Asian Case Reports in Pediatrics
Vol.04 No.03(2016), Article ID:19038,6
pages
10.12677/ACRP.2016.43004
Clinical Observation of Children’s Idiopathic Pulmonary Hemosiderosis: A Case Report
Fang Chen1, Xiaodan Li2, Li Deng*
Department of Respiratory, Guangzhou Women’s and Children’s Medical Center, Guangzhou Medical University, Guangzhou Guangdong

Received: Nov. 1st, 2016; accepted: Nov. 22nd, 2016; published: Nov. 25th, 2016
Copyright © 2016 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



ABSTRACT
Object: The objective was to investigate the causes and treatment of idiopathic pulmonary hemosiderosis. Method: The clinical data of one child with the disease of idiopathic pulmonary hemosiderosis in our department were retrospectively analyzed. Result: The child was transferred to our department because of coughing the sputum with blood streaks repeatedly, and the CT showed the mass-like and ground-glass-like shadow of lung. We can find siderophages in the bronchoalveolar lavage fluid. After the treatment of oral corticosteroid in long term, the child hasn’t coughed the sputum with blood streaks again and the CT showed that the lesion has been absorbed. Conclusion: Whether the child is combining anemia or not, if he has hemoptysis repeatly, we should consider that he is possible to have the disease of idiopathic pulmonary hemosiderosis and have differential diagnosis and treat him early.
Keywords:Idiopathic Pulmonary Hemosiderosis, Children, Diagnosis, Treatment

一例儿童特发性肺含铁血黄素沉着症诊疗观察
陈芳,黎晓丹,邓力*
广州医科大学附属广州市妇女儿童医疗中心呼吸科,广东 广州

