Hans Journal of Biomedicine
Vol.
11
No.
01
(
2021
), Article ID:
39866
,
6
pages
10.12677/HJBM.2021.111002
YAP/TAZ蛋白调控病毒侵染与免疫的 研究进展
邹丰,苟洪伟,黄金华,李晨辉,赵铁军*
浙江师范大学化学与生命科学学院,浙江 金华

收稿日期:2020年12月25日;录用日期:2021年1月8日;发布日期:2021年1月20日

摘要
YAP/TAZ介导的Hippo信号通路在生命进程中扮演重要角色,它主要调控器官大小,胚胎发育,细胞增殖以及肿瘤发生等。最新研究发现Hippo信号通路关键蛋白YAP/TAZ在调控人类病毒侵染及免疫反应中发挥非常重要的作用。文章总结了YAP/TAZ蛋白的病理学特征,重点介绍YAP/TAZ蛋白在调控人类病毒侵染、复制、诱发疾病以及免疫调控中的功能及其机理,以期为病毒致病机制研究及治疗手段开发提供新的见解和思路。
关键词
YAP/TAZ蛋白,Hippo信号通路,病毒侵染,免疫,发病机理
Research Progress of YAP/TAZ Protein Regulating Virus Infection and Immunity
Feng Zou, Hongwei Gou, Jinhua Huang, Chenhui Li, Tiejun Zhao*
College of Chemistry and Life Sciences, Zhejiang Normal University, Jinhua Zhejiang

Received: Dec. 25th, 2020; accepted: Jan. 8th, 2021; published: Jan. 20th, 2021

ABSTRACT
Accumulating evidence showed that YAP/TAZ mediated Hippo pathway plays a critical role in biological processes, including the control of organ size, embryonic development, cell proliferation, and cancer development. The Hippo signaling pathway key protein YAP/TAZ has been found to play a very important role in human virus infection and immunity. This paper summarizes the pathologic characteristics of YAP/TAZ protein, and focuses on the functions and mechanisms of YAP/TAZ protein in the regulation of human virus infection, replication, disease induction and immune regulation. It might give new insights into the research of viral disease and its therapy.
Keywords:YAP/TAZ, Hippo Signaling Pathway, Virus Infection, Immunity, Pathogenesis

Copyright © 2021 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
大量研究表明Hippo通路参与调控细胞增殖,器官大小 [1] [2]。近年来,Hippo信号通路在疾病调控中发挥着越来越重要的作用,尤其是YAP/TAZ蛋白对病毒侵染及免疫反应的调控受到广泛关注。研究报道Hippo/YAP通路在调节一种表达CXCR2的髓源性抑制细胞(MDSCs)的C-X-C基序趋化因子配体5 (CXCL5)时具有非自主功能。当癌细胞中YAP过度活化时,CXCL5分泌增强,进而募集更多MDSCs,促进肿瘤发生。在胰腺导管腺癌中,YAP促进MDSCs分化积累,调节免疫抑制;而当YAP或MDSCs缺失时,免疫反应增强 [3]。另有研究表明,YAP能够引导肿瘤相关巨噬细胞TAMs向免疫抑制/亲肿瘤M2表型分化。在小鼠肝脏肿瘤模型中,单个肿瘤起始细胞可通过YAP诱导CCL2和CSF1表达,促进髓源性抑制细胞和M2巨噬细胞的招募,进而促进肿瘤发生 [4]。文章总结了YAP/TAZ蛋白对病毒侵染的调控机制,同时综述了YAP/TAZ与免疫调控的研究进展。
2. Hippo通路及YAP/TAZ蛋白
