Hans Journal of Surgery
Vol.
10
No.
04
(
2021
), Article ID:
45347
,
9
pages
10.12677/HJS.2021.104010
不同淋巴结清扫方式对cIA期 肺腺癌患者围手术期的 影响分析
衣腾飞1*,李烁1,秦坤2,邵盛腾1,高俊琼3,刘玉洪1#
1青岛大学附属医院胸外科,山东 青岛
2青岛大学附属医院急诊科,山东 青岛
3青岛大学附属医院麻醉科,山东 青岛

收稿日期:2021年8月16日;录用日期:2021年9月15日;发布日期:2021年9月22日

摘要
目的:研究系统性纵隔淋巴结切除术(systematic mediastinal lymph node dissection, SMLD)、淋巴结采样(lymph node sampling, LNS)和叶特异性淋巴结清扫术(lobe-specific lymph node dissection, L-SND)对胸腔镜肺癌切除术患者的影响,进而探索临床IA期(Clinical Stage IA, cIA)肺腺癌患者最佳淋巴结清扫方式。方法:采用回顾性队列研究,利用“医渡云”系统收集603例2015年6月~2019年6月在青岛大学附属医院胸外科行胸腔镜手术的cIA期肺腺癌患者的临床资料。采用IBM SPSS 23.0软件完成统计学分析,计量资料的比较采用单因素方差分析或Kruskal-Wallis H检验;计数资料的比较采用χ2检验或Fisher’s确切概率法。单因素logistic回归分析pN的危险因素,并将有统计学意义的因素纳入多因素logistic回归分析。结果:肿瘤的大小、CTR、组织学类型、pN、术后并发症、置管时间以及住院时间在SMLD、LNS和L-SND三组间具有统计学差异,且多因素Logistic回归分析显示吸烟史(OR = 2.989,95%CI = 1.436~6.219,P = 0.03)、CTR (OR = 0.043,95%CI = 0.004~0.426,P = 0.007)是pN的独立危险因素。结论:CTR = 0或术中快速冰冻病理示AIS的T1a-3aN0M0患者可不进行淋巴结清扫;0 < CTR ≤ 0.5或术中快速冰冻病理示MIA的T1a-3aN0M0或0.5 < CTR < 1的T1aN0M0患者推荐LNS或L-SND;而0.5 < CTR ≤ 1的T2a-3aN0M0或CTR = 1的T1a-3aN0M0或术中快速冰冻病理示IDA尤其是PPA、MPA的患者则推荐SMLD。
关键词
cIA期肺腺癌,系统性纵隔淋巴结切除术,淋巴结采样,叶特异性淋巴结清扫术

Analysis of the Influence of Different Lymph Node Dissection Methods on the Perioperative Period of cIA Stage Lung Adenocarcinoma Patients
Tengfei Yi1*, Shuo Li1, Kun Qin2, Shengteng Shao1, Junqiong Gao3, Yuhong Liu1#
1Department of Thoracic Surgery, The Affiliated Hospital of Qingdao University, Qingdao Shandong
2Emergency Department, The Affiliated Hospital of Qingdao University, Qingdao Shandong
3Department of Anesthesiology, The Affiliated Hospital of Qingdao University, Qingdao Shandong

Received: Aug. 16th, 2021; accepted: Sep. 15th, 2021; published: Sep. 22nd, 2021

ABSTRACT
Objective: To study the effects of systematic mediastinal lymph node dissection (SMLD), lymph node sampling (LNS) and lobe-specific lymph node dissection (L-SND) on patients underwent video-assisted thoracoscopic surgery, and then explore an optimal lymph node dissection for patients with clinical stage IA (cIA) lung adenocarcinoma. Methods: In this retrospective cohort study, from June 2015 to