收稿日期:2016年11月1日;录用日期:2016年11月22日;发布日期:2016年11月25日

摘 要
目的:探讨原发性肺含铁血黄素沉着症原因和治疗方法。方法:对我科发现一例特发性肺含铁血黄素沉着症病例进行临床资料的回顾分析。结果:患儿因反复咳血丝痰一年余来我院住院,CT显示肺部团片状磨玻璃影,肺泡灌洗液可见含铁血黄素巨噬细胞。长期口服激素治疗后无咳血丝痰,CT显示病变吸收。结论:儿童出现反复咯血,不管是否合并贫血,我们应该积极考虑原发性肺含铁血黄素沉着症的可能,进行早期鉴别诊断和治疗。
关键词 :特发性肺含铁血黄素沉着症,儿童,诊断,治疗

1. 引言
原发性肺含铁血黄素沉着症(idiopathic pulmonary hemosiderosis, IPH)是以反复肺泡毛细血管出血、含铁血黄素沉着为病理特征的肺小血管出血性疾病,临床中以反复出现呼吸系统症状和小细胞低色素性贫血为发病表现。目前具体病因未明,临床中仍存在对该病缺乏认识或认识不足,影响患儿肺纤维化过程及预后 [1] [2] 。现就我院发现的一例病例进行报道,从临床特征、诊断等方面分析该病,目的在于加深对该病的认识,提高该疾病有效的早期诊治,提高患儿的生存率。
2. 病例报道
病史:患儿,男,7岁,因“反复咳血丝痰1年余”于2015年8月6日来我院门诊就诊。既往反复咳血丝痰,量少,晨起及感冒咳嗽后加重,血丝痰增多,色鲜红,无发热,运动后偶见喘息,无气促、发绀,无乏力、面色苍白等。曾多次外院住院治疗,外院胸部CT检查提示:双肺散在片状磨玻璃影,支气管镜检查提示支气管未见异常,支气管内膜炎症不明显,总IgE 1359IU/ML,粉尘螨(+++),皮肤点刺试验阳性,考虑“肺尘螨”,予抗过敏等对症支持治疗,病情仍反复,遂来我院。来我院时精神胃纳可,大小便正常。
入院查体:体温36.8℃,呼吸15次/min,心率98次/min,血压105/80 mmHg,意识清楚,面色红润,咽部充血,双肺呼吸音稍粗,未闻及干、湿啰音,心率98次/min,律齐,心音有力,未闻及杂音,腹软,肝脾肋下未触及。
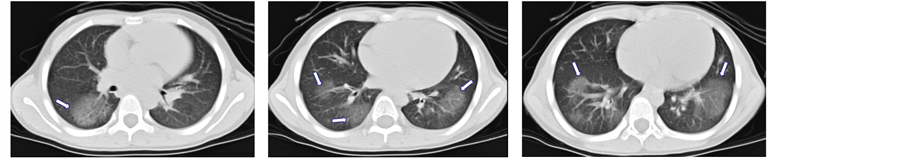
入院后完善相关检查,血常规检查提示:白细胞7.3*10~9/L,红细胞4.63*10~12/L,血红蛋白128g/L,血小板329*10~9/L,中性粒细胞百分比52%,单核细胞百分比10%,淋巴细胞百分比31%,嗜酸性粒细胞百分比7%。肝肾功能、生化等未见异常。胸部平扫+增强CT检查提示:双侧胸廓对称,双肺纹理增强,右肺中、下叶及左肺下叶见团片状磨玻璃影,余肺未及异常,双肺门不大,增强扫描未见异常强化灶,气管及双侧主支气管管腔通畅,未见扩张狭窄段,腔内未见异常密度影;纵隔影无偏移,内未见异常钙化灶,增强扫描未见明确占位性病变或肿大淋巴结影,心影及大血管形态正常;双侧胸未见胸腔积液及胸膜增厚征象(如图1(a)-(c))。行纤维支气管镜检查,镜下见:声门正常,活动度好,主气管管腔通畅,软骨环清晰,气管下段右壁可见一盲端口,隆突光滑、增宽,位置居中;右主支气管通畅,粘膜稍充血,右中叶开口狭窄,2.8 mm内镜通过困难,未见异物及赘生物堵塞;左主支气管通畅,各叶支气管
 (a) (b) (c)
(a) (b) (c)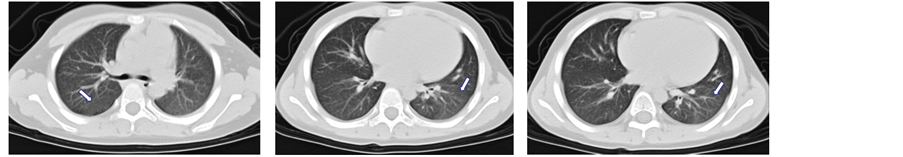 (d) (e) (f)
(d) (e) (f)
Figure 1. (a)-(c) CT shows that the ground-glass-like shadow can be seen in the right middle lobe, right lower lobe, and left lower lobe; (d)-(f) after the treatment of oral corticosteroid for 3 months, the CT shows that the lesion has been absorbed
图1. (a)-(c)提示右肺中、下叶及左肺下叶见团片状磨玻璃影;(d)-(f)为激素治疗后3月复查胸部CT提示右肺中、下叶及左肺下叶病变基本吸收
开口正常,粘膜稍充血,未见异物及赘生物堵塞;予温生理盐水灌洗双下肺叶,术毕退镜。取肺泡灌洗液检查:一般细菌、结核及真菌涂片、培养、病毒检测,支原体、衣原体检测等均为阴性。取肺泡灌洗液普鲁士蓝染色后镜下可见含铁血黄素巨噬细胞(如图2)。
临床诊断诊断:特发性肺含铁血黄素沉着症。开始口服甲泼尼松片16 mg,qd顿服。患儿症状明显改善,咳血丝痰逐渐改善,1月后患儿已无咳血丝痰,病情稳定2月后进行激素逐渐减量,口服激素治疗3月后复查CT提示右肺中、下叶及左肺下叶病变基本吸收(图1(d)-(f))。
3. 讨论
IPH是常发生于儿童并且目前病因未明的一类疾病,国外报道IPH的年发病率0.24~1.26例/100万 [1] [2] 。儿童和成年人均可发病,但大部分集中在儿童,而在儿童中80%在10岁以前发病 [3] [4] ,另外有报道新生儿也可发病 [5] [6] 。其可能的病因有研究者认为与自身免疫,过敏,基因遗传或者环境因素等有关 [7] 。其中自身免疫可能是最为重要的因素。已有报道显示麸质过敏性肠病,乳糜泻,疱疹样皮炎,肾小球性肾炎,类风湿性关节炎,以及对牛奶过敏可能伴随着IPH [8] [9] [10] 。尽管在本病例中患儿尘螨特异性IgE明显升高,嗜酸性粒细胞百分比也升高,尘螨阳性,提示可能合并尘螨过敏外,并未提示过敏体征,过去既往史也未见异常。具体病因仍不明确。
IPH是小儿反复咯血的常见原因 [11] ,但目前国内外尚未制定关于IPH的统一诊断标准,普遍认为IPH是一类排它性诊断疾病。目前诊断主要依据应包括1)反复发作咳血、缺铁性贫血和双肺浸润影三联征;2) X线胸片或高分辨率胸部CT扫描(HRCT)显示弥散性肺浸润和肺间质的改变;3)痰、肺泡灌洗液(BALF)、胃液或肺组织中找到巨噬细胞中充满含铁血黄素颗粒(HLMs)或组织病理学检查显示肺泡含铁血黄素沉积是确诊的依据;4)除外其他继发性肺含铁血黄素沉着症,如血管炎、风湿性疾病、免疫缺陷病、血管球性肾炎、肺结核、支气管异物、血管畸形和反复支气管肺炎等。其中具备1)和2)条可以作为疑似病例,按IPH 治疗;具备1)~4)条则可确诊 [12] 。牛奶蛋白过敏(Heiner Syndrome)和乳糜泻(Lane Hamilton Syndrome)也可以作为诊断评估的一部份 [13] [14] [15] 。但在我们报道的病例中,患儿就诊时以及回顾既往外院的就诊记录并不提示合并贫血,外周血检测中血红蛋白浓度不低,这也是未能早期诊断的重要原因,这也提示我们IPH并不一定伴有贫血,这可能与血常规的检测时间窗或者出血量较少有关。
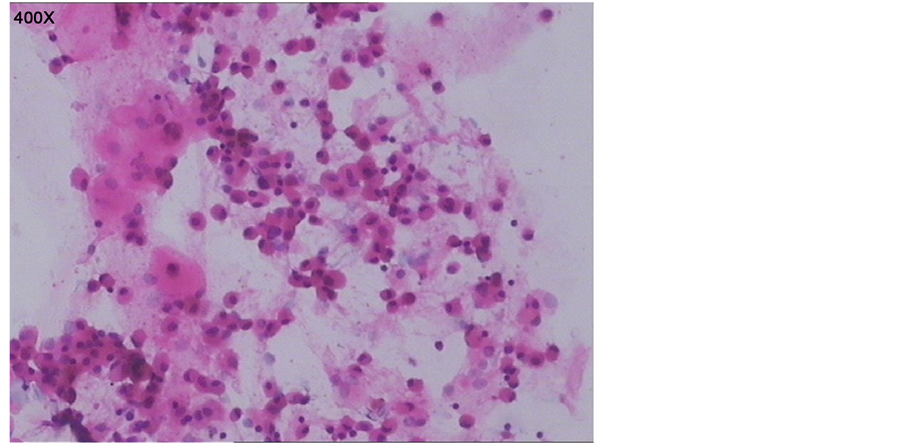
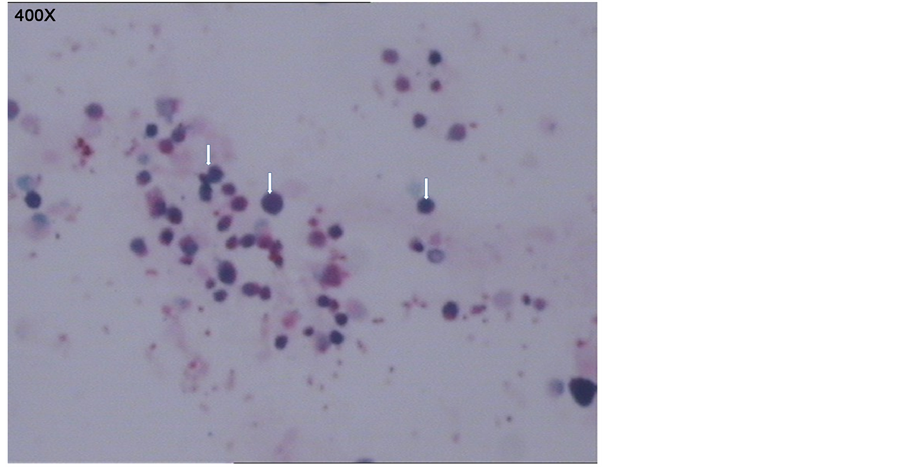
Figure 2. Pathological diagnosis: many siderophages, few exfoliated epithelial cells, lymphocyte can be seen in the bronchoalveolar lavage fluid, but no heterocysts. Special stains: iron stain 9+
图2. 病理诊断:肺泡灌洗液送检涂片镜下见多量含铁血黄素细胞及少量脱落上皮细胞、淋巴细胞,未见明显异型细胞。特染:含铁染色9+
胸部影像学表现取决于出血量、出血频度、病期和病程。HRCT对于肺纤维化早期诊断具有重要指导意义。在急性发作期,肺部出血量不同影像学检查也提示明显差异,CT显示肺纹理增多、模糊,肺野透亮度普遍减低呈磨玻璃影,可见小片状密度增高影,亦可融合成大片状影 [16] 。当出血量范围较局限时,表现为肺腺泡密度增高影及小叶中心或全小叶磨玻璃影,病变以小叶间隔为界;但出血量较大时,则表现为小叶与小叶融合病变,沿支气管树走行方向分布,边界欠清,融合病变中心密度较高,出现斑片状实变影 [16] 。在慢性稳定期,由于肺泡间质含铁血黄素沉着,纤维组织增生,小叶间隔及肺泡壁明显增厚,不同程度肺间质纤维化,呈弥漫分布粟粒状颗粒。而在慢性病程中急性发作时,则可见不对称分布大片状致密影 [17] 。最后由于反复肺泡出血、肺间质含铁血黄素大量沉着,导致肺间质广泛纤维化,在CT上变现为肺纹理增粗弥漫的网结状或粗条状影 [10] 。
随着疾病的进展,肺间质内形成广泛的纤维化,最后导致呼吸衰竭以及慢性肺源性心脏病。为此早期诊断,早期治疗尤为重要。目前已有大量研究表明糖皮质激素治疗IPH,可以有效降低该疾病的发病率和由于急性肺泡大出血引起的致死率,以及有效地控制肺纤维化的进程 [18] [19] 。为此,早期诊断对疾病预后具有重要影响。目前全身激素推荐用量起始2个月泼尼松龙<1 mg/kg/day,然后再根据具体病情逐渐减量 [20] 。但也有一些研究建议甲基强的松龙1~2 mg/kg/day [21] [22] 。另外,免疫抑制剂主要用于激素不敏感的患者,Yoachimescu 等研究者发现硫唑嘌呤联合糖皮质激素可发挥更好的疗效,特别是在控制IHP急性发作过程中具有良好疗效 [3] 。除了咪唑嘌呤,有文献报道用羟化氯喹、环磷酰胺治疗儿童或成人IPH [23] [24] [25] 。比较少见的治疗方案,如静脉输注丙种球蛋白、血浆净化分离法、甲氨喋呤、n-乙酰半胱氨酸、麦考酚酯、6-巯基嘌呤等也见有相关报道 [26] 。在我们治疗该名患儿中,考虑患儿肺部出血量较小,全身状况较好,我们予甲泼尼龙16 mg (1 mg/kg/day)起始剂量进行口服治疗,2个月后减量为12 mg/day,第3个月继续减量改8 mg/day甲泼尼龙口服治疗。期间患儿病情控制良好,未见咳血丝痰,无喘促,复查CT也提示肺部出血部位明显吸收。
总的来说,在儿童中出现反复咯血,不管是否合并贫血我们应该积极考虑IPH的可能,早期鉴别诊断,以免漏诊,延误病情。一旦明确诊断后应该早期进行全身激素系统治疗,并定期随访观察,及时调整及监测全身激素用量。
文章引用
陈 芳,黎晓丹,邓 力. 一例儿童特发性肺含铁血黄素沉着症诊疗观察
Clinical Observation of Children’s Idiopathic Pulmonary Hemosiderosis: A Case Report[J]. 亚洲儿科病例研究, 2016, 04(03): 17-22. http://dx.doi.org/10.12677/ACRP.2016.43004
参考文献 (References)
- 1. Kjellman, B., Elinder, G., Garwicz, S., et al. (1984) Idiopathic Pulmonary Haemosiderosis in Swedish Children. Acta Paediatrica Scandinavica, 73, 584-588.