Hippo通路是一条首先在果蝇中被发现的信号通路。Hippo通路在哺乳动物中同样高度保守,调节细胞接触抑制及肿瘤发生 [5] [6]。在哺乳动物细胞中,Hippo通路由TAOK1-3激酶磷酸化,进而激活MST1/2起始 [7]。随后,MST1/2通过与调节蛋白SAV1互作从而激活LATS1/2激酶活性 [8]。此外,MST1/2还可促进MOB1与LATS1/2互作,而MOB1的磷酸化可导致LATS1/2被完全激活 [9]。研究发现,神经纤维肿瘤抑制因子NF2通过MST1/2-SAV1复合物促进LATS1/2磷酸化 [10]。同样MAP4K家族、环腺苷酸(cAMP)也能激活LATS1/2活性,而LPA和S1P会抑制LATS1/2活性 [11] [12] [13]。LATS1/2激活可以直接磷酸化YAP和TAZ,使其滞留在胞浆中,从而抑制下游靶基因表达。LATS1/2激酶可以磷酸化YAP五个关键位点(TAZ四个),其中,YAPSer127/381位,TAZSer89/311位与蛋白入核及蛋白降解密切相关 [1]。YAPSer127/TAZS89位磷酸化后与14-3-3蛋白结合,使YAP蛋白定位在细胞质中。而YAPS381/TAZS311位磷酸化引起酪蛋白激酶1(CK1δ/ε)诱导的进一步磷酸化,从而使YAP/TAZ招募SCFβ-TRCP E3连接酶,从而走向泛素化及蛋白酶体依赖的降解途径 [14]。相反,当激酶模块失活时,低磷酸化的YAP/TAZ可以穿梭到细胞核,并通过与转录因子TEAD相互作用而充当转录共激活因子。TEAD是哺乳动物Hippo信号通路中关键的转录激活因子,而YAP被认为是TEAD的转录共激活因子,二者在细胞核内互作,诱导与细胞增殖,分化,发育和凋亡相关的下游靶基因表达,如结缔组织生长因子(CTGF)、富半胱氨酸诱导因子61(CYR61)、神经肽-1(NRP1)等 [15],最终影响细胞生长,增殖及迁移(见图1)。
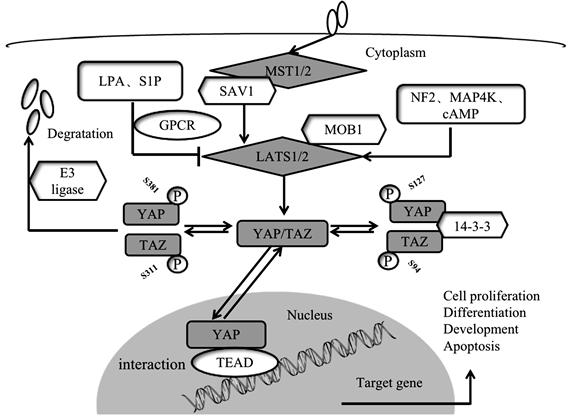
Figure 1. The Hippo signaling pathway and its key protein YAP/TAZ
图1. Hippo信号通路及其关键蛋白YAP/TAZ
3. YAP/TAZ病理学功能
YAP/TAZ蛋白异常调控会影响细胞增殖,凋亡,迁移和分化,从而导致包括癌症在内的多种疾病产生。在肝癌,食道癌,胃癌,前列腺癌,结肠癌,肺癌,乳腺癌等人类癌症中,检测到癌细胞中YAP/TAZ过表达及其在细胞核内定位 [16] [17] [18]。在肝癌,结肠癌,食道癌,卵巢癌病人中,YAP/TAZ过表达与患者不良预后密切相关 [19] [20]。在癌症发生过程中,Hippo信号通路异常调控主要是通过影响上游激酶LATS1/2和MST1/2的活性,从而导致YAP/TAZ低磷酸化及其富集于核内 [16]。
在哺乳动物中,YAP/TAZ蛋白也调控一些非肿瘤型疾病。例如,在一个长期神经受损的动物模型中,研究发现YAP/TAZ主要聚集在核内,由此导致外周神经损伤水平上升。研究发现在特异性缺失YAP/TAZ的小鼠心脏模型中,小鼠会表现出心脏发育缺陷的生理现象。此外,YAP过表达会激活心肌细胞增殖,而成年小鼠心脏中缺失SAV则会增强YAP表达,从而激活心肌细胞增殖 [21]。
越来越多研究表明,Hippo通路YAP/TAZ蛋白在调控人类病毒侵染诱导的肿瘤疾病、调控免疫反应等方面发挥关键作用。
4. YAP/TAZ蛋白对病毒侵染的调控作用
常见的人类病毒,例如HBV、HPV、KSHV、EBV、ZIKV等,均能通过调控Hippo信号通路,尤其通过调控YAP/TAZ蛋白的表达、磷酸化水平及影响其核定位从而促进疾病的发生。首先,病毒侵染后可通过其编码蛋白直接影响YAP/TAZ的表达,促进肿瘤发生。如HBV编码的HBx蛋白与CREB结合激活YAP启动子促进YAP表达。除了影响启动子活力外,HBx蛋白也能通过下调miRNA-375促进YAP表达 [22],preS2蛋白则通过抑制miRNA-338-3p促进TAZ蛋白表达,从而促进肝癌细胞的增殖及迁移 [23]。此外,HBx互作蛋白HBXIP还通过激活转录因子c-Myb上调肝癌细胞中YAP的表达,促进肝癌发生 [24]。EBV病毒感染通过其编码的LMP1蛋白促进TAZ表达,从而调控鼻咽癌发生 [25]。然而,在ZIKV感染模型中沉默YAP/TAZ表达,则可抑制ZIKV复制、增值,最终影响疾病发生 [26]。其次,病毒也能通过影响YAP磷酸化水平从而调控疾病发生。如HPV编码E6蛋白可下调YAPS397位磷酸化水平,从而促进宫颈癌发生 [27]。研究表明YAP/TAZ最终发挥功能一般通过入核后激活下游靶基因表达,从而促进肿瘤发生。如HPV编码的E6蛋白与PDZ结构蛋白互作后调控YAP的核定位,从而影响宫颈癌发生 [28]。这样的调控机制同样发生在EBV病毒上。EBV病毒编码的LMP1蛋白通过与凝溶胶蛋白相互作用抑制LATS1/2磷酸化,从而稳定了YAP/TAZ并促进其核转移,最终诱发鼻咽癌 [25]。最后,病毒也通过影响Hippo通路上游激酶活性进而影响YAP/TAZ活力,从而促进肿瘤疾病发生。例如KSHV编码的vGPCR蛋白通过抑制LATS1/2激酶活性,从而激活YAP/TAZ,最终促进肿瘤细胞增殖和转化 [29]。
综上,常见人类病毒主要通过其编码的病毒蛋白调控YAP/TAZ蛋白表达、上游激酶及自身磷酸化水平、以及YAP/TAZ蛋白的核定位等促进肿瘤等疾病的发生。
