Hans Journal of Medicinal Chemistry
Vol.
09
No.
03
(
2021
), Article ID:
44565
,
7
pages
10.12677/HJMCe.2021.93014
新型TK抑制剂达克替尼在肺腺癌细胞增殖和凋亡的影响
郭李玲1,毛 俊2
1甘肃医学院附属医院全科医学科,甘肃 平凉
2甘肃医学院基础医学院生物化学与分子生物学,甘肃 平凉

收稿日期:2021年7月5日;录用日期:2021年7月19日;发布日期:2021年8月16日

摘 要
目的:探究新型TK抑制剂达克替尼对人肺腺癌A549细胞增殖与侵袭作用及机制。方法:人肺腺癌A549细胞为研究对象,根据预实验结果,将人肺腺癌细胞系A549分成3组,即空白对照组,低剂量组和高剂量组。RT-PCR检测EFGR和凋亡蛋白Caspase-3和Caspase-9的表达,流式细胞仪检测细胞的分裂周期和细胞凋亡率,CCK-8检测细胞的增殖。结果:空白对照组,低剂量组和高剂量组的EFGR,Caspase-3和Caspase-9 mRNA相对表达量相对量比较具有统计学差异(P < 0.05);空白对照组的EFGR mRNA相对表达量相对量要明显高于低剂量组和高剂量组(P < 0.05),空白对照组的Caspase-3和Caspase-9 mRNA相对表达量要明显低于低剂量组和高剂量组(P < 0.05),低剂量组和高剂量组的EFGR,Caspase-3和Caspase-9 mRNA相对表达量相对量比较无统计学差异(P > 0.05)。低剂量组和高剂量组的G0/G1期比例明显高于空白对照组(P < 0.05);S期和G2期比例明显低于空白对照组(P < 0.05)。低剂量组和高剂量组的总凋亡率明显高于空白对照组(P < 0.05);高剂量组晚期凋亡率明显高于低剂量组(P < 0.05)。药物处理2天后,空白对照组的细胞增值率要明显高于低剂量组和高剂量组(P < 0.05);药物处理3天后,空白对照组的细胞增值率要明显高于低剂量组和高剂量组(P < 0.05)。结论:低剂量达克替尼可以抑制肺腺癌细胞的增殖,促进肺腺癌细胞的凋亡,并且可以抑制EFGR的表达,可以作为耐药性肺腺癌的治疗方案。
关键词
达克替尼,肺腺癌,增殖,凋亡

Effect of a Novel TK Inhibitor Dacomitinib on Proliferation and Apoptosis of Lung Adenocarcinoma Cells
Liling Guo1, Jun Mao2
1Department of General Medicine, The Affiliated Hospital of Gansu Medical College, Pingliang Gansu
2Department of the Biochemistry and Molecular Biology, College of the Basic Medicine, Gansu Medical College, Pingliang Gansu

Received: Jul. 5th, 2021; accepted: Jul. 19th, 2021; published: Aug. 16th, 2021

ABSTRACT
Objective: The objective is to investigate the proliferation and invasion of human lung adenocarcinoma A549 cells induced by a novel TK inhibitor, dacomitinib, and its mechanism. Methods: Human lung adenocarcinoma cell line A549 was divided into three groups according to the preliminary results, namely the blank control group, the low-dose group and the high-dose group. The expression of EFGR and Caspase-3 and Caspase-9 was detected by RT-PCR. The cycle of cell division and apoptotic rate of the cell were detected by flow cytometry. The cell proliferation was detected by CCK-8. Results: The relative expressions of EFGR, caspase-3 and caspase-9 mRNA in blank control group, low-dose group and high-dose group were statistically different (P < 0.05).The relative expression of EFGR mRNA in the blank control group was significantly higher than that in the low-dose group and the high-dose group (P < 0.05). The relative expression of Caspase-3 and caspase-9 mRNA in the blank control group was significantly lower than that in the low-dose group and high-dose group (P < 0.05). There was no significant difference in the relative expression of EFGR, caspase-3 and caspase-9 mRNA between the low-dose group and the high-dose group (P > 0.05). The proportions of G0/G1 phase in low-dose group and high-dose group were significantly higher than those in blank control group (P < 0.05), while the proportions of S phase and G2 phase were significantly lower than those in blank control group (P < 0.05). The total apoptotic rate of the low-dose group and the high-dose group significantly higher than that of the blank control group (P < 0.05), and the late apoptotic rate of the high-dose group was significantly higher than that of the low-dose group (P < 0.05). After 2 days of drug treatment, the cell increment rate of blank control group was significantly higher than that of low-dose group and high-dose group (P < 0.05); after 3 days of drug treatment, the cell increment rate of blank control group was significantly higher than that of low-dose group and high-dose group (P < 0.05). Conclusion: Low-dose of dacomitinib can inhibit the proliferation of lung adenocarcinoma cells, promote the apoptosis of lung adenocarcinoma cells, and inhibit the expression of EFGR. It can be used as a therapeutic regimen for drug-resistant lung adenocarcinoma.
Keywords:Dacomitinib, Lung Adenocarcinoma, Proliferation, Apoptosis

Copyright © 2021 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
肺癌是临床上的多发病,尤其是随着生活环境的改变,生活压力的激增,人们饮食习惯的改变,肺癌的发病率明显升高。据流行病学统计结果显示,全球每年新增的肺癌患者约几万例,多见于发展中国家 [1] [2] [3]。目前对于肺癌患者的治疗,化疗方案是较为理想的治疗方案。有研究表明,表皮生长因子受体酪氨酸激酶抑制剂(Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors, EGFR-TKIs)是比较有效的治疗方案之一,比如吉非替尼等 [4],但是,该类治疗方案也存在一定的问题,比如药物耐药性的问题 [5]。并且与表皮生长因子(EFGR)的表达量相关,为了解决这个问题,第二代TKI应运而生,代表药物是达克替尼。有研究表明,达克替尼可以抑制EFGR的表达,从而达到抑制耐药性的作用 [6]。但是,关于达克替尼的细胞学实验较少,因此,本研究以人肺腺癌A549细胞为研究对象,验证达克替尼的生物学行为,以期为新型TK抑制剂达克替尼治疗人肺腺癌提供理论依据。
2. 材料与方法
2.1. 肺腺癌细胞系A549的培养
细胞系购自北京索莱宝生物有限公司,均经过str检测,证明细胞没有被污染。
2.2. 实验分组
根据预实验结果,将人肺腺癌细胞系A549分成3组,即空白对照组,低剂量组和高剂量组。低剂量组细胞给予1 μmol/L的达克替尼进行处理3 h;高剂量组细胞给予10 μmol/L的达克替尼进行处理3 h;空白对照组细胞给予等计量的PBS溶液进行对照处理。
2.3. 实时荧光定量PCR检测Akt1、Caspase-3和Caspase-9 mRNA的表达水平
收集上述3组细胞,加入1 mL Trizol试剂,充分混合后,提取细胞总RNA,室温条件下根据逆转录试剂盒说明书,将总RNA逆转录为cDNA,−20℃低温冰箱保存。然后根据荧光定量PCR试剂盒说明书,加入cDNA和USP-22基因和ERK5的引物模板(如表1所示),置于Applied Biosystems PCR仪进行反应,设置条件按照说明书。统计并记录各样本CT值。
Table 1. The primer sequence of target gene
表1. 目的基因的引物序列
2.4. 细胞凋亡水平
利用流式细胞仪进行检测,加入AV-PI试剂盒的混合制剂,然后流式细胞仪上机测试,验证细胞的凋亡水平。
2.5. 细胞增殖能力
利用CCK-8试剂盒检测细胞增殖能力,加入CCK-8试剂盒的混合制剂,利用酶标仪进行测定,波长为450 nm。
2.6. 统计学方法
计数资料用 ± s形式表示,SPSS 20.0软件进行统计学分析,3组的RT-PCR数据结果,细胞增值率和细胞凋亡率比较采用单因素方差分析,组间的比较采用q检验,认为P < 0.05有统计学意义。
3. 结果
3.1. EFGR,Caspase-3和Caspase-9的mRNA相对表达量
结果表明,空白对照组,低剂量组和高剂量组的EFGR,Caspase-3和Caspase-9的mRNA相对表达量比较具有统计学差异(F = 10.209, 9.892, 11.092; P < 0.05);其中低剂量组和高剂量组的EFGR的mRNA相对表达量要明显低于空白对照组(P < 0.05),Caspase-3和Caspase-9的mRNA相对表达量要明显高于空白对照组(P < 0.05) (见图1)。

Figure 1. The relative mRNA expression of EFGR, caspase-3 and caspase-9. Note: ** indicates statistical difference (P < 0.05); # indicates no statistical difference (P > 0.05)
图1. EFGR,Caspase-3和Caspase-9的mRNA相对表达量。注:**表示具有统计学差异(P < 0.05);#表示没有统计学差异(P > 0.05)
3.2. 3组细胞周期比较结果
结果表明,低剂量组和高剂量组的G0/G1期比例明显高于空白对照组(P < 0.05);S期和G2期比例明显低于空白对照组(P < 0.05) (见图2)。
3.3. 3组细胞凋亡率比较结果
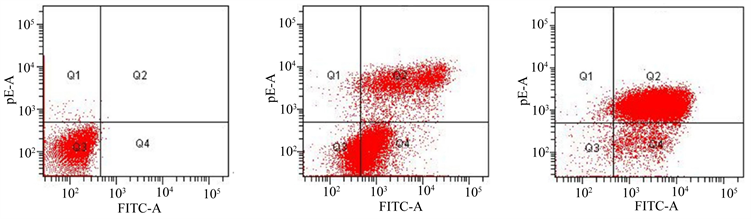
结果表明,低剂量组和高剂量组的总凋亡率明显高于空白对照组(P < 0.05);高剂量组晚期凋亡率明显高于低剂量组(P < 0.05) (见图3)。
3.4. 3组细胞增殖率比较结果
结果显示,药物处理2天后,空白对照组的细胞增值率要明显高于低剂量组和高剂量组(q = 2.293, 2.775; P < 0.05);药物处理3天后,空白对照组的细胞增值率要明显高于低剂量组和高剂量组(q = 2.887, 2.461; P < 0.05) (见图4)。
 (a) (b)(c)
(a) (b)(c)
Figure 2. The comparison of cell cycle among three groups. (a) The blank control group; (b) The low-dose group; (c) The high-dose group
图2. 3组细胞周期比较结果。(A) 空白对照组;(B) 低剂量组;(C) 高剂量组
 (a) (b)(c)
(a) (b)(c)
Figure 3. The comparison of apoptosis rate among three groups. (a) The blank control group; (b) The low-dose group; (c) The high-dose group
图3. 3组细胞凋亡率比较结果。(a) 空白对照组;(b) 低剂量组;(c) 高剂量组
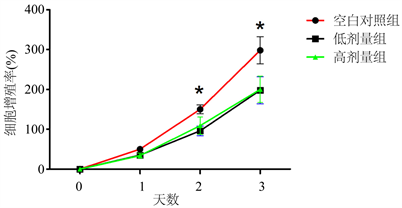
Figure 4. The comparison of proliferation rate among three groups. Note: * indicates statistical difference (P < 0.05)
图4. 3组细胞增值率比较结果。注:*表示具有统计学差异(P < 0.05)
4. 讨论
本研究结果表明,根据预实验结果给予1 μmol/L低剂量的达克替尼和10 μmol/L高剂量的达克替尼两种浓度进行处理,并且加入等量PBS溶液进行对比研究。RT-PCR检测EFGR和凋亡蛋白Caspase-3和Caspase-9的表达。流式细胞仪检测细胞的分裂周期和细胞凋亡率,CCK-8检测细胞的增殖。结果表明,达克替尼处理人肺腺癌A549细胞,可以明显降低EFGR mRNA相对表达量和蛋白表达量,增加Caspase-3和Caspase-9 mRNA相对表达量和蛋白表达量。并且,低剂量组和高剂量组的G0/G1期比例明显高于空白对照组(P < 0.05);低剂量组和高剂量组的总凋亡率明显高于空白对照组(P < 0.05);药物处理3天后,空白对照组的细胞增值率要明显高于低剂量组和高剂量组(P < 0.05)。说明,达克替尼可以抑制细胞增殖,促进细胞凋亡。可以作为EFGR阳性的肺腺癌A549细胞的治疗药物。
对于肺腺癌患者而言,尤其是晚期肺腺癌,表皮生长因子受体(Epidermal Growth Factor Receptor, EFGR)突变是其耐药性的主要特点 [7] [8] [9]。周玮玮等人 [10] 利用量子点(QDs)荧光探针检测人肺腺癌组织,结果表明,晚期肺腺癌组织可见EFGR的高表达。史张等人 [11] 对581例肺腺癌患者进行检测,可见132例EFGR的高突变,结果表明,EFGR的高突变与肺腺癌的增殖和侵袭有关。本研究在前人的基础上,利用第二代表皮生长因子受体酪氨酸激酶抑制剂(Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors, EGFR-TKIs)达克替尼进行治疗,结果表明,达克替尼可以抑制EFGR的mRNA相对表达水平和蛋白表达水平,说明达克替尼可以抑制EFGR的表达。
既往研究表明,Caspase-3和Caspase-9是与细胞凋亡密切相关,因此,也有学者称他们为“凋亡相关蛋白”,将Caspase-3和Caspase-9作为细胞凋亡的指示分子 [12] [13] [14]。本研究以Caspase-3作为凋亡的靶点,结果表明,达克替尼可以促进Caspase-3的mRNA相对表达水平和蛋白表达水平,说明达克替尼可以促进肺腺癌细胞的凋亡。另外,本研究利用CCK-8试剂盒检测细胞的增殖,结果表明,药物处理2天后,空白对照组的细胞增值率要明显高于低剂量组和高剂量组(P < 0.05);药物处理3天后,空白对照组的细胞增值率要明显高于低剂量组和高剂量组(P < 0.05)。结果说明,达克替尼可以抑制肺腺癌细胞的增殖。并且与达克替尼的剂量无关。
综上所述,低剂量达克替尼可以抑制肺腺癌细胞的增殖,促进肺腺癌细胞的凋亡,并且可以抑制EFGR的表达,可以作为耐药性肺腺癌的治疗方案。
文章引用
郭李玲,毛 俊. 新型TK抑制剂达克替尼在肺腺癌细胞增殖和凋亡的影响
Effect of a Novel TK Inhibitor Dacomitinib on Proliferation and Apoptosis of Lung Adenocarcinoma Cells[J]. 药物化学, 2021, 09(03): 112-118. https://doi.org/10.12677/HJMCe.2021.93014
参考文献
- 1. Jordan, E.J., Kim, H.R., Arcila, M.E., et al. (2017) Prospective Comprehensive Molecular Characterization of Lung Adenocarcinomas for Efficient Patient Matching to Approved and Emerging Therapies. Cancer Discovery, 7, 15 p. https://doi.org/10.1158/2159-8290.CD-16-1337
- 2. Velcheti, V., Hida, T., Reckamp, K.L., et al. (2017) Phase 2 Study of Lenvatinib in Patients with RET Fusion-Positive Adenocarcinoma of the Lung. European Journal of Cancer, 72, S178. https://doi.org/10.1016/S0959-8049(17)30651-2
- 3. Feng, M., Zhu, J., Liang, L., et al. (2017) Diagnostic Value of Tumor Markers for Lung Adenocarcinoma-Associated Malignant Pleural Effusion: A Validation Study and Me-ta-Analysis. International Journal of Clinical Oncology, 22, 283-290. https://doi.org/10.1007/s10147-016-1073-y
- 4. Ono, A., Kenmotsu, H., Watanabe, M., et al. (2014) Mutant Al-lele Frequency Predicts the Efficacy of EGFR-TKIs in Lung Adenocarcinoma Harboring the L858R Mutation. Annals of Oncology, 25, 1948-1953. https://doi.org/10.1093/annonc/mdu251
- 5. Kim, I.A., Lee, J.S., Kim, H.J., et al. (2018) Cumulative Smoking Dose Affects the Clinical Outcomes of EGFR-Mutated Lung Adenocarcinoma Patients Treated with EGFR-TKIs: A Retrospective Study. BMC Cancer, 18, Article No. 768. https://doi.org/10.1186/s12885-018-4691-0
- 6. Zhou, F., Ma, W., Li, W., et al. (2018) Thick-Wall Cavity Predicts Worse Progression-Free Survival in Lung Adenocarcinoma Treated with First-Line EGFR-TKIs. BMC Cancer, 18, Article No. 1033. https://doi.org/10.1186/s12885-018-4938-9
- 7. Chen, H., Yang, X., Liu, H., et al. (2017) Correlation between Serum Tumor Markers and Efficacy of First-Line EGFR-TKIs in Patients with Advanced Lung Adenocarcinoma. Chi-nese Journal of Lung Cancer, 1, 76-87.
- 8. Ma, X., Zhu, H., Guo, H., et al. (2016) Risk Factors of Brain Metastasis during the Course of EGFR-TKIs Therapy for Patients with EGFR-Mutated Advanced Lung Adenocarcinoma. Onco-target, 7, 81906-81917. https://doi.org/10.18632/oncotarget.11918
- 9. Ming-Szu, H., Jr-Hau, L., Lin, Y.C., et al. (2016) The Content of Mutant EGFR DNA Correlates with Response to EGFR-TKIs in Lung Adenocarcinoma Patients with Common EGFR Mutations. Medicine, 95, e3991. https://doi.org/10.1097/MD.0000000000003991
- 10. 周玮玮, 王磊, 苗玉, 等. 量子点荧光探针检测肺腺癌组织中EGFR的表达[J]. 标记免疫分析与临床, 2018, 25(5): 736-739.
- 11. 史张, 宋长恩, 施睿峰, 等. 肺腺癌CT及临床特征与EGFR 19号外显子突变的相关性研究[J]. 临床放射学杂志, 2017, 36(4): 490-494.
- 12. Bose, T.O., Pham, Q.M., Jellison, E.R., et al. (2013) CD11a Regulates Effector CD8 T Cell Differentiation and Central Memory Development in Response to Infection with Listeria Monocytogenes. Infection and Immunity, 81, 1140-1151. https://doi.org/10.1128/IAI.00749-12
- 13. Tai, W., Chen, Z. and Cheng, K. (2013) Expression Profile and Func-tional Activity of Peptide Transporters in Prostate Cancer Cells. Molecular Pharmaceutics, 10, 477-487. https://doi.org/10.1021/mp300364k
- 14. Ferrone, S. and Marincola, F.M. (1995) Loss of HLA Class I Antigens by Melanoma Cells: Molecular Mechanisms, Functional Significance and Clinical Relevance. Immunology Today, 16, 487-494. https://doi.org/10.1016/0167-5699(95)80033-6
