Advances in Analytical Chemistry
Vol.
14
No.
01
(
2024
), Article ID:
81390
,
14
pages
10.12677/AAC.2024.141002
基于大环分子主–客体相互作用的超分子聚合物网络研究进展
李文婷,刘傲冉
浙江师范大学化学与材料科学学院,浙江 金华
收稿日期:2023年11月22日;录用日期:2024年2月21日;发布日期:2024年2月28日

摘要
高分子链间通过非共价交联作用能够形成超分子聚合物网络(SPNs),这类材料具有动态可逆性、刺激响应性、自愈性和形状记忆等优点,目前已在高分子科学、超分子化学、自适应材料和生物医学材料等诸多领域取得广泛应用。基于大环主体的主客体相互作用是构筑超分子聚合物网络的一类重要非共价驱动力,本文按照大环主体的种类介绍了不同类型主–客体作用交联的超分子聚合物网络的构筑策略,以及它们的刺激响应组装行为和功能调控相关研究进展。同时,讨论了该领域面临的问题和挑战,为发展其他新颖超分子聚合物网络材料提供参考。
关键词
超分子化学,自组装,主–客体相互作用,超分子聚合物网络,刺激响应性

The Review Focuses on the Supramolecular Polymer Network Formed by Macrocyclic Molecules through Host-Guest Interactions
Wenting Li, Aoran Liu
College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua Zhejiang
Received: Nov. 22nd, 2023; accepted: Feb. 21st, 2024; published: Feb. 28th, 2024

ABSTRACT
The noncovalent crosslinking of polymer chains can lead to the formation of supramolecular polymer networks (SPNs) featuring with unique dynamic, stimuli-responsive, self-healing and shape memory etc, which have enabled widespread applications in polymer science, supramolecular chemistry, adaptive materials as well as biomedical materials. The macrocyclic host based host-guest interactions are an important class of non covalent driving force for constructing SPNs. This review mainly summarize the representative construction strategies for SPNs crosslinked by different host-guest interactions according to the types of macrocyclic hosts, as well as the research progress related to their stimuli-responsive assembly behavior and functional regulation. Meanwhile, the challenges and perspectives will be discussed to provide valuable references for the development of other novel SPNs materials.
Keywords:Supramolecular Chemistry, Self-Assembly, Host-Guest Interaction, Supramolecular Polymeric Network, Stimuli Responses

Copyright © 2024 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 前言
超分子聚合物源自于超分子化学和聚合物科学,是由两者整合而形成的一个发展迅速的研究方向 [1] [2] [3] 。超分子聚合物网络则是通过非公价相互作用交联而成的软材料 [4] [5] [6] [7] 。与共价聚合物网络相比,超分子聚合物网络具有易回收、自修复、刺激响应性和形状记忆等优点 [8] [9] [10] [11] [12] 。同时非共价相互作用亦赋予了超分子聚合物网络可逆性和刺激响应性,使其在构建自愈材料、药物传输系统、记忆材料、高粘性吸附系统和超分子聚合物电解质等方面得到广泛应用 [13] - [28] 。
对于超分子聚合物网络,其优良性能大多依赖于非共价相互作用,包括:氢键 [29] [30] [31] [32] ,主–客体作用 [33] - [38] ,金属配位 [39] [40] ,π-π堆积 [41] [42] ,静电作用 [43] 和卤键 [44] 等。在以上非共价相互作用中,基于大环分子的主–客体识别备受关注。最为经典的大环体系分别为:冠醚,环糊精,杯芳烃,葫芦脲以及柱芳烃。本文总结了近年来功能性超分子聚合物网络的进展,并依据大环主体分子的种类逐一归类并讨论。
2. 基于冠醚主–客体作用的超分子聚合物网络
冠醚(Crown ether, CE)作为第一代大环主体分子,已被广泛应用于构筑各种超分子结构 [45] [46] 。2009年,Huang等人设计并合成了末端含二苯并24-冠-8大环(DB24C8)单元的星形四臂聚己内酯1,以及末端含二苄基仲铵盐(DBAS)单元的线形双臂聚己内酯2;并基于二者之间的冠醚–仲胺盐主客体交联作用构筑了一类超分子聚合物网络3 [47] (图1)。由于超分子相互作用固有的热敏性以及DBAS的酸/碱刺激响应性,使得超分子聚合物网络3中的DB24C8-DBAS的主客体交联作用具有热和酸/碱双重刺激响应性。这类超分子聚合物网络为今后构筑其他类型刺激响应性超分子聚合物凝胶提供了广泛思路。
2013年,Huang和他的同事报道了通过聚对苯乙炔(PPE)聚合物4和同二位DBAS二聚体5的悬垂DB24C8单元之间的主客体相互作用交联而成的超分子聚合物网络6 [48] 。如图2所示,与分子4相比,6的荧光强度较弱。当受到外界刺激(K+、Cl−、pH、加热)时,6的结构被破坏后荧光强度增强,因此可用于制备多基质荧光传感器。
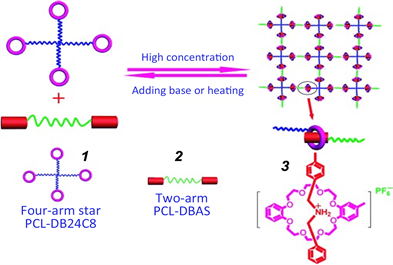
Figure 1. Schematic representation for the fabrication of responsive supramolecular networks from four-arm star PCL-DB24C8 1 and two-arm PCL-DBAS 2 as a result of molecular recognition between dibenzo [24] crown-8 3 and dibenzylammonium salt (DBAS) moieties