5. YAP/TAZ蛋白与免疫
天然免疫系统通过区别自身和非自身,构成对抗病毒侵染的第一道防线。研究表明Hippo通路中YAP蛋白负调控天然抗病毒免疫且YAP对天然免疫的调控独立于上游激酶。天然免疫细胞可通过细胞内受体识别病毒,如RIG-I,cGAS等。病毒侵染后,胞内受体RIG-I和cGAS可激活IKKε及TBK1激酶,进而招募IRF3,促进IRF3磷酸化、二聚化及入核,从而诱导其效应基因IFNs表达。而YAP通过抑制IRF3二聚化及其核定位负调控抗病毒天然免疫。研究表明,当YAP被敲除后,天然免疫增强,病毒载量下降 [30]。此外,病毒侵染宿主后,宿主也能通过IKKε影响YAP磷酸化,下调YAP表达,进而增强天然抗病毒免疫反应 [31]。研究表明,YAP/TAZ也能与TBK1直接作用,抑制TBK1第63位赖氨酸泛素化,消除病毒诱导TBK1激活,从而抑制天然免疫 [2]。
此外,YAP蛋白在抗肿瘤免疫中也发挥重要作用。YAP能直接调控PD-L1转录,使得PD-L1与免疫识别受体PD-1相互作用,避免免疫监视。YAP也能通过促进肿瘤分泌因子的表达,进而促进免疫抑制细胞的招募,抑制免疫反应对肿瘤细胞的调控;此外,YAP通过激活CAFs促进肿瘤免疫抑制。研究表明CAFs通过释放大量的免疫抑制细胞因子,促进免疫逃避。综上,YAP负调控天然抗病毒免疫及抗肿瘤免疫 [32]。
6. 展望
文章概括了Hippo信号通路YAP/TAZ蛋白在控制细胞增殖,肿瘤发生以及病毒诱导疾病、影响免疫反应等方面的作用。文章旨在阐明病毒诱导肿瘤与Hippo通路YAP/TAZ蛋白之间的联系,推广Hippo通路YAP/TAZ参与调控的肿瘤发病机理,从而为寻找治疗疾病靶点提供一些参考,以便研发相关药物,加快临床治疗进程。
虽然有许多关键问题未被阐明,但我们感兴趣之处在于是否可以通过人为干预Hippo信号通路从而治疗由病毒感染引起的疾病。用药物调控Hippo信号通路组分可能会为预防病毒性疾病提供一些线索。例如VP和VGLL4等药物能够抑制YAP和TEAD之间相互作用。研究表明VP药物对卵巢癌细胞增值呈现时间和剂量抑制作用,并能抑制癌细胞的迁移和侵袭。而VGLL4与YAP直接竞争结合TEAD从而抑制肺癌细胞增值 [33] [34]。因此,我们可以着眼于研发更多靶向YAP/TAZ的药物,通过抑制YAP/TAZ表达、磷酸化修饰,抑制YAP/TAZ与其上下游蛋白的互作,从而阻碍病毒编码蛋白对YAP/TAZ调控,从而实现抑制人类病毒感染、传播及致病的作用,对预防病毒侵染具有重要生理意义。
基金项目
国家自然科学基金项目(31970173);国家级大学生创新创业训练计划(202010345019)。
文章引用
邹 丰,苟洪伟,黄金华,李晨辉,赵铁军. YAP/TAZ蛋白调控病毒侵染与免疫的研究进展
Research Progress of YAP/TAZ Protein Regulating Virus Infection and Immunity[J]. 生物医学, 2021, 11(01): 8-13. https://doi.org/10.12677/HJBM.2021.111002
参考文献
- 1. Zhao, B., Li, L., Lei, Q., et al. (2010) The Hippo-YAP Pathway in Organ Size Control and Tumorigenesis: An Updated Version. Genes & Development, 24, 862-874. https://doi.org/10.1101/gad.1909210
- 2. Zhang, Q., Meng, F., Chen, S., et al. (2017) Hippo Signalling Governs Cytosolic Nucleic Acid Sensing through YAP/TAZ-Mediated TBK1 Blockade. Nature Cell Biology, 19, 362-374. https://doi.org/10.1038/ncb3496
- 3. Hong, L., Li, X., Zhou, D., et al. (2018) Role of Hippo Signaling in Regulating Immunity. Cellular & Molecular Immunology, 15, 1003-1009. https://doi.org/10.1038/s41423-018-0007-1
- 4. Taha, Z., van Rensburg, H.J.J. and Yang, X. (2018) The Hippo Pathway: Immunity and Cancer. Cancers (Basel), 10, 94. https://doi.org/10.3390/cancers10040094