June 2019, the clinical date of 603 patients with cIA lung adenocarcinoma who underwent video-assisted thoracoscopic surgery in the department of Thoracic Surgery of The Affiliated Hospital of Qingdao University were collected by using the “Medical Du Yun” system. IBM SPSS 23.0 statistical software was used to complete the statistical analysis, the measurement data were compared by one-way ANOVA or Kruskal-Wallis H test, and the counting data were compared by chi-square test or Fisher exact probability test. Univariate analysis was performed on the risk factors of pN, then the factors with statistically significant were included in multivariate logistic regression analysis. Results: There were statistically significant differences in tumor size, CTR, histological type, pN, postoperative complications, time of intubation and time of hospital stay among SMLD, LNS and L-SND groups. Multivariate logistic regression analysis showed that smoking (OR = 2.989, 95%CI = 1.436~6.219, P = 0.03) and CTR (OR = 0.043, 95%CI = 0.004~0.426, P = 0.007) were independent risk factors of pN. Coclusions: For T1a-3aN0M0 patients with CTR = 0 or AIS, lymph node dissection may not be performed; LNS or L-SND is recommended when T1a- 3aN0M0 patients with 0 < CTR ≤ 0.5 or MIA and T1aN0M0 patients with 0.5 < CTR < 1; however, SMLD is recommended for T2a-3aN0M0 with 0.5 < CTR ≤ 1 or T1a-3aN0M0 with CTR = 1, or patients with IDA, especially PPA and MPA, as shown by intraoperative rapid freezing pathology.
Keywords:Clinical Stage IA Lung Adenocarcinoma, Systematic Mediastinal Lymph Node Dissection, Lymph Node Sampling, Lobe-Specific Lymph Node Dissection

Copyright © 2021 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 前言
肺癌是全球发病率和病死率最高的恶性肿瘤 [1] ,且非小细胞肺癌(Non-small cell lung cancer, NSCLC)占绝大多数 [2]。解剖性肺叶切除加系统性纵隔淋巴结清扫已成为NSCLC的标准术式,而淋巴结转移与否是影响肺癌分期和预后不良的重要因素。根据欧洲胸心外科(European Society of Thoracic surgeons, ESTS)指南 [3] ,淋巴结清扫方式包括系统性纵隔淋巴结切除术(systematic mediastinal lymph node dissection, SMLD)、淋巴结采样(lymph node sampling, LNS)、叶特异性淋巴结清扫术(lobe-specific lymph node dissection, L-SND)、选择性淋巴结活检和扩大性淋巴结清扫等。