时候,恶性程度及淋巴结转移的程度可能会相对高一点。大多数病例的行为和形态不足以证明所有病变都是普遍恶性的,尽管本研究中部分患者存在黏膜下浸润和结缔组织增生,这增加了潜在侵袭行为的可能性,但该疾病目前预后良好,无p53蛋白过表达或Ki-67的高标记指数,即使在浸润深的肿瘤中也很少发生转移和死亡 [11] [26] 。胃底腺型胃癌是否是由泌酸腺腺瘤发展而来的也存在争议,GOGA为良性肿瘤,但有较高的黏膜下浸润的风险(60%) [27] 。从分子生物学上看,似乎将GOGA视为GA-FG的前驱病变而非其浸润前状态更为可信,这些都有待于更多病例及更深入的分子病理的研究。也许,只有在将来报告更多长期复发的病例以及伴随的免疫组织化学和分子基础时,才能对该疾病进行更深入的了解与认识。
总之,GOGA是一种良性上皮性肿瘤,而GA-FG是一种预后较好、生长缓慢的低度恶性肿瘤,是否存在黏膜下层浸润是二者鉴别诊断的要点。GOGT发病机制及预后均异于传统的早期分化型胃腺癌,肿瘤体积小,细胞异型性小,易漏诊,且该类疾病的报道数量尚少,积累相应的病例可以提高临床及病理医师对该疾病的认识与了解,从而提高疾病的检出率,并给予对应治疗措施。
文章引用
刘婉婷,谷海燕,魏良洲. 胃泌酸腺肿瘤的临床、内镜及组织学特点
Clinical, Endoscopic and Histological Features of Gastric Acid Gland Tumors[J]. 临床医学进展, 2023, 13(10): 16387-16393. https://doi.org/10.12677/ACM.2023.13102293
参考文献
- 1. Nagtegaal, I.D., Odze, R.D., Klimstra, D., Paradis, V., Rugge, M., Schirmacher, P., Washington, K.M., Carneiro, F. and Cree, I.A. (2020) The 2019 WHO Classification of Tumours of the Digestive System. Histopathology, 76, 182-188. https://doi.org/10.1111/his.13975
- 2. Krooks, J., Thaker, H., Qiu, S., Reep, G. and He, J. (2023) Oxyntic Gland Adenoma in a Patient with Refractory Reflux. Cureus, 15, e38577. https://doi.org/10.7759/cureus.38577
- 3. Iwamuro, M., Kusumoto, C., Nakagawa, M., Matsueda, K., Kobayashi, S., Yoshioka, M., Inaba, T., Toyokawa, T., Sakaguchi, C., Tanaka, S., Tanaka, T. and Okada, H. (2022) Endoscopic Features of Oxyntic Gland Adenoma and Gastric Adenocarcinoma of the Fundic Gland Type Differ between Patients with and without Helicobacter pylori Infection: A Retrospective Observational Study. BMC Gastroenterology, 22, Arti-cle No. 294. https://doi.org/10.1186/s12876-022-02368-w
- 4. Li, C., Wu, X., Yang, S., Yang, X., Yao, J. and Zheng, H. (2020) Gastric Adenocarcinoma of the Fundic Gland Type: Clinicopathological Features of Eight Patients Treated with Endoscopic Submucosal Dissection. Diagnostic Pathology, 15, Article No. 131. https://doi.org/10.1186/s13000-020-01047-2
- 5. Yu, Y.J., Zheng, J.Q., Xu, J., Gan, M.F., Li, H., Fu, G.X., Weng, S.X., Wang, J.C. and Hu, X.T. (2023) Clinical and Pathological Characteristics of Gastric Adenocarcinoma of Fundic Gland Type. Chinese Medical Journal, 103, 1560-1562.