- 5. Piccolo, S., Dupont, S. and Cordenonsi, M. (2014) The Biology of YAP/TAZ: Hippo Signaling and Beyond. Physiological Reviews, 94, 1287-1312. https://doi.org/10.1152/physrev.00005.2014
- 6. Zhao, B., Wei, X., Li, W., et al. (2007) Inactivation of YAP Oncoprotein by the Hippo Pathway Is Involved in Cell Contact Inhibition and Tissue Growth Control. Genes & Development, 21, 2747-2761. https://doi.org/10.1101/gad.1602907
- 7. Boggiano, J.C., Vanderzalm, P.J. and Fehon, R.G. (2011) Tao-1 Phosphorylates Hippo/MST Kinases to Regulate the Hippo-Salvador-Warts Tumor Suppressor Pathway. Developmental Cell, 21, 888-895. https://doi.org/10.1016/j.devcel.2011.08.028
- 8. Oka, T., Mazack, V. and Sudol, M. (2008) Mst2 and Lats Kinases Regulate Apoptotic Function of Yes Kinase-Associated Protein (YAP). Journal of Biological Chemistry, 283, 27534-27546. https://doi.org/10.1074/jbc.M804380200
- 9. Ni, L., Zheng, Y., Hara, M., Pan, D. and Luo, X. (2015) Structural Basis for Mob1-Dependent Activation of the Core Mst-Lats Kinase Cascade in Hippo Signaling. Genes & Development, 29, 1416-1431. https://doi.org/10.1101/gad.264929.115
- 10. Yin, F., Yu, J., Zheng, Y., et al. (2013) Spatial Organization of Hippo Signaling at the Plasma Membrane Mediated by the Tumor Suppressor Merlin/NF2. Cell, 154, 1342-1355. https://doi.org/10.1016/j.cell.2013.08.025
- 11. Yu, F.X., Zhao, B., Panupinthu, N., et al. (2012) Regulation of the Hippo-YAP Pathway by G-Protein-Coupled Receptor Signaling. Cell, 150, 780-791. https://doi.org/10.1016/j.cell.2012.06.037
- 12. Yu, F.X., Zhang, Y., Park, H.W., et al. (2013) Protein Kinase A Activates the Hippo Pathway to Modulate Cell Proliferation and Differentiation. Genes & Development, 27, 1223-1232. https://doi.org/10.1101/gad.219402.113
- 13. Meng, Z., Moroishi, T., Mottier-Pavie, V., et al. (2015) MAP4K Family Kinases Act in Parallel to MST1/2 to Activate LATS1/2 in the Hippo Pathway. Nature Communications, 6, Article No. 8357. https://doi.org/10.1038/ncomms9357
- 14. Zhao, B., Li, L., Tumaneng, K., Wang, C.Y. and Guan, K.L. (2010) A Coordinated Phosphorylation by Lats and CK1 Regulates YAP Stability through SCFβ-TRCP. Genes & Development, 24, 72-85. https://doi.org/10.1101/gad.1843810
- 15. Zhao, B., Ye, X., Yu, J., et al. (2008) TEAD Mediates YAP-Dependent Gene Induction and Growth Control. Genes & Development, 22, 1962-1971. https://doi.org/10.1101/gad.1664408
- 16. Moroishi, T., Hansen, C.G. and Guan, K.L. (2015) The Emerging Roles of YAP and TAZ in Cancer. Nature Reviews Cancer, 15, 73-79. https://doi.org/10.1038/nrc3876
- 17. Kang, W., Cheng, A.S., Yu, J., et al. (2016) Emerging Role of Hippo Pathway in Gastric and Other Gastrointestinal Cancers. World Journal of Gastroenterology, 22, 1279-1288. https://doi.org/10.3748/wjg.v22.i3.1279
- 18. Zhang, L., Yang, S., Chen, X., et al. (2015) The Hippo Pathway Effector YAP Regulates Motility, Invasion, and Castration-Resistant Growth of Prostate Cancer Cells. Molecular and Cellular Biology, 35, 1350-1362. https://doi.org/10.1128/MCB.00102-15
- 19. Harvey, K.F., Zhang, X. and Thomas, D.M. (2013) The Hippo Pathway and Human Cancer. Nature Reviews Cancer, 13, 246-257. https://doi.org/10.1038/nrc3458
- 20. Xia, Y., Chang, T., Wang, Y., et al. (2014) YAP Promotes Ovarian Cancer Cell Tumorigenesis and Is Indicative of a Poor Prognosis for Ovarian Cancer Patients. PLoS One, 9, e91770. https://doi.org/10.1371/journal.pone.0091770
- 21. Gomez, M., Gomez, V. and Hergovich, A. (2014) The Hippo Pathway in Disease and Therapy: Cancer and Beyond. Clinical and Translational Medicine, 3, 22. https://doi.org/10.1186/2001-1326-3-22
- 22. Zhang, T., Zhang, J., You, X., et al. (2012) Hepatitis B Virus X Protein Modulates Oncogene Yes-Associated Protein by CREB to Promote Growth of Hepatoma Cells. Hepatology, 56, 2051-2059. https://doi.org/10.1002/hep.25899
- 23. Liu, P., Zhang, H., Liang, X., et al. (2015) HBV preS2 Promotes the Expression of TAZ via miRNA-338-3p to Enhance the Tumorigenesis of Hepatocellular Carcinoma. Oncotarget, 6, 29048-29059. https://doi.org/10.18632/oncotarget.4804
- 24. Wang, Y., Fang, R., Cui, M., et al. (2017) The Oncoprotein HBXIP Up-Regulates YAP through Activation of Transcription Factor c-Myb to Promote Growth of Liver Cancer. Cancer Letters, 385, 234-242. https://doi.org/10.1016/j.canlet.2016.10.018
- 25. He, J., Tang, F., Liu, L., et al. (2017) Positive Regulation of TAZ Expression by EBV-LMP1 Contributes to Cell Proliferation and Epithelial-Mesenchymal Transition in Nasopharyngeal Carcinoma. Oncotarget, 8, 52333-52344. https://doi.org/10.18632/oncotarget.13775