但随着胸部CT的发展和健康查体意识的提高,越来越多早期NSCLC被发现,尤其是cIA期;加上术前很难明确判断纵隔淋巴结受累情况,致使早期NSCLC术中采取何种淋巴结清扫方式尚存争议。一项随机多中心前瞻性研究(ACOSOG Z0030trial) [4] 中,Darling认为SMLD和LNS在提高术后总生存期(Overall survival, OS)及无进展生存期(Progression free survival, PFS),降低局部复发和远处转移率方面均无统计学差异;而David [5] 通过研究发现淋巴结的切除数目影响患者OS和疾病特异性生存率(DSS),但最佳淋巴结切除数目尚不清楚。因此,本文将回顾性分析青岛大学附属医院603例cIA期肺腺癌的临床资料,探索胸腔镜根治术下何种淋巴结清扫方式可使患者获益更大。
2 资料与方法
2.1. 研究对象
采用回顾性队列研究,选取2015年6月~2019年6月于青岛大学附属医院胸外科接受胸腔镜根治术的603例cIA期肺腺癌患者,其中SMLD组214例,LNS组189例,L-SND组200例。纳入标准:1) 年龄 < 70岁;2) 单一病变的原发肺腺癌;3) 肿瘤最大径 ≤ 3 cm;4) 胸部CT显示肺门、纵隔淋巴结长轴径 ≤ 1 cm;5) 无远处转移。排除标准:1) 接受免疫治疗或新辅助治疗;2) 既往其他系统恶性肿瘤病史;3) 过去6个月内有严重心脏病、心肌梗死病史;4) 间质性肺炎、肺纤维化或严重肺气肿患者。
2.2. 淋巴结清扫方式的定义
淋巴结站别根据美国胸科协会(American Thoracic Society, ATS) [6] 定义。2006年ESTS [3] 指南将SMLD定义为至少清扫6组淋巴结,包括同侧3组以上的纵隔淋巴结,其中包括隆突下淋巴结;并对上述位置的淋巴结及其周围脂肪组织一并完全切除。Adachi [7] 等人将L-SND定义为根据肿瘤所在肺叶清扫特异的纵隔和肺门淋巴结,肺门及肺内淋巴结在肿瘤所在肺叶切除时被一并清扫。但由于右肺中叶淋巴引流模式尚不清楚,故不采取L-SND。LNS指对术前及术中发现的具有代表性或异常淋巴结进行切除。例如ACOSOG Z0030 trial [4] ,左肺NSCLC行第5、6、7和10组淋巴结采样;右肺NSCLC行第2R、4R、7和10组淋巴结采样。这与本研究的淋巴结采样基本一致。但也有研究左侧采样第5、6和7组淋巴结,右侧采样第4、7和10组淋巴结 [8]。
2.3. 放射学和组织学定义
肿瘤实性成分比值(Consolidation Tumor Ratio, CTR)定义为CT纵隔窗实性成分最大径与肺窗肿瘤最大径之比。腺癌亚型依据国际肺癌研究协会、欧洲呼吸学会及美国胸科学会发布的LADC分类系统:腺泡型–优势腺癌(APA)、乳头状型–优势腺癌(PPA)、微乳头状型–优势腺癌(MPA)、实体型–优势腺癌(SPA)和伏壁型–优势腺癌(LPA)。肿瘤的临床和病理分期均依据第8版美国癌症联合委员会(AJCC)癌症分期系统确定 [9]。
2.4. 统计学处理
数据分析采用IBM SPSS 23.0统计软件,计量资料(偏态分布)采用中位数表示,计量资料(正态分布)采用单因素方差分析,并应用LSD-t和SNK-Q进行事后检验;计量资料(偏态分布)及等级资料采用Kruskal-Wallis H检验;计数资料采用χ2检验或Fisher’s确切概率法;P值 < 0.05认为有统计学差异。两两组间术后指标的比较采用Mann-Whitney U检验或χ2检验,P < 0.017认为有统计学意义。
3. 结果
3.1. 患者的基线特征
所有患者中,男性200人,女性占66.8%;平均年龄是54岁;大多数为不吸烟者,约占81.8%;肿瘤平均直径是1.37 cm,且多数位于右肺上叶和左肺上叶,分别占34.5%和29.0%;CTR平均值为0.58;病理显示原位腺癌(AIS)43例,微浸润性腺癌(MIA) 149例,浸润性腺癌(IDA) 411例;在IDA亚型中,APA、PPA、MPA、LPA、SPA分别约占63.7%、18.2%、2.2%、13.6%、2.2%;术后病理证实6.3%肺腺癌患者发生淋巴结转移,其中pN1、pN2、pN1 + pN2各有16例、7例、15例(如表1)。
Table 1. Baseline characteristics of the three groups
表1. 三组患者的基线特征
3.2. 术后指标的组间比较
患者的年龄、性别、饮酒史、吸烟史、合并症以及肿瘤位置等基线特征在不同淋巴结清扫方式间均无统计学差异,而pN、术后并发症、置管时间及住院时间等术后指标在不同组间具有统计学差异。虽SMLD组pN发生率高于L-SND和LNS组,L-SND组pN发生率高于LNS组,但L-SND组分别与SMLD组、LNS组相比,术后病理转移阳性淋巴结的概率并无统计学差异(P > 0.017)。另外,SMLD组术后并发症发生率较高,其中房颤15例、心律失常7例、肺炎13例、肺漏气7例、乳糜胸2例。相比LNS和L-SND组,术后并发症发生率高且均有统计学差异;而LNS与L-SND两组间无差异(P > 0.017) (如表2)。