图1. 基于二苯并 [24] 冠-8 (DB24C8) 1和二苄基铵盐2部分之间的分子识别,四臂星型PCL-DB24C8和两臂PCL-DBAS构建响应性超分子网络3的示意图

Figure 2. Cartoon representation of the DB24C8-containing polymer 4 and the homoditopic DBAS dimer 5. Also shown in schematic fashion is the formation of the supramolecular conjugated polymeric network 6 and its disassembly promoted by different chemical and physical stimuli
图2. 含DB24C8的聚合物4、同二位DBAS二聚体5以及超分子聚合物网络6的形成和刺激响应示意图
相比于单重氢键或这配位作用,多重氢键或者配位作用的强度显著提高了几个数量级,而后两者已经被证明能够显著提高相应提高SPN的机械性能(图3(a))。而具有立体空腔的穴醚与紫精客体之间的主客体作用也比冠醚–仲胺盐主客体作用显著增强约3个数量级,因此,利用穴醚–紫精主客体作用有望发展出新一代具有优异机械性能的SPNs材料。2023年,Yan等人首次构筑了一种基于穴醚主客体交联作用的超分子聚合物网络 [49] 。如图3(b)所示,通过分别将穴醚大环和紫精模块分别修饰到聚合物7以及交联剂分子8中,再利用二者之间形成的强主客体作用制备了具有不同交联密度超分子聚合物网络SPNs-1-5。研究表明,得益于穴醚与紫精模块之间具有高结合常数的主客体交联作用,所构筑的超分子聚合物网络表现出良好的结构稳定性,并能够有效抵抗外力作用下的机械变形。

Figure 3. (a) Various noncovalent interactions with different binding constants construct SPNS with tunable mechanical properties through their different binding capacities. (b) Chemical structures and corresponding cartoons of CP 7 and bicaquat crosslinkers 8, and schematic representation of SPNS formed by Cryptand-based host-guest recognition of crosslinks
图3. (a)不同结合常数的各种非共价相互作用通过其不同的结合能力构建具有可调机械特性的SPN。(b) CP 7和双百草枯交联剂8的化学结构和相应的卡通图,以及通过基于cryptand的主客体识别交联的SPN形成的示意图
3. 基于环糊精主–客体作用的超分子聚合物网络
环糊精(Cyclodextrin, CD)是一类呈环形,外部亲水而内部空腔相对疏水的水溶性大环主体分子,已被广泛应用于在水介质中构建超分子聚合物材料 [50] [51] 。
2018年,Harada等人利用α-CD与偶氮苯的特异性主客体识别作用,设计并构筑了一款基于 [2] 拟轮烷的光响应拓扑交联聚合物水凝胶致动器 [52] 。如图4所示,赖氨酸修饰α-CD 9并与含偶氮苯基团的客体10可以形成 [2] 拟轮烷11,再进一步通过其与交联剂分子的缩聚反应可以获得一类具有独特拓扑连接的聚 [2] 轮烷水凝胶12。研究表明, [2] 轮烷结构在该聚合物网络中起到可移动连接的作用,使得水凝胶12的机械性能得到增强,能够显示出2800%的断裂应变。此外,在UV或可见光照射条件下,可以诱导偶氮苯单元发生可逆光异构化,并改变了 [2] 轮烷连接体的结构,从而导致聚合物网络变形。
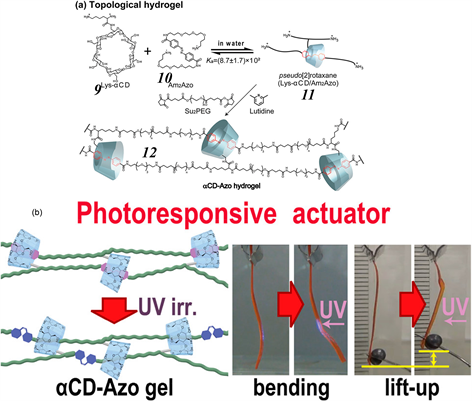
Figure 4. Chemical structures of the host 9, guest 10, pseudorotaxane 11, and the rotaxane-containing cross-linked SPN 12. (b) Schematic illustration of the photo-induced transition from 13 to a deformed state 14
图4. 主体9、客体10、拟轮烷11和含轮烷的交联SPN 12的化学结构。(b) 光诱导从13到变形状态14转变的示意图
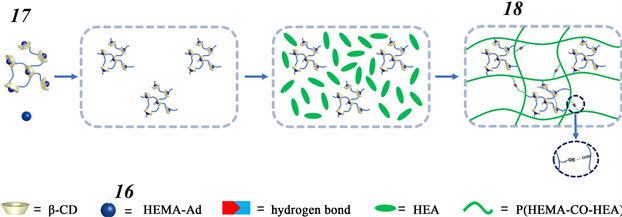
Figure 5. Structures of poly(β-CD) 15 and HEMA-modified Ad 16. Also shown in schematic form is the host−guest complex 17 formed from 15 and 16, as well as the double network 18 produced through these interactions in conjunction with hydrogen bonding
图5. 聚(β-CD) 15和HEMA修饰的Ad 16的结构。还以示意图的形式示出了由15和16形成的主客体复合物17,以及通过这些相互作用与氢键结合产生的双网络18
2019年,受动物肌肉的吸引,Zhang和他的同事们制备了一种基于β-CD与Ad主−客体相互作用的自愈弹性材料 [53] 。如图5所示,交联聚合的β-CD 15与被甲基丙烯酸羟乙酯(HEMA)修饰过的Ad 16可形成主客体络合物17。然后在在丙烯酸2-羟乙酯(HEA)的刺激下发生聚合,得到双网络弹性材料18。该材料的网络通过强氢键以及主客体相互作用进行交联,为此所得产物不仅具有高强度还具有高弹性。同时,当材料被切割或发生断裂时,仍能实现自我愈合。
2023年,Tang和他的团队通过β-环糊精(βCD)和金刚烷(Ad)的主客体预组装策略构建了一种超分子甲壳素基(SMCT)水凝胶 [54] 。在该策略中,先将羧乙基甲壳素接枝Ad (CECT-Ad)与醛基修饰βCD (βCD-CHO)进行预组装形成主客体复合物,然后利用其与羧乙基甲壳素接枝己二酸二酰肼(CECT-ADH)间的酰腙偶联反应形成SMCT水凝胶(图6)。通过一系列研究证实了可逆主客体交联能够赋予SMCT水凝胶高度动态的网络结构。与具有相似力学性能的共价交联的甲壳素基(CCT)水凝胶相比,SMCT水凝胶可以更好地适应不规则形状的伤口。
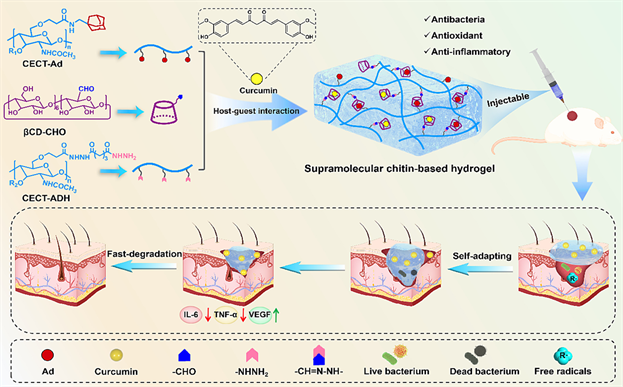
Figure 6. Chemical structures of host CECT-Ad, guest βCD-CHO, pseudotaxane, and cross-linked SPN containing rotaxane. (b) Schematic diagram of the light-induced transition from to the deformed state
图6. 基于βCD/Ad主客体相互作用的SMCT水凝胶的设计策略及其促进伤口愈合过程示意图