- 26. Garcia, G., Paul, S., Beshara, S., et al. (2020) Hippo Signaling Pathway Has a Critical Role in Zika Virus Replication and in the Pathogenesis of Neuroinflammation. The American Journal of Pathology, 190, 844-861. https://doi.org/10.1016/j.ajpath.2019.12.005
- 27. He, C., Mao, D., Hua, G., et al. (2015) The Hippo/YAP Pathway Interacts with EGFR Signaling and HPV Oncoproteins to Regulate Cervical Cancer Progression. EMBO Molecular Medicine, 7, 1426-1449. https://doi.org/10.15252/emmm.201404976
- 28. Strickland, S.W., Brimer, N., Lyons, C., et al. (2018) Human Papillomavirus E6 Interaction with Cellular PDZ Domain Proteins Modulates YAP Nuclear Localization. Virology, 516, 127-138. https://doi.org/10.1016/j.virol.2018.01.003
- 29. Liu, G., Yu, F.X., Kim, Y.C., et al. (2015) Kaposi Sarcoma-Associated Herpesvirus Promotes Tumorigenesis by Modulating the Hippo Pathway. Oncogene, 34, 3536-3546. https://doi.org/10.1038/onc.2014.281
- 30. Wang, S., Xie, F., Chu, F., et al. (2017) YAP Antagonizes Innate Antiviral Immunity and Is Targeted for Lysosomal Degradation through IKKɛ-Mediated Phosphorylation. Nature Immunology, 18, 733-743. https://doi.org/10.1038/ni.3744
- 31. Kim, N., Park, Y.Y., Joo, C.H. and Kim, H.S. (2018) Relief of YAP-Mediated Inhibition by IKKɛ Promotes Innate Antiviral Immunity. Cellular & Molecular Immunology, 15, 642-644. https://doi.org/10.1038/cmi.2017.97
- 32. White, S.M., Murakami, S. and Yi, C. (2019) The Complex Entanglement of Hippo-Yap/Taz Signaling in Tumor Immunity. Oncogene, 38, 2899-2909. https://doi.org/10.1038/s41388-018-0649-6
- 33. Feng, J., Gou, J., Jia, J., Yi, T., Cui, T. and Li, Z. (2016) Verteporfin, a Suppressor of YAP-TEAD Complex, Presents Promising Antitumor Properties on Ovarian Cancer. OncoTargets and Therapy, 9, 5371-5381. https://doi.org/10.2147/OTT.S109979
- 34. Zhang, W., Gao, Y., Li, P., et al. (2014) VGLL4 Functions as a New Tumor Suppressor in Lung Cancer by Negatively Regulating the YAP-TEAD Transcriptional Complex. Cell Research, 24, 331-343. https://doi.org/10.1038/cr.2014.10
NOTES
*通讯作者。