- 2. Ohga, S., Takahashi, K., Miyazaki, S., et al. (1995) Idiopathic Pulmonary Haemosiderosis in Japan: 39 Possible Cases from a Survey Questionnaire. European Journal of Pediatrics, 154, 994-995.
- 3. Ioachimescu, O.C., Sieber, S. and Kotch, A. (2004) Idiopathic Pulmonary Haemosiderosis Revisited. European Respiratory Journal, 24, 162-170. http://dx.doi.org/10.1183/09031936.04.00116302
- 4. Le Clainche, L., Le Bourgeois, M., Fauroux, B., et al. (2000) Long-Term Outcome of Idiopathic Pulmonary Hemosiderosis in Children. Medicine (Baltimore), 79, 318-326. http://dx.doi.org/10.1097/00005792-200009000-00005
- 5. Gutierrez, S., Shaw, S., Huseni, S., et al. (2014) Extracorporeal Life Support for a 5-Week-Old Infant with Idiopathic Pulmonary Hemosiderosis. European Journal of Pediatrics, 173, 1573-1576. http://dx.doi.org/10.1007/s00431-013-2130-4
- 6. Limme, B., Nicolescu, R. and Misson, J.P. (2014) Neonatal Pulmonary Hemosiderosis. Case Reports in Pediatrics, 2014, 463973.
- 7. Agata, H., Kondo, N., Fukutomi, O., et al. (1997) Pulmonary Hemosiderosis with Hypersensitivity to Buckwheat. Annals of Allergy, Asthma & Immunology, 78, 233-237. http://dx.doi.org/10.1016/S1081-1206(10)63394-7
- 8. Morgan, P.G. and Turner-Warwick, M. (1981) Pulmonary Haemosiderosis and Pulmonary Haemorrhage. British Journal of Diseases of the Chest, 75, 225-242. http://dx.doi.org/10.1016/0007-0971(81)90001-2
- 9. Bhatia, S., Tullu, M.S., Vaideeswar, P., et al. (2011) Idiopathic Pulmonary Hemosiderosis: Alveoli Are an Answer to Anemia. Journal of Postgraduate Medicine, 57, 57-60. http://dx.doi.org/10.4103/0022-3859.74290
- 10. Khorashadi, L., Wu, C.C., Betancourt, S.L., et al. (2015) Idiopathic Pulmonary Haemosiderosis: Spectrum of Thoracic Imaging Findings in the Adult Patient. Clinical Radiology, 70, 459-465. http://dx.doi.org/10.1016/j.crad.2014.11.007
- 11. 陈和斌, 陆小霞, 蒋鲲. 儿童反复咯血的病因及临床诊治分析[J]. 中国当代儿科杂志, 2014, 16(03): 281-284.
- 12. 蔡栩栩, 尚云晓. 特发性肺含铁血黄素沉着症诊断和治疗进展[J]. 实用儿科临床杂志, 2011, 26(16): 1231-1234.
- 13. Nacaroglu, H.T., Sandal, O.S., Bag, O., et al. (2015) Association of Celiac Disease with Idiopathic Pulmonary Hemosiderosis. Lane Hamilton Syndrome. Iranian Journal of Pediatrics, 25, e3312. http://dx.doi.org/10.5812/ijp.3312
- 14. Sethi, G.R., Singhal, K.K., Puri, A.S., et al. (2011) Benefit of Gluten-Free Diet in Idiopathic Pulmonary Hemosiderosis in Association with Celiac Disease. Pediatric Pulmonology, 46, 302-305. http://dx.doi.org/10.1002/ppul.21357
- 15. Torres, M.J., Giron, M.D., Corzo, J.L., et al. (1996) Release of Inflammatory Mediators after Cow’s Milk Intake in a Newborn with Idiopathic Pulmonary Hemosiderosis. Journal of Allergy and Clinical Immunology, 98, 1120-1123. http://dx.doi.org/10.1016/S0091-6749(96)80201-6
- 16. 陈霞, 侯振洲. 儿童特发性肺含铁血黄素沉着症HRCT表现分析[J]. 医学影像学杂志, 2015(06): 1112-1114.
- 17. 李海燕. 儿童特发性肺含铁血黄素沉着症的CT表现[J]. 中国药物与临床, 2013, 13(08): 1047-1048.
- 18. Green, R.J., Ruoss, S.J., Kraft, S.A., et al. (1996) Pulmonary Capillaritis and Alveolar Hemorrhage. Update on Diagnosis and Management. Chest, 110, 1305-1316. http://dx.doi.org/10.1378/chest.110.5.1305
- 19. Kiper, N., Gocmen, A., Ozcelik, U., et al. (1999) Long-Term Clinical Course of Patients with Idiopathic Pulmonary Hemosiderosis (1979-1994): Prolonged Survival with Low-Dose Corticosteroid Therapy. Pediatric Pulmonology, 27, 180-184. http://dx.doi.org/10.1002/(SICI)1099-0496(199903)27:3<180::AID-PPUL5>3.0.CO;2-8
- 20. Gencer, M., Ceylan, E., Bitiren, M. and Koc, A. (2007) Two Sisters with Idiopathic Pulmonary Hemosiderosis. Canadian Respiratory Journal, 14, 490-493. http://dx.doi.org/10.1155/2007/150926
- 21. Sherani, K.M., Upadhyay, H.N., Sherani, F.K., et al. (2015) Idiopathic Pulmonary Hemosiderosis Presenting in an Adult: A Case Report and Review of the Literature. Lung India, 32, 395-397. http://dx.doi.org/10.4103/0970-2113.159594
- 22. Inayama, M., Hino, H., Takezaki, A., et al. (2007) [A Case of Adult Onset Idiopathic Pulmonary Hemosiderosis Markedly Improved by Steroid Therapy]. Nihon Kokyuki Gakkai Zasshi, 45, 971-976.
- 23. Taytard, J., Nathan, N., de Blic, J., et al. (2013) New Insights into Pediatric Idiopathic Pulmonary Hemosiderosis: The French RespiRare((R)) Cohort. Orphanet Journal of Rare Diseases, 8, 161. http://dx.doi.org/10.1186/1750-1172-8-161
- 24. Zhang, X., Wang, L., Lu, A., et al. (2010) Clinical Study of 28 Cases of Paediatric Idiopathic Pulmonary Haemosiderosis. Journal of Tropical Pediatrics, 56, 386-390. http://dx.doi.org/10.1093/tropej/fmq010
- 25. Sant’Anna, C.C., Horta, A.A., Tura, M.T., et al. (2007) [Idiopathic Pulmonary Hemosiderosis Treated with Azathioprine in a Child]. Jornal Brasileiro de Pneumologia, 33, 743-746. http://dx.doi.org/10.1590/S1806-37132007000600020
- 26. Chin, C.I., Kohn, S.L., Keens, T.G., Margetis, M.F. and Kato, R.M. (2015) A Physician Survey Reveals Differences in Management of Idiopathic Pulmonary Hemosiderosis. Orphanet Journal of Rare Diseases, 10, 98. http://dx.doi.org/10.1186/s13023-015-0319-5
NOTES
*通讯作者。