- 6. Nabil, T.M. and Moawad, U.K., (2021) Histo-morphological, Ultrastructural and Morphometrical Age-Related Changes of Fundic Region of New Zealand Rabbits (Oryctolagus cuniculus). Anatomia, Histologia, Embryologia, 50, 876-887. https://doi.org/10.1111/ahe.12731
- 7. Gómez-Santos, L., Alonso, E., Díaz-Flores, L., Madrid, J.F. and Sáez, F.J. (2017) Transdifferentiation of Mucous Neck Cells into Chief Cells in Fundic Gastric Glands Shown by GNA Lectin Histochemistry. Tissue and Cell, 49, 746-750. https://doi.org/10.1016/j.tice.2017.10.007
- 8. Ueyama, H., Yao, T., Nakashima, Y., Hirakawa, K., Oshiro, Y., Hirahashi, M., Iwashita, A. and Watanabe, S. (2010) Gastric Adenocarcinoma of Fundic Gland Type (Chief Cell Pre-dominant Type): Proposal for a New Entity of Gastric Adenocarcinoma. The American Journal of Surgical Pathology, 34, 609-619. https://doi.org/10.1097/PAS.0b013e3181d94d53
- 9. Meng, X.Y., Yang, G., Dong, C.J. and Zheng, R.Y. (2021) Gastric Adenocarcinoma of the Fundic Gland: A Review of Clinicopathological Characteristics, Treatment and Prognosis. Rare Tumors, 13, 1-7. https://doi.org/10.1177/20363613211060171
- 10. Matsumoto, K., Ueyama, H., Yao, T., Iwano, T., Yamamoto, M., Utsunomiya, H., Uchida, R., Abe, D., Oki, S., Suzuki, N., Ikeda, A., Yatagai, N., Akazawa, Y., Takeda, T., Ueda, K., Asaoka, D., Hojo, M. and Nagahara, A. (2022) Endoscopic Features of Gastric Epithelial Neoplasm of Fundic Gland Mucosa Lineage. Diagnostics, 12, Article 2666. https://doi.org/10.3390/diagnostics12112666
- 11. Yang, M., Sun, X., Chen, Y. and Yang, P. (2023) Twenty Cases of Gastric Adenocarcinoma of the Fundic Gland Type. Scandinavian Journal of Gastroenterology, 58, 744-750. https://doi.org/10.1080/00365521.2022.2164213
- 12. Sato, Y., Fujino, T., Kasagawa, A., Morita, R., Ozawa, S.I., Matsuo, Y., Maehata, T., Yasuda, H., Takagi, M. and Itoh, F. (2016) Twelve-Year Natural History of a Gastric Adeno-carcinoma of Fundic Gland Type. Clinical Journal of Gastroenterology, 9, 345-351. https://doi.org/10.1007/s12328-016-0680-5
- 13. 王涛, 罗华. 主细胞为主型胃泌酸腺肿瘤9例报道并文献复习[J]. 重庆医科大学学报, 2022, 47(2): 209-212.
- 14. Wang, J.Z., Huang, J.F. and He, Z.M. (2023) Gastric Adeno-carcinoma of Fundic Gland Type: A Clinicopathological Analysis of Five Cases. Chinese Journal of Pathology, 52, 838-840.