4. 基于杯芳烃主–客体作用的超分子聚合物网络
杯芳烃是一类苯酚–甲醛环状低聚物,可以很容易地在其上边缘、下边缘甚至桥连键上进行功能化修饰,并能对其主–客体作用进行调节。因此,基于杯芳烃的主客体作用被广泛应用于构筑各种超分子组装体,包括将其作为非共价交联作用构筑主客体超分子聚合物网络结构SPN [55] [56] 。
2012年,Pappalardo和同事报道了一种基于杯 [5] 芳烃主客体作用构建的SPN [57] 。如图7所示制备了杯 [5] 芳烃修饰的聚苯醚聚合物19和1,10-癸二胺客体20,组合而成的SPN 21。AFM分析表明,当19和20的摩尔比为2:1时,可以用来覆盖表面的均匀、连续的厚度接近均匀的网络。此外,还发现通过改变主客体比可以调节体系的荧光性质。最后,通过连续添加碱和酸可以诱导21的分解与组装。
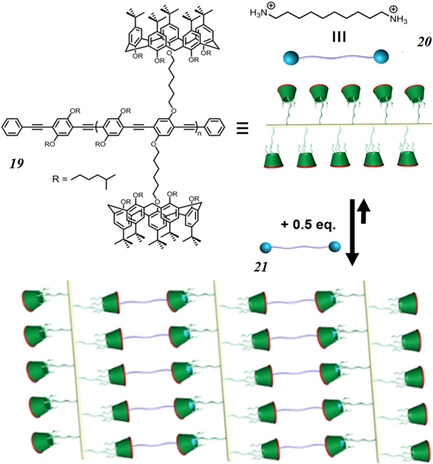
Figure 7. Schematic view of the calyx [5] arene-containing PPE system 19, the 1,10-decanediyldiammonium guest 20, and the SPN 21 produced via their host-guest-driven self-assembly
图7. 含杯 [5] 芳烃的PPE系统19、1,10-癸二基二铵guest 20和SPN 21的示意图,通过宿主–客体驱动的自组装产生

Figure 8. Chemical structures of amphiphilic sulfonatocalix [4] arene 22 and MV2+-containing poly (vinyl alcohol) polymer 23. Also shown in schematic form is the supramolecular cross-linked hydrogel 23 prepared through 23 with secondary assembled micelles from 24
图8. 磺基杯 [4] 芳烃22和含MV2+的聚乙烯醇聚合物23的化学结构图,以及由24的二次组装胶束制备的超分子交联水凝胶23
2015年,Liu等人合成了一种两亲性的磺化杯 [4] 芳烃22,其能够进行自组装得到球形胶束组装体,再进一步与侧链含紫精单元(MV2+)的聚乙烯醇23通过主客体作用交联得到具有三维网络结构的超分子交联水凝胶24 (图8) [58] 。整体过程分为:① 22的初始自组装;② 通过主–客体作用与23进行二次组装。研究表明,形成的水凝胶24具有刺激响应性,可以在温度、氧化还原和离子强度变化等不同刺激条件下,表现出可逆或不可逆的凝胶化行为。
5. 基于葫芦脲的主–客体作用的超分子聚合物网络
葫芦脲是一类含有甘脲单元的桶状大环化合物,其外部具有亲水性而内部空腔则具有疏水性,可以在水相中与多种客体分子形成主客体络合物,并且,通过改变甘脲单元的数量可获得具有空腔大小的葫芦脲。目前,葫芦脲已经广泛用于构筑水相主客体作用体系,并在聚合物组装等方面具有重要应用 [59] [60] 。
2015年,Scherman等人报道了一种基于DNA杂交和CB [8] 与苯丙氨酸主–客体作用的双重网络水凝胶材料 [61] 。如图9所示,将苯丙氨酸功能化的羧甲基纤维素25,线性DNA连接物26,Y-形DNA支架27,以及CB [8] 大环进行混合组装,可以得到双网络结构的水凝胶28,其中包括一个由25和CB [8] 主客体作用交联形成的网络和第二个由26和27之间碱基对互补作用交联形成的网络。研究表明,该双网络水凝胶28具有双重刺激响应性,在核酸酶作用下可以使其中的DNA网络解离但保持主客体交联网络29,而在纤维素酶作用下则可以使主客体交联网络解离但保持DNA网络30。并且,由于29与30可相互渗透,使得双网络水凝胶28同时具备良好的热稳定性,机械强度,拉伸能力延展性等。
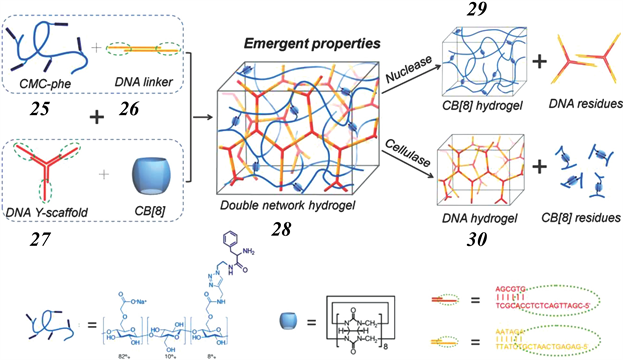
Figure 9. Schematic representation for the double network hydrogels with interpenetrating DNA and host-guest supramolecular systems
图9. 具有DNA杂化和主客体交联双网络结构的水凝胶构筑过程示意图
2018年,Scherman等人受到蜘蛛丝的启发,开发了一种以CB [8] 异三元主客体作用交联形成的超收缩纤维 [62] 。如图10所示,对聚酰胺类聚合物31进行紫精功能化,再修饰二氧化硅纳米颗粒上,将其与修饰了萘基的羟乙基纤维素聚合物32以及CB [8] 大环进行混合,两种聚合物中的紫精和萘环单元可以同时被CB [8] 空腔包结,从而形成主客体作用交联的水凝胶。将该水凝胶在水相中进一步进行紫外光交联,得到具有双网络结构的超收缩纤维33。
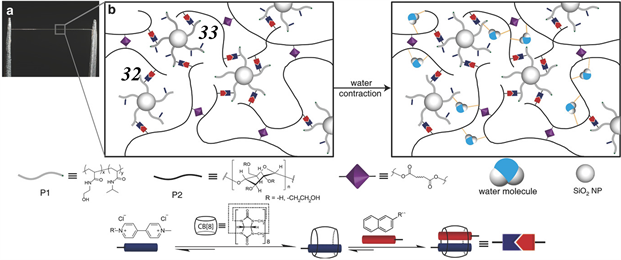
Figure 10. Chemical structures of 31 and 32 and schematic illustration of the supercontractile fiber 33 undergoing supercontraction at high humidity. Also shown is a photograph of the supercontractile fiber
图10. 31和32的化学结构和超收缩纤维33在高湿条件下的超收缩示意图。图中还显示了超收缩纤维的照片
2018年,Zhao及其团队开发了一种通用的方法来制造基于DNA四面体的超分子纳米凝胶,可用于靶向递送化学和光动力药物 [68] 。其纳米凝胶具有尺寸可调、主客体竞争响应和DNA酶响应特性。同时DNA四面体作为骨架具有良好的生物相容性和稳定性,主客体超分子连接体提供刺激响应性。DNA适体AS1411的引入赋予纳米凝胶优异的细胞靶向能力,进一步帮助DNA纳米凝胶有效进入癌细胞。

Figure 11. The formation of supramolecular DNA nano gel and its chemical and optical power combination therapy on cancer cells
图11. 超分子DNA纳米凝胶的形成及其对癌症细胞的化学和光动力联合治疗示意图
6. 基于柱芳烃的主–客体作用的超分子聚合物网络
柱芳烃是一类相对较新的大环主体分子,其相对刚性的独特柱状结构,自2008年被首次合成以来,已经快速发展成为了一类重要的大环主体分子,被广泛应用于构筑各种主客体超分子体系和功能材料 [63] [64] [65] 。
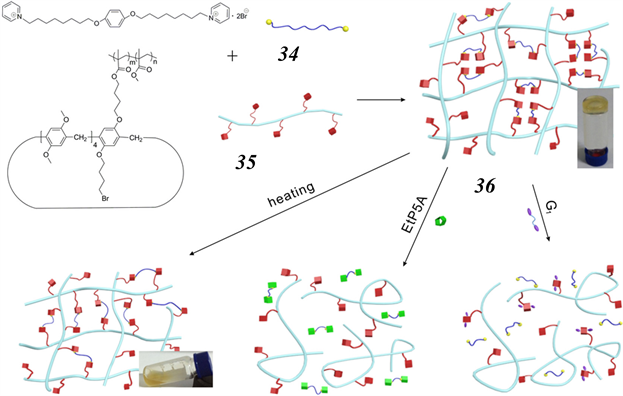
Figure 12. Schematic representation of the multi-responsive supramolecular gel 36 constructed from the bis (pyridinium) dicationic guest 34 and copolymer 35
图12. 由双吡啶客体34和共聚物35构建的多响应性超分子凝胶36的示意图