Table 2. pN, perioperative complications, intubation time and hospital time were compared among groups
表2. pN、围手术期并发症、置管时间和住院时间的组间比较
3.3. 肿瘤大小和CTR预测pN
分析发现0 ≤ CTR ≤ 0.5的211例患者术后病理证实淋巴结均未发生转移,无论肿瘤的直径大小;实性成分为主的333例患者中,当肿瘤直径在0~1 cm时无淋巴结转移,直径在2~3 cm时均出现转移阳性淋巴结,且随着直径增大淋巴结转移率逐渐升高;当CTR = 1时,无论肿瘤大小,均会出现淋巴结转移且随着肿瘤增大淋巴结转移率也逐渐升高。详见图1。
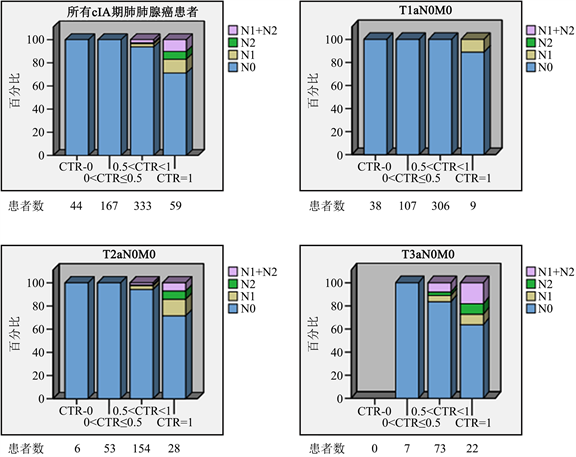
Figure 1. Prevalence of lymph node metastasis according to tumor size and CTR (CTR, consolidation tumor ratio)
图1. 肿瘤大小和CTR预测淋巴结转移
3.4. pN的危险因素分析
根据是否出现pN分为两组,将年龄、性别、饮酒史、吸烟史、合并症、肿瘤大小、CTR、病变部位、手术方式和组织学类型进行单因素Logistic回归分析,结果发现年龄(P = 0.004,OR = 0.932)、吸烟史(P < 0.001,OR=4.627)、CTR (P < 0.001,OR = 0.006)、手术方式(P = 0.013,OR = 1.934)及组织学类型(P < 0.001,OR = 0.630)在两组间均具有统计学差异;然后将这5个因素纳入多因素Logistic回归分析发现:吸烟史(OR = 2.989,95%CI = 1.436~6.219,P = 0.03)、CTR (OR = 0.043,95%CI = 0.004~0.426,P = 0.007)是pN的独立危险因素(如表3)。
Table 3. Multivariate Logistic regression analysis of pathological lymph nodemetastasis in 603 patients with cIA lung adenocarcinoma
表3. 603例cIA期肺腺癌病理淋巴结转移的多因素Logistic回归分析
4. 讨论
充分的淋巴结评估在NSCLC准确分期和预后评估中起至关重要的作用。随着早期NSCLC检测率不断提高,部分学者认为SMLD可能并非所有早期肺癌患者所必需。本研究中,pGGO或GGO为主的T1a-3aN0M0和实性成分为主的T1aN0M0患者术后病理均未发现转移阳性淋巴结,而实性成分为主的T2a-3aN0M0和纯实性成分的T1a-3aN0M0患者术后均出现pN,且随着肿瘤增大淋巴结转移率逐渐增高。与Zhang [10] 等人的研究结果基本一致。因此,CTR = 0或术中快速冰冻示AIS的T1a-3aN0M0患者可不进行淋巴结清扫;0 < CTR ≤ 0.5或术中快速冰冻示MIA的T1a-3aN0M0或0.5 < CTR < 1的T1aN0M0患者可考虑LNS或L-SND。Hughes [11] 等人曾报道SMLD的cI期NSCLC相对non-SMLD患者,前者并未提高肺癌分期的准确性;Maniwa et al. [12] 分析129例NSCLC的临床资料发现L-SND和SMLD组在OS和DFS上无统计学差异且pN2发生率大致相等。相反,Darling [4]认为SMLD能够发现更多隐匿阳性淋巴结;Keller [13] 通过研究也发现SMLD识别转移阳性淋巴结的能力高于LNS,有助于提高肺癌病理分期,且更广泛的淋巴结清扫能够切除微转移淋巴结,进而改善预后。当然,有学者主张广泛的纵隔探查和彻底的淋巴结切除会释放更多的细胞因子和生长因子,可能会刺激肿瘤细胞再生,不利于患者预后。SMLD组和LNS组相比,pN及cN/pN up-staging均有统计学意义(P < 0.017);但L-SND组分别与SMLD组、LNS组相比,pN均无统计学差异(P > 0.017)。因此,L-SND有望作为替代性淋巴结清扫方式,不仅能够避免LNS过程中遗漏可能发生转移的淋巴结,同时减少了淋巴结的清扫程度,保留大部分淋巴结的防御功能,降低患者术后并发症发生率及局部复发和远处转移的风险。
