Advances in Clinical Medicine
Vol.
13
No.
03
(
2023
), Article ID:
63242
,
8
pages
10.12677/ACM.2023.133675
非酒精性脂肪性肝病患者肝脏脂肪含量与 铁含量的相关性
朱文妹,李晖*
华北理工大学附属医院核磁室,河北 唐山
收稿日期:2023年2月24日;录用日期:2023年3月19日;发布日期:2023年3月29日

摘要
目的:磁共振mDIXON-Quant技术定量评估非酒精性脂肪肝病(non alcoholic fatty liver disease, NAFLD)患者肝脏脂肪含量与铁含量的相关性。方法:选取2021年3月至2022年9月在华北理工大学附属医院就诊经超声或CT检查诊断为脂肪肝并且无过量饮酒史的患者48例,作为非酒精性脂肪肝组;另外选取同时期健康体检者54名,作为对照组。所有受试者均进行上腹部mDIXON-Quant检查,由2名放射科医生(测量数据前均进行过培训)采用双盲法通过Philips ISP (Intellispace Portal)工作站对脂肪分数(Fat faction, FF)图、R2*图进行数据的测量。应用SPSS21.0软件对所有数据进行统计学分析。结果:两名医师所测得的数据可信度及一致性均良好(组内相关系数及alpha系数均 > 0.9)。脂肪肝组与正常对照组间年龄及性别无统计学差异(P > 0.05)。脂肪肝组体质量指数(Body mass index, BMI)、肝脏FF值、肝脏R2*值均高于正常对照组,差异具有统计学意义(P < 0.001)。Logistic回归分析显示肝脏R2*值(OR = 1.198, 95% CI = 1.071~1.339, P < 0.05)、BMI (OR = 1.955, 95% CI = 1.422~2.687, P < 0.05)是NAFLD的独立危险因素,随着肝脏R2*值及BMI值的增加,脂肪肝的风险增加。本组48例NAFLD患者肝脏脂肪分数与肝脏铁含量存在低度正相关性(rs = 0.307, P < 0.05)。结论:mDIXON-Quant可用于评估NAFLD患者肝脏铁含量,且可信度及一致性高。NAFLD患者肝脏铁含量高于正常人,且其肝脏脂肪含量与肝脏铁含量呈低度正相关。R2*值及BMI是NAFLD独立危险因素。
关键词
mDIXON-Quant,肝脏,铁含量,非酒精性脂肪肝病

Correlation between Liver Fat Content and Iron Content in Patients with Non Alcoholic Fatty Liver Disease
Wenmei Zhu, Hui Li*
Nuclear Magnetic Room, Affiliated Hospital of North China University of Science and Technology, Tangshan Hebei
Received: Feb. 24th, 2023; accepted: Mar. 19th, 2023; published: Mar. 29th, 2023

ABSTRACT
Objective: Quantitative evaluation of the correlation between liver fat content and iron content in patients with non alcoholic fatty liver disease (NAFLD) by magnetic resonance mDIXON-Quant technique. Methods: Forty-eight patients who were diagnosed with fatty liver by ultrasound or CT examination and had no history of excessive drinking at the Affiliated Hospital of North China University of Technology from March 2021 to September 2022 were selected as non alcoholic fatty liver group; an additional 54 healthy volunteers from the same period were selected as the control group. All subjects underwent an upper abdomen mDIXON-Quant examination and data were measured by two radiologists (who were trained before measuring the data) with double blind method via Philips ISP (Intellispace Portal) workstation for fat fraction mapping and R2* mapping. Data were analyzed by SPSS21.0. Results: The data obtained by the two radiologists were consistent and reliable (intra-class correlation coefficient and alpha coefficient > 0.9). There was no significant difference in age and gender between the two groups (P > 0.05). The differences in body mass index (BMI), liver FF and liver R2* values between the two groups were statistically significant (P < 0.001). Logistic regression analysis showed that liver R2* values (OR = 1.198, 95% CI = 1.071~1.339, P < 0.05), BMI (OR = 1.955, 95% CI = 1.422~2.2687, P < 0.05) were independent risk factors for NAFLD, and the risk of fatty liver increased with increasing liver R2* value and BMI. There was a low positive correlation between liver fat fraction and liver iron content in our group of 48 patients with NAFLD (rs = 0.307, P < 0.05). Conclusions: The mDIXON-Quant can be used to assess liver iron content in non alcoholic fatty liver with high reliability and consistency. Patients with NAFLD had higher liver iron levels than normal and their liver fat content had a low positive correlation with liver iron content. R2* values and BMI were independent risk factors for NAFLD.
Keywords:mDIXON-Quant, Liver, Iron Content, Non Alcoholic Fatty Liver Disease