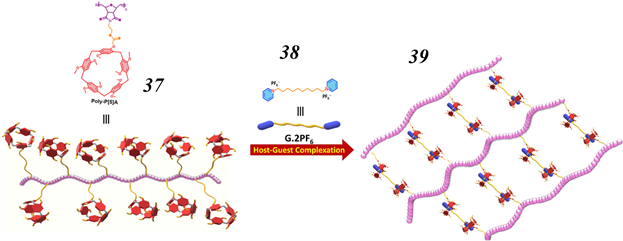
Figure 13. Schematic views showing the chemical structures of the pillar [5] arene-bearing polymer 37 and its ditopic bispyridinium guest 38. Also shown in cartoon representation is the SPN 39 obtained as the result of host-guest driven self-assembly and its response to anions
图13. 柱 [5] 芳烃聚合物37及其双异构双吡啶基客体38的化学结构的示意图。SPN 39是由主客体驱动的自组装及其对阴离子的反应而得到的
2016年,Liao和同事报道了基于柱 [5] 芳烃–吡啶鎓盐主–客体识别的多响应超分子交联凝胶 [66] 。如图12所示,将双(吡啶)阳离子客体34作为侧基添加到含柱 [5] 芳烃的聚甲基丙烯酸甲酯共聚物35中,生成了一种超分子交联聚合物凝胶36。在CHCl3中,这种凝胶可以通过添加竞争主体、乙基取代柱 [5] 芳烃(EtP5A)或竞争客体丁二腈来转化为相应的溶胶形式。
2019年,Arunachalam和他的同事报告了一种基于柱 [5] 芳烃–吡啶盐宿主−客体识别基序的阴离子响应型SPN [67] 。如图13所示,先通过柱 [5] 芳烃合成柱 [5] 芳烃悬垂聚合物37。后将37与双(吡啶鎓)客体38在CHCl3/CH3COCH3 (1:1, v/v)的混合物中混合,可得SPN 39。所得的SPN可通过添加四丁基氯化铵控制其解离。
7. 结论与展望
本综述主要介绍了基于大环主客体作用构筑超分子聚合物网络SPN这一研究领域的重要进展。得益于大环主客体作用的可逆性和刺激响应性,赋予了所构筑SPN材料多种独特性质如动态可逆性、自愈性以及形状记忆等。另一方面,大多数SPN由PA、PAAM、PEG、多糖和PS等共价聚合物进行制备,这意味着它们通常能够表现出良好的化学稳定性和机械完整性。尽管已经取得许多重要进展,但是当前SPN的发展仍然面临一些挑战。首先,仅有部分构成SPN的大环组分(CDs和CB[n])是商品化试剂,许多大环如冠醚、杯[n]芳烃和柱[n]芳烃等,以及相应的共价聚合物需要繁杂的合成。其次,目前用于构筑SPN的大环组分和共价聚合物的类型还比较有限,十分有必要发展更多种类的大环和共价聚合物用于构筑SPN。最后,除了环糊精和葫芦脲外,鼓励发展更多基于其他类型大环的SPN并应用于生物医药领域。
文章引用
李文婷,刘傲冉. 基于大环分子主–客体相互作用的超分子聚合物网络研究进展
The Review Focuses on the Supramolecular Polymer Network Formed by Macrocyclic Molecules through Host-Guest Interactions[J]. 分析化学进展, 2024, 14(01): 7-20. https://doi.org/10.12677/AAC.2024.141002
参考文献
- 1. Brunsveld, L., Folmer, B.J.B., Meijer, E.W. and Sijbesma, R.P. (2001) Supramolecular Polymers. Chemical Reviews, 101, 4071-4098. https://doi.org/10.1021/cr990125q
- 2. De Greef, T.F.A., Smulders, M.M.J., Wolffs, M., Schenning, A.P.H.J., Sijbesma, R.P. and Meijer, E.W. (2009) Supramolecular Polymerization. Chemical Reviews, 109, 5687-5754. https://doi.org/10.1021/cr900181u
- 3. Fox, J.D. and Rowan, S.J. (2009) Supramolecular Polymerizations and Main-Chain Supramolecular Polymers. Macromolecules, 42, 6823-6835. https://doi.org/10.1021/ma901144t
- 4. Seiffert, S. and Sprakel, J. (2012) Physical Chemistry of Supramolecular Polymer Networks. Chemical Society Reviews, 41, 909-930. https://doi.org/10.1039/C1CS15191F
- 5. Besenius, P. and Cormack, P.A.G. (2012) Supramolecular Chemistry in Poly-mer Networks. Wiley, Hoboken. https://doi.org/10.1002/9780470661345.smc140
- 6. Voorhaar, L. and Hoogenboom, R. (2016) Supramolecular Polymer Networks: Hydrogels and Bulk Materials. Chemical Society Reviews, 45, 4013-4031. https://doi.org/10.1039/C6CS00130K
- 7. Wang, R., Sing, M.K., Avery, R.K., Souza, B.S., Kim, M. and Olsen, B.D. (2016) Classical Challenges in the Physical Chemistry of Polymer Networks and the Design of New Materials. Accounts of Chemical Research, 49, 2786-2795. https://doi.org/10.1021/acs.accounts.6b00454
- 8. Herbst, F., Dohler, D., Michael, P. and Binder, W.H. (2013) Self-Healing Polymers via Supramolecular Forces. Macromolecular Rapid Communications, 34, 203-220. https://doi.org/10.1002/marc.201200675
- 9. An, S.Y., Arunbabu, D., Noh, S.M., Song, Y.K. and Oh, J.K. (2015) Re-cent Strategies to Develop Self-Healable Crosslinked Polymeric Networks. Chemical Communications, 51, 13058-13070. https://doi.org/10.1039/C5CC04531B
- 10. Zhang, G., Zhao, Q., Zou, W., Luo, Y. and Xie, T. (2016) Unusual Aspects of Supramolecular Networks: Plasticity to Elasticity, Ultrasoft Shape Memory, and Dynamic Mechanical Properties. Advanced Functional Materials, 26, 931-937. https://doi.org/10.1002/adfm.201504028
- 11. Jiang, Z.C., Xiao, Y.Y., Kang, Y., Pan, M., Li, B.J. and Zhang, S. (2017) Shape Memory Polymers Based on Supramolecular Interactions. ACS Applied Materials & Interfaces, 9, 20276-20293. https://doi.org/10.1021/acsami.7b03624
- 12. Wu, X., Wang, J., Huang, J. and Yang, S. (2019) Robust, Stretchable, and Self Healable Supramolecular Elastomers Synergistically Cross-Linked by Hydrogen Bonds and Coordination Bonds. ACS Ap-plied Materials & Interfaces, 11, 7387-7396. https://doi.org/10.1021/acsami.8b20303
- 13. Liao, X., Chen, G. and Jiang, M. (2013) Hydrogels Locked by Molecular Recognition Aiming at Responsiveness and Functionality. Polymer Chemistry, 4, 1733-1745. https://doi.org/10.1039/C2PY20693E
- 14. Hart, L.R., Harries, J.L., Greenland, B.W., Colquhoun, H.M. and Hayes, W. (2013) Healable Supramolecular Polymers. Polymer Chemistry, 4, 4860-4870. https://doi.org/10.1039/c3py00081h
- 15. Kaitz, J.A., Possanza, C.M., Song, Y., Diesendruck, C.E., Spiering, A.J.H., Meijer, E.W. and Moore, J.S. (2014) Depolymerizable, Adaptive Supramolecular Polymer Nanoparticles and Networks. Poly-mer Chemistry, 5, 3788-3794. https://doi.org/10.1039/C3PY01690K
- 16. Saboktakin, M.R. and Tabatabaei, R.M. (2015) Supramolecular Hydrogelsas Drug Delivery Systems. International Journal of Biological Macromolecules, 75, 426-436. https://doi.org/10.1016/j.ijbiomac.2015.02.006
- 17. Callari, M., Thomas, D.S. and Stenzel, M.H. (2016) The Dual-Role of Pt(iv) Complexes as Active Drug and Crosslinker for Micelles Based on β-Cyclodextrin Grafted Polymer. Journal of Materials Chemistry B, 4, 2114-2123. https://doi.org/10.1039/C5TB02429C
- 18. Heinzmann, C., Weder, C. and de Espinosa, L.M. (2016) Supramolecular Polymer Adhesives: Advanced Materials Inspired by Nature. Chemical Society Reviews, 45, 342-358. https://doi.org/10.1039/C5CS00477B
- 19. Amabilino, D.B., Smith, D.K. and Steed, J.W. (2017) Supramolecular Materi-als. Chemical Society Reviews, 46, 2404-2420. https://doi.org/10.1039/C7CS00163K
- 20. Lu, W., Le, X., Zhang, J., Huang, Y. and Chen, T. (2017) Supramolecular Shape Memory Hydrogels: A New Bridge between Stimuli-Responsive Poly-mers and Supramolecular Chemistry. Chemical Society Reviews, 46, 1284-1294. https://doi.org/10.1039/C6CS00754F
- 21. Webber, M.J. and Langer, R. (2017) Drug Delivery by Supramolecular Design. Chemical Society Reviews, 46, 6600-6620. https://doi.org/10.1039/C7CS00391A
- 22. Lowenberg, C., Balk, M., Wischke, C., Behl, M. and Lendlein, A. (2017) Shape-Memory Hydrogels: Evolution of Structural Principles to Enable Shape Switching of Hydrophilic Polymer Networks. Accounts of Chemical Research, 50, 723-732. https://doi.org/10.1021/acs.accounts.6b00584
- 23. Huynh, T.P., Sonar, P. and Haick, H. (2017) Advanced Materials for Use in Soft Self-Healing Devices. Advanced Materials, 29, Article ID: 1604973. https://doi.org/10.1002/adma.201604973
- 24. Zhao, R., Zhao, T., Jiang, X., Liu, X., Shi, D., Liu, C., Yang, S. and Chen, E.-Q. (2017) Thermoplastic High Strain Multishape Memory Polymer: Side-Chain Polynorbornene with Columnar Liquid Crystalline Phase. Advanced Materials, 29, Article ID: 1605908. https://doi.org/10.1002/adma.201605908
- 25. Huang, G., Li, F., Zhao, X., Ma, Y., Li, Y., Lin, M., Jin, G., Lu, T.J., Genin, G.M. and Xu, F. (2017) Functional and Biomimetic Materials for Engineering of the Three-Dimensional Cell Microenvironment. Chemical Reviews, 117, 12764-12850. https://doi.org/10.1021/acs.chemrev.7b00094
- 26. Yan, X., Liu, Z., Zhang, Q., Lopez, J., Wang, H., Wu, H.C., Niu, S., Yan, H., Wang, S., Lei, T., Li, J., Qi, D., Huang, P., Huang, J., Zhang, Y., Wang, Y., Li, G., Tok, J. B., Chen, X. and Bao, Z. (2018) Quadruple H-Bonding Cross-Linked Supramolecular Polymeric Materials as Substrates for Stretchable, Antitearing, and Self-Healable Thin Film Electrodes. Journal of the American Chemical Society, 140, 5280-5289. https://doi.org/10.1021/jacs.8b01682
- 27. Dahlke, J., Tepper, R., Geitner, R., Zechel, S., Vitz, J., Kampes, R., Popp, J., Hager, M.D. and Schubert, U.S. (2018) A Healing Ionomer Crosslinked by a Bis-Bidentate Halogen Bond Linker: A Route to Hard and Healable Coatings. Polymer Chemistry, 9, 2193-2197. https://doi.org/10.1039/C8PY00149A
- 28. Zhou, B., Jo, Y.H., Wang, R., He, D., Zhou, X., Xie, X. and Xue, Z. (2019) Self-Healing Composite Polymer Electrolyte Formed via Su-pramolecular Networks for High-Performance Lithium-Ion Batteries. Journal of Materials Chemistry A, 7, 10354-10362. https://doi.org/10.1039/C9TA01214A
- 29. Schäfer, S. and Kickelbick, G. (2018) Double Reversible Networks: Im-provement of Self-Healing in Hybrid Materials via Combination of Diels-Alder Cross-Linking and Hydrogen Bonds. Macro-molecules, 51, 6099-6110. https://doi.org/10.1021/acs.macromol.8b00601
- 30. Hua, Z., Wilks, T.R., Keogh, R., Herwig, G., Stavros, V.G. and O’Reilly, R.K. (2018) Entrapment and Rigidification of Adenine by a Photo Cross-Linked Thymine Network Leads to Fluores-cent Polymer Nanoparticles. Chemistry of Materials, 30, 1408-1416. https://doi.org/10.1021/acs.chemmater.7b05206
- 31. Chang, X., Geng, Y., Cao, H., Zhou, J., Tian, Y., Shan, G., Bao, Y., Wu, Z.L. and Pan, P. (2018) Dual-Crosslink Physical Hydrogels with High Toughness Based on Synergistic Hydrogen Bond-ing and Hydrophobic Interactions. Macromolecular Rapid Communications, 39, e1700806. https://doi.org/10.1002/marc.201700806
- 32. Wang, Y.J., Zhang, X.N., Song, Y., Zhao, Y., Chen, L., Su, F., Li, L., Wu, Z.L. and Zheng, Q. (2019) Ultrastiff and Tough Supramolecular Hydrogels with a Dense and Robust Hydrogen Bond Network. Chemistry of Materials, 31, 1430-1440. https://doi.org/10.1021/acs.chemmater.8b05262
- 33. McKee, J.R., Appel, E.A., Seitsonen, J., Kontturi, E., Scherman, O.A. and Ikkala, O. (2014) Healable, Stable and Stiff Hydrogels: Combining Conflicting Properties Using Dynamic and Selective Three-Component Recognition with Reinforcing Cellulose Nanorods. Advanced Func-tional Materials, 24, 2706-2713. https://doi.org/10.1002/adfm.201303699