- 15. Hidaka, Y., Mitomi, H., Saito, T., Takahashi, M., Lee, S.Y., Matsumoto, K., Yao, T. and Watanabe, S. (2013) Alteration in the Wnt/β-Catenin Signaling Pathway in Gastric Neoplasias of Fundic Gland (Chief Cell Predomi-nant) Type. Human Pathology, 44, 2438-2448. https://doi.org/10.1016/j.humpath.2013.06.002
- 16. Ishizu, K., Hashimoto, T., Naka, T., Yatabe, Y., Kojima, M., Kuwata, T., Nonaka, S., Oda, I., Esaki, M., Kudo, M., Gotohda, N., Yoshida, T., Yoshikawa, T. and Sekine, S. (2021) APC Mutations Are Common in Adenomas But Infrequent in Ade-nocarcinomas of the Non-Ampullary Duodenum. Journal of Gastroenterology, 56, 988-998. https://doi.org/10.1007/s00535-021-01823-x
- 17. Kakumoto, A., Kuroda, H., Jamiyan, T., Shimakawa, T. and Masunaga, A. (2021) Gastric Adenocarcinoma of the Fundic Gland Type: A Case Report. American Journal of Case Reports, 22, e933474. https://doi.org/10.12659/AJCR.933474
- 18. Afolabi, H.A., Salleh, S.M., Zakaria, Z., Ch’ng, E.S., Mohd Nafi, S.N., Abdul Aziz, A.A.B., Irekeola, A.A., Wada, Y. and Al-Mhanna, S.B. (2022) A GNAS Gene Mutation’s Inde-pendent Expression in the Growth of Colorectal Cancer: A Systematic Review and Meta-Analysis. Cancers, 14, Article 5480. https://doi.org/10.3390/cancers14225480
- 19. Cho, H., Hashimoto, T., Naka, T., Yatabe, Y., Oda, I., Saito, Y., Yoshikawa, T. and Sekine, S. (2022) Activating KRAS and GNAS Mutations in Heterotopic Submucosal Glands of the Stomach. Journal of Gastroenterology, 57, 333-343. https://doi.org/10.1007/s00535-022-01863-x
- 20. Je, E.M., An, C.H., Chung, Y.J., Yoo, N.J. and Lee, S.H. (2015) GNAS Mutation Affecting Codon 201 Is Rare in Most Human Tumors. Pathology & Oncology Research, 21, 859-860. https://doi.org/10.1007/s12253-015-9919-6
- 21. Nong, Y., Zhang, Y., Zhang, Y., Pan, L., Chen, J., Zhu, C., Han, L., Li, A. and Liu, S. (2020) Analysis of Genetic Alterations Identifies the Frequent Mutation of GNAS in Colorectal Laterally Spreading Tumors. Cancer Communications, 40, 636-640. https://doi.org/10.1002/cac2.12085
- 22. Yang, G. (2022) Microsatellite Instability/Mismatch Repair Deficiency and Activation of the Wnt/β-Catenin Signaling Pathway in Gastric Adenocarcinoma of the Fundic Gland: A Case Report. Medicine, 101, e30311. https://doi.org/10.1097/MD.0000000000030311
- 23. Iwamuro, M., Kusumoto, C., Nakagawa, M., Kobayashi, S., Yoshioka, M., Inaba, T., Toyokawa, T., Hori, S., Tanaka, S., Matsueda, K., Tanaka, T. and Okada, H. (2021) Endoscop-ic Resection Is a Suitable Initial Treatment Strategy for Oxyntic Gland Adenoma or Gastric Adenocarcinoma of the Fun-dic Gland Type. Scientific Reports, 11, Article No. 7375. https://doi.org/10.1038/s41598-021-86893-w
- 24. Imamura, K., Yao, K., Nimura, S., Tanabe, H., Kanemitsu, T., Miyaoka, M., Ono, Y., Ueki, T. and Iwashita, A. (2021) Characteristic Endoscopic Findings of Gastric Adenocarcinoma of Fundic-Gland Mucosa Type. Gastric Cancer, 24, 1307-1319. https://doi.org/10.1007/s10120-021-01208-2
- 25. Japanese Gastric Cancer Association (2011) Japanese Gastric Cancer Treatment Guidelines 2010 (Ver. 3). Gastric Cancer, 14, 113-123. https://doi.org/10.1007/s10120-011-0042-4
- 26. Miyazawa, M., Matsuda, M., Yano, M., Hara, Y., Arihara, F., Horita, Y., Matsuda, K., Sakai, A. and Noda, Y. (2016) Gastric Adenocarcinoma of the Fundic Gland (Chief Cell-Predominant Type): A Review of Endoscopic and Clinicopathological Features. World Journal of Gastroenterology, 22, 10523-10531. https://doi.org/10.3748/wjg.v22.i48.10523
- 27. 潘升华. 第5版WHO消化系统肿瘤中泌酸腺腺瘤及胃底腺型腺癌的介绍及解读[C]//2020年浙江省医学会病理学术大会. 2020年浙江省医学会病理学术大会论文汇编. 2020: 325-327.
NOTES
*通讯作者Email: weiliangzhou62@162.com