对于上叶肿瘤,往往发生上纵隔淋巴结转移,当肺门或上纵隔淋巴结为阴性时,几乎不会出现隆突下淋巴结转移;下叶肿瘤也几乎不发生上纵隔淋巴结转移 [14] [15] [16]。NSCLC的叶特异性淋巴结转移模式自20世纪90年代开始被描述,但L-SND的有效性尚存争议 [17] [18]。Haruki [19] 等人通过分析876名接受手术治疗的cI期NSCLC的临床资料,阐明了淋巴结转移的分布和发生率,发现纵隔淋巴结转移的总发生率为9.1%,且GGO为主的cI期NSCLC并未出现肺门及纵隔淋巴结转移,认为cI期NSCLC选择L-SND是可行的。而Cao et al. [20] 利用SEER数据库收集并分析了3269例 ≤2 cm的I期NSCLC临床数据得出:淋巴结清扫组的LCSS和OS优于非淋巴结清扫组,≥4个区域淋巴结清扫优于1~3个区域淋巴结清扫;但此研究未考虑肿瘤的GGO占比及患者是否有合并症。
我们分析发现SMLD组术后心律失常发生率尤其是房颤明显高于LNS和L-SND组。有研究 [21] 回顾性分析了379例pT1a-2aN0M0肺癌患者,结果显示SMLD和L-SND组患者的3年及5年生存率均无统计学差异(P > 0.05);但在手术时间、术中失血量、置管时间及住院时间具有统计学差异(P < 0.01),并且L-SND可明显降低术后并发症(P < 0.05)。与Maniwa et al. [12] 的研究结果不一致,认为SMLD和L-SND组的术后并发症发生率无显著差异(P > 0.05)。另外,我们发现SMLD组的置管时间和住院时间均长于LNS和L-SND组,且有统计学意义(P < 0.001);这可能是SMLD会损伤纵隔内的血管、神经或淋巴结构,从而导致术后并发症的发生,如心律失常、复发性神经损伤或乳糜胸,进而延长了置管时间及住院时间 [4]。
本研究存在一定局限性:首先,由于本研究是单一机构的回顾性研究,所以选择偏倚和时间趋势偏倚是不可避免的;其次,由于肺叶特异性淋巴结转移情况比较复杂,需要前瞻性研究来解释;作为首例三期试验的JCOG1413临床试验 [22] 于2017年1月开始,其结论有助于阐释L-SND对临床I-II期NSCLC的生存获益;最后,本研究着重评估三种淋巴结清扫方式对cIA期肺腺癌患者的短期疗效,尚未进行生存分析以评估长期效果。
综上所述,在cIA期肺腺癌患者中,CTR = 0或术中快速冰冻示AIS的T1a-3aN0M0患者可不行淋巴结清扫;0 < CTR ≤ 0.5或术中快速冰冻示MIA的T1a-3aN0M0或0.5 < CTR < 1的T1aN0M0患者推荐LNS或L-SND;0.5 < CTR ≤ 1的T2a-3aN0M0或CTR = 1的T1a-3aN0M0或术中快速冰冻示IDA尤其是PPA、MPA的患者则推荐SMLD。因此,为减少cIA期肺腺癌患者过度的手术损伤及围手术期痛苦,LNS或L-SND能够取代SMLD,成为部分cIA期肺腺癌纵隔淋巴结清扫可选择的清扫方式。但未来尚需前瞻性研究来提供有力的证据。
文章引用
衣腾飞,李 烁,秦 坤,邵盛腾,高俊琼,刘玉洪. 不同淋巴结清扫方式对cIA期肺腺癌患者围手术期的影响分析
Analysis of the Influence of Different Lymph Node Dissection Methods on the Perioperative Period of cIA Stage Lung Adenocarcinoma Patients[J]. 外科, 2021, 10(04): 54-62. https://doi.org/10.12677/HJS.2021.104010
参考文献
- 1. Bray, F., et al. (2018) Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians, 68, 394-424. https://doi.org/10.3322/caac.21492
- 2. Barta, J.A., Powell, C.A. and Wisnivesky, J.P. (2019) Global Epidemiol-ogy of Lung Cancer. Annals of Global Health, 85, 8. https://doi.org/10.5334/aogh.2419
- 3. Lardinois, D., et al. (2006) ESTS Guidelines for Intraoperative Lymph Node Staging in Non-Small Cell Lung Cancer. European Journal of Cardio-Thoracic Surgery, 30, 787-792. https://doi.org/10.1016/j.ejcts.2006.08.008
- 4. Darling, G.E., et al. (2011) Randomized Trial of Mediastinal Lymph Node Sampling versus Complete Lymphadenectomy during Pulmonary Resec-tion in the Patient with N0 or N1 (Less than Hilar) Non-Small Cell Carcinoma: Results of the American College of Sur-gery Oncology Group Z0030 Trial. The Journal of Thoracic and Cardiovascular Surgery, 141, 662-670. https://doi.org/10.1016/j.jtcvs.2010.11.008