- 34. Ma, X. and Zhao, Y. (2015) Biomedical Applications of Supramolecular Systems Based on Host-Guest Interactions. Chemical Reviews, 115, 7794-7839. https://doi.org/10.1021/cr500392w
- 35. Yang, X., Yu, H., Wang, L., Tong, R., Akram, M., Chen, Y. and Zhai, X. (2015) Self-Healing Polymer Materials Constructed by Macrocycle-Based Host-Guest Interactions. Soft Matter, 11, 1242-1252. https://doi.org/10.1039/C4SM02372B
- 36. Yu, Z., Zhang, J., Coulston, R.J., Parker, R.M., Biedermann, F., Liu, X., Scherman, O.A. and Abell, C. (2015) Supramolecular Hydrogel Microcapsules via Cucurbituril Host-Guest Interactions with Triggered and UV-Controlled Molecular Permeability. Chemical Science, 6, 4929-4933. https://doi.org/10.1039/C5SC01440A
- 37. Fu, T., Li, Z., Zhang, Z., Zhang, X. and Wang, F. (2017) Supramolecular Cross-Linking and Gelation of Conjugated Polycarbazoles via Hydrogen Bond Assisted Molecular Tweezer/Guest Complexa-tion. Macromolecules, 50, 7517-7525. https://doi.org/10.1021/acs.macromol.7b01149
- 38. Xiao, T., Xu, L., Zhou, L., Sun, X.-Q., Lin, C. and Wang, L. (2019) DynamicHydrogels Mediated by Macrocyclic Host-Guest Interactions. Journal of Materials Chemistry B, 7, 1526-1540. https://doi.org/10.1039/C8TB02339E
- 39. Bentz, K.C. and Cohen, S.M. (2018) Supramolecular Metallopolymers: From Linear Materials to Infinite Networks. Angewandte Chemie International Edition, 57, 14992-15001. https://doi.org/10.1002/anie.201806912
- 40. Liu, H., Peng, H., Xin, Y. and Zhang, J. (2019) Metal-Organic Frameworks: A Universal Strategy towards Super-Elastic Hydrogels. Polymer Chemistry, 10, 2263-2272. https://doi.org/10.1039/C9PY00085B
- 41. Hart, L.R., Hunter, J.H., Nguyen, N.A., Harries, J.L., Greenland, B.W., Mac-kay, M.E., Colquhoun, H.M. and Hayes, W. (2014) Multivalency in Healable Supramolecular Polymers: The Effect of Supra-molecular Cross-Link Density on the Mechanical Properties and Healing of Non-Covalent Polymer Networks. Polymer Chem-istry, 5, 3680-3688. https://doi.org/10.1039/C4PY00292J
- 42. Hayes, W. and Greenland, B.W. (2015) Donor-Acceptor π-π Stacking Interac-tions: From Small Molecule Complexes to Healable Supramolecular Polymer Networks. In: Seiffert, S., Ed., Supramolecular Polymer Networks and Gels, Springer International Publishing, Cham, 143-166.
- 43. Wang, H., Ji, X., Li, Y., Li, Z., Tang, G. and Huang, F. (2018) An ATP/ATPase Responsive Supramolecular Fluorescent Hydrogel Constructed via Electrostatic Interac-tions between Poly(Sodium p-Styrenesulfonate) and a Tetraphenylethene Derivative. Journal of Materials Chemistry B, 6, 2728-2733. https://doi.org/10.1039/C8TB00366A
- 44. Tepper, R., Bode, S., Geitner, R., Jager, M., Gorls, H., Vitz, J., Dietzek, B., Schmitt, M., Popp, J., Hager, M.D. and Schubert, U.S. (2017) Polymeric Halogen-Bond-Based Donor Systems Showing Self-Healing Behavior in Thin Films. Angewandte Chemie International Edition, 56, 4047-4051. https://doi.org/10.1002/anie.201610406
- 45. Pedersen, C.J. (1988) The Discovery of Crown Ethers. Science, 241, 536-540. https://doi.org/10.1126/science.241.4865.536
- 46. Price, T.L. and Gibson, H.W. (2011) Supramolecular Polymer Chemis-try. Wiley-VCH, Amsterdam.
- 47. Ge, Z., Hu, J., Huang, F. and Liu, S. (2009) Responsive Supramolecular Gels Constructed by Crown Ether Based Molecular Recognition. Angewandte Chemie International Edition, 48, 1798-1802. https://doi.org/10.1002/anie.200805712
- 48. Ji, X., Yao, Y., Li, J., Yan, X. and Huang, F. (2013) A Supramolecular Cross Linked Conjugated Polymer Network for Multiple Fluorescent Sensing. Journal of the American Chemical Society, 135, 74-77. https://doi.org/10.1021/ja3108559
- 49. Liu, Y.H., Wan, J.J., Zhao, X.Y., et al. (2023) Highly Strong and Tough Supra-molecular Polymer Networks Enabled by Cryptand-Based Host-Guest Recognition. Angewandte Chemie International Edition, 62, e202302370. https://doi.org/10.1002/anie.202302370
- 50. Yasen, W., Dong, R., Zhou, L., Wu, J., Cao, C., Aini, A. and Zhu, X. (2017) Synthesis of a Cationic Supramolecular Block Copolymer with Covalent and Noncovalent Polymer Blocks for Gene Delivery. ACS Applied Materials & Interfaces, 9, 9006-9014. https://doi.org/10.1021/acsami.6b15919
- 51. Minato, K., Mayumi, K., Maeda, R., Kato, K., Yokoyama, H. and Ito, K. (2017) Mechanical Properties of Supramolecular Elastomers Prepared from Polymer-Grafted Polyrotaxane. Polymer, 128, 386-391. https://doi.org/10.1016/j.polymer.2017.02.090
- 52. Takashima, Y., Hayashi, Y., Osaki, M., Kaneko, F., Yamaguchi, H. and Harada, A. (2018) A Photoresponsive Polymeric Actuator Topologically Cross-Linked by Movable Units Based on a Rotaxane. Macromolecules, 51, 4688-4693. https://doi.org/10.1021/acs.macromol.8b00939
- 53. Hou, J.B., Zhang, X.Q., Wu, D., Feng, J.F., Ke, D., Li, B.J. and Zhang, S. (2019) Tough Self-Healing Elastomers Based on the Host-Guest Inter-action of Polycyclodextrin. ACS Applied Materials & Interfaces, 11, 12105-12113. https://doi.org/10.1021/acsami.9b00626
- 54. Shi, W.W., Zhang, D.Q., Han, L.Y., et al. (2023) Supramolecular Chi-tin-Based Hydrogels with Self-Adapting and Fast-Degradation Properties for Enhancing Wound Healing. Carbohydrate Poly-mers, 323, Article ID: 121374. https://doi.org/10.1016/j.carbpol.2023.121374
- 55. Zhu, W., Gou, P. and Shen, Z. (2008) Applications of Calixarenes in Polymer Synthesis. Macromolecular Symposia, 261, 74-84. https://doi.org/10.1002/masy.200850110
- 56. Villari, V., Gattuso, G., Notti, A., Pappalardo, A. and Micali, N. (2012) Self Assembled Calixarene Derivative as a Supramolecular Poly-mer. The Journal of Physical Chemistry B, 116, 5537-5541. https://doi.org/10.1021/jp300848n
- 57. Pappalardo, A., Bal-listreri, F.P., Destri, G.L., Mineo, P.G., Tomaselli, G.A., Toscano, R.M. and Trusso Sfrazzetto, G. (2012) Supramolecular Polymer Networks Based on Calixarene Tethered Poly(p-phenyleneethynylene). Macromolecules, 45, 7549-7556. https://doi.org/10.1021/ma3015239
- 58. Wang, K.P., Chen, Y. and Liu, Y. (2015) A Polycation-Induced Secondary As-sembly of Amphiphilic Calixarene and Its Multi-Stimuli Responsive Gelation Behavior. Chemical Communications, 51, 1647-1649. https://doi.org/10.1039/C4CC08721F
- 59. Marquez, C., Hudgins, R.R. and Nau, W.M. (2004) Mechanism of Host-Guest Complexation by Cucurbituril. Journal of the American Chemical Society, 126, 5806-5816. https://doi.org/10.1021/ja0319846
- 60. Angelos, S., Yang, Y.W., Patel, K., Stoddart, J.F. and Zink, J.I. (2008) pH Re-sponsive Supramolecular Nanovalves Based on Cucurbituril Pseudorotaxanes. Angewandte Chemie International Edition, 47, 2222-2226. https://doi.org/10.1002/anie.200705211
- 61. Li, C., Rowland, M.J., Shao, Y., Cao, T., Chen, C., Jia, H., Zhou, X., Yang, Z., Scherman, O.A. and Liu, D. (2015) Responsive Double Network Hydrogels of Interpenetrating DNA and CB Host-Guest Supramolecular Systems. Advanced Materials, 27, 3298-3304. https://doi.org/10.1002/adma.201501102
- 62. Wu, Y., Shah, D.U., Wang, B., Liu, J., Ren, X., Ramage, M.H. and Scher-man, O.A. (2018) Biomimetic Supramolecular Fibers Exhibit Water Induced Supercontraction. Advanced Materials, 30, e1707169. https://doi.org/10.1002/adma.201707169
- 63. Wang, P., Xing, H., Xia, D. and Ji, X. (2015) A Novel Supramolecular Polymer Gel Constructed by Crosslinking Pillararene-Based Supramolecular Polymers through Metal-Ligand Interactions. Chemical Communications, 51, 17431-17434. https://doi.org/10.1039/C5CC07252B
- 64. Wang, Y., Ping, G. and Li, C. (2016) Efficient Complexation between Pillararenes and Neutral Guests: From Host-Guest Chemistry to Functional Materials. Chemical Communications, 52, 9858-9872. https://doi.org/10.1039/C6CC03999E
- 65. Wang, Y., Sun, C.-L., Niu, L.-Y., Wu, L.Z., Tung, C.-H., Chen, Y.-Z. and Yang, Q.-Z. (2017) Photoresponsive AA/BB Supramolecular Polymers Comprising Stiff-Stilbene Based Guests and Bispil-lararenes. Polymer Chemistry, 8, 3596-3602. https://doi.org/10.1039/C7PY00326A
- 66. Chang, J., Zhao, Q., Kang, L., Li, H., Xie, M. and Liao, X. (2016) Multiresponsive Supramolecular Gel Based on Pillararene-Containing Polymers. Macro-molecules, 49, 2814-2820. https://doi.org/10.1021/acs.macromol.6b00270
- 67. Boominathan, M., Kiruthika, J. and Aru-nachalam, M. (2019) Construction of Anion-Responsive Crosslinked Polypseudorotaxane Based on Molecular Recognition of Pillararene. Journal of Polymer Science, Part A: Polymer Chemistry, 57, 1508-1515. https://doi.org/10.1002/pola.29413
- 68. Yang, H., Duan, Z.Z., Liu, F.B., Zhao, Y.Y. and Liu, S. (2023) Cucur-bituril-Based Supramolecular DNA Nanogel for Targeted Codelivery of Chemo/Photodynamic Drugs. ACS Macro Letters, 12, 295-301. https://doi.org/10.1021/acsmacrolett.2c00763