Copyright © 2023 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
NAFLD是目前欧美国家最常见的慢性肝脏疾病,在中国的患病率亦已达到了29.2%,且呈不断上升的趋势 [1] [2]。多病因和复杂发病机制致使临床上尚无针对NAFLD的有效治疗药物,其发生发展涉及包括氧化应激、胰岛素抵抗(Insulin Resistance, IR)等多种危险因素 [3]。近年研究表明 [4] [5],大多数NAFLD患者存在着肝脏铁沉积,铁与NAFLD的发生存在着紧密联系,且在NAFLD进展、预后中都起着重要作用,因此对肝脏铁含量的定量评估可以为临床相关疾病诊疗及预后评估等方面提供一定的帮助 [6] [7]。本研究拟采用mDIXON-Quant技术定量评估NAFLD患者肝脏铁含量,探讨其肝脏脂肪含量与铁含量相关性。
2. 资料与方法
2.1. 研究对象
选取2021年3月至2022年9月在华北理工大学附属医院就诊并经超声或CT检查诊断为脂肪肝且无过量饮酒史为非酒精性脂肪肝的患者及同时期正常健康志愿者。纳入标准:1) 年龄 ≥ 18岁;2) 符合2018年中华医学会肝病学分会脂肪肝和酒精性肝病学组提出的《非酒精性脂肪性肝病防治指南(2018年更新版)》 [8] 中NAFLD的诊断标准;3) 同时期超声或CT检查无脂肪肝的健康体检者作为正常对照组;4) 临床资料完整。所有患者均行上腹部MRI检查,包括mDIXON-Quant序列。排除标准:1) 有MRI检查禁忌征者;2) 原发性或继发性血液系统疾病导致肝脏铁沉积者;3) 肝脏肿瘤切除术后或因恶性肿瘤正在接受放化疗的患者;4) 饮酒史:过去12个月每周饮用乙醇(酒精)男性 ≥ 210 g,女性 ≥ 140 g;5) 肝炎病毒携带者及患者。6) 糖尿病史及高血压病史。最终纳入102例患者,年龄18~87岁,平均年龄(55 ± 13)岁;其中NAFLD组48例,男性17例,女性31例,平均年龄(57.15 ± 12.45)岁,BMI:27.19 ± 3.06 kg/m2;正常组54例,男性18例,女性36例,平均年龄(53.98 ± 14.25)岁,BMI:21.29 ± 2.67 kg/m2。本研究经医院伦理委员会批准,所有受试者均知情同意。
2.2. 仪器与方法
应用荷兰Philips Ingenia 3.0T MRI扫描仪,检查者禁食4~6 h,扫描前对患者进行呼吸培训。所有检查者行仰卧位,选择3D mDIXON-Quant梯度回波序列以及常规T2WI、同反相位及弥散加权序列扫描。扫描参数:mDIXON-Quant序列参数:重复时间5.7 ms,回波时间1.02 ms,回波时间间隔1.3 ms,层厚6 mm,视野400 mm × 350 mm × 231 mm,体素2.5 mm × 2.5 mm × 6 mm,激励次数1,敏感性编码2,低反转角(3˚)减少T1偏倚,扫描6个回波对T2*衰减进行校正,屏气扫描,扫描时间16 s。
2.3. 图像分析与测量
采用Philips ISP (Intellispace Portal)工作站处理MRI数据,两名放射科医师(测量数据前均进行过培训)采用双盲法分析图像质量并进行数据测量,在肝脏FF图像于肝内各段(共八段)各画取一面积约300 mm2感兴趣区(region of interest, ROI),放置时对照同反相位图像,尽可能避开肝边缘、肝内胆管、血管等结构,复制FF图像上的ROI并置于R2*图像上,FF图与R2*图上的ROI尽可能为肝脏同一层面的相同位置,记录各ROI内FF值及R2*值,计算每位医师所测得肝各段FF值及R2*值的平均值作为肝总体的FF值及R2*值(图1~4)。