- 5. David, E.A., et al. (2017) Does Lymph Node Count Influence Survival in Surgically Resected Non-Small Cell Lung Cancer? The Annals of Thoracic Surgery, 103, 226-235. https://doi.org/10.1016/j.athoracsur.2016.05.018
- 6. Mountain, C.F. (1997) Revisions in the International System for Staging Lung Cancer. Chest, 111, 1710-1717. https://doi.org/10.1378/chest.111.6.1710
- 7. Adachi, H., et al. (2017) Lobe-Specific Lymph Node Dissection as a Standard Procedure in Surgery for Non-Small Cell Lung Cancer: A Propensity Score Matching Study. Journal of Tho-racic Oncology, 12, 85-93. https://doi.org/10.1016/j.jtho.2016.08.127
- 8. Dezube, A.R., et al. (2020) Analysis of Lymph Node Sampling Minimums in Early Stage Non-Small-Cell Lung Cancer. Seminars in Thoracic and Cardiovascular Surgery. https://doi.org/10.1053/j.semtcvs.2020.11.007
- 9. Wankhede, D. (2021) Evaluation of Eighth AJCC TNM Sage for Lung Cancer NSCLC: A Meta-Analysis. Annals of Surgical Oncology, 28, 142-147. https://doi.org/10.1245/s10434-020-09151-9
- 10. Zhang, Y., et al. (2020) Segment Location and Ground Glass Opacity Ratio Reliably Predict Node-Negative Status in Lung Cancer. The Annals of Thoracic Surgery, 109, 1061-1068. https://doi.org/10.1016/j.athoracsur.2019.10.072
- 11. Hughes, M.J., Chowdhry, M.F., Woolley, S.M. and Walker, W.S. (2011) In Patients Undergoing Lung Resection for Non-Small Cell Lung Cancer, Is Lymph Node Dissection or Sampling Superior? Interactive CardioVascular and Thoracic Surgery, 13, 311-315. https://doi.org/10.1510/icvts.2011.268979
- 12. Maniwa, T., et al. (2013) Recurrence of Mediastinal Node Cancer after Lobe-Specific Systematic Nodal Dissection for Non-Small-Cell Lung Cancer. European Journal of Car-dio-Thoracic Surgery, 44, e59-e64. https://doi.org/10.1093/ejcts/ezt195
- 13. Yoon, H.Y., et al. (2018) Prognosis of Multi-Level N2-Positive Non-Small Cell Lung Cancer According to Lymph Node Staging Using Endobronchial Ultrasound-Transbronchial Bi-opsy. Thoracic Cancer, 9, 684-692. https://doi.org/10.1111/1759-7714.12629
- 14. Aokage, K., et al. (2010) Subcarinal Lymph Node in Upper Lobe Non-Small Cell Lung Cancer Patients: Is Selective Lymph Node Dissection Valid? Lung Cancer, 70, 163-167. https://doi.org/10.1016/j.lungcan.2010.02.009
- 15. Deng, H.Y., et al. (2020) Lobe-Specific Lymph Node Dissec-tion for Clinical Early-Stage (cIA) Peripheral Non-Small Cell Lung Cancer Patients: What and How? Annals of Surgical Oncology, 27, 472-480. https://doi.org/10.1245/s10434-019-07926-3
- 16. Wu, Y., et al. (2020) Metastatic Patterns of Mediastinal Lymph Nodes in Small-Size Non-Small Cell Lung Cancer (T1b). Frontiers in Surgery, 7, Article ID: 580203. https://doi.org/10.3389/fsurg.2020.580203
- 17. Okada, M., et al. (1998) Proposal for Reasonable Mediastinal Lymphadenectomy in Bronchogenic Carcinomas: Role of Subcarinal Modes in Selective Dissection. The Journal of Thoracic and Cardiovascular Surgery, 949-953. https://doi.org/10.1016/S0022-5223(98)70045-5
- 18. Tsitsias, T., et al. (2021) New N1/N2 Classification and Lobe Specific Lymphatic Drainage: Impact on Survival in Patients with Non-Small Cell Lung Cancer Treated with Sur-gery. Lung Cancer, 151, 84-90. https://doi.org/10.1016/j.lungcan.2020.11.005
- 19. Haruki, T., et al. (2015) Mediastinal Nodal Involvement in Pa-tients with Clinical Stage I Non-Small-Cell Lung Cancer: Possibility of Rational Lymph Node Dissection. Journal of Thoracic Oncology, 10, 930-936. https://doi.org/10.1097/JTO.0000000000000546
- 20. Cao, J., et al. (2018) Prognostic Impact of Lymphadenecto-my on Outcomes of Sublobar Resection for Stage IA Non-Small Cell Lung Cancer https://doi.org/10.1016/j.jtcvs.2018.03.122
- 21. 杨耀湘. 非小细胞肺癌患者ALK、EGFR、KRAS基因的检测及临床病理特征[J]. 中国现代药物应用, 2018, 12(8): 219-220.
- 22. Hishida, T., et al. (2018) A Randomized Phase III Trial of Lobe-Specific vs. Systematic Nodal Dissection for Clinical Stage I-II Non-Small Cell Lung Cancer (JCOG1413). Japanese Journal of Clinical Oncology, 48, 190-194. https://doi.org/10.1093/jjco/hyx170
NOTES
*第一作者。
#通讯作者。