Figure 1. Shows the normal control subject, female, 45 years old, with a BMI of 18.4 kg/m2. On the FF diagram, an ROI was placed on segments II, IV and VIII, and the FF values were 1.57%, 1.92%, 2.4% respectively
图1. 所示正常对照组受试者,女,45岁,BMI为18.4 kg/m2。在FF图上肝II段、IV段、VIII段分别放置1个ROI,其FF值分别为1.57%、1.92%、2.4%
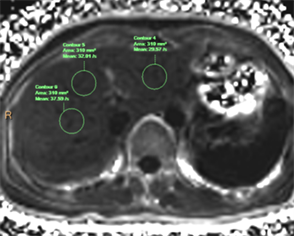
Figure 2. Shows the same subject, On the R2* diagram, an ROI was placed on segments II, IV and VIII, and the R2* values were 29.57−1 S, 32.01−1 S, 37.59−1 S respectively
图2. 所示同一受试者,在R2*图上肝II段、IV段、VIII段相同位置分别放置1个ROI,其R2*值分别为29.57−1 S、32.01−1 S、37.59−1 S

Figure 3. Fatty liver group, male, 56 years old, BMI 30.33 kg/m2. One ROI was placed in section IV, Section VII and Section VIII of FF map respectively, the FF values were 23.62%, 25.99% and 28.2% respectively
图3. 脂肪肝组受试者,男,56岁,BMI为30.33 kg/m2。在FF图肝IV段、VII段、VIII段分别放置1个ROI,其FF值分别为23.62%、25.99%、28.2%
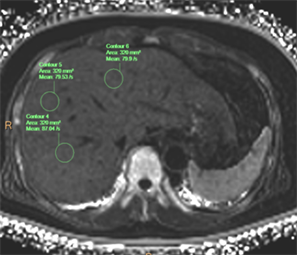
Figure 4. Shows the same subject, an ROI was placed in section IV, section VII and section VIII of R2* map respectively, the R2* values were 79.9−1 S, 87.04−1 S and 79.53−1 S, respectively
图4. 同一受试者,在R2*图肝IV段、VII段、VIII段分别放置1个ROI,其R2*值分别为79.9−1 S、87.04−1 S、79.53−1 S
2.4. 统计学方法
采用Shpiro-Wilk检验分析计量资料的正态性,计量资料用 或M ± QR表示;计量资料组间差异比较采用独立样本t检验或非参数Mann-Whitney U检验。定性资料组间比较采用χ2检验。采用SPSS 21.0信度分析检验2名医师测量数据的可信度,alpha系数 > 0.9表示信度极高;应用组内相关系数(intraclass correlation coefficient, ICC)表示测量结果的重复性,ICC > 0.75表示重复性非常高。采用logistic回归方法分析肝脏铁含量、BMI与NAFLD的关系。采用Spearman分析脂肪肝组肝脏脂肪含量与铁含量的相关性,相关系数0 < rs ≤ 0.4为低度相关,0.4 < rs ≤ 0.7为中度相关,0.7 < rs ≤ 1为高度相关。P < 0.05为差异有统计学意义。
3. 结果
3.1. 两名影像医师测量数据可信度及重复性分析
两名影像医师肝脏FF值及R2*值的alpha系数均大于0.9,ICC值均大于0.75,均具有良好的可信度及重复性,取两名医师测量数据的平均值进行后续分析。见表1。
Table 1. Liver FF and R2* values determined by different radiologist and their comparison ( )
表1. 不同医师测定的肝脏FF值、R2*值及其比较( )
注:1) *:M ± QR。2) #:Mann-Whitney U检验。
3.2. 脂肪肝组及正常组研究对象基线资料及R2*值的比较
脂肪肝组及正常组性别、年龄均无统计学差异(P > 0.05),脂肪肝组BMI、肝脏脂肪含量及铁含量均高于正常组,差异具有统计学意义(P < 0.05)。见表2。
Table 2. Comparison of baseline data between normal group and fatty liver group ( )
表2. 正常组及脂肪肝组研究对象基线资料的比较( )
注:1) *:M ± QR。2) #:Mann-Whitney U检验。
3.3. Logistic回归分析
结果显示肝脏铁含量、BMI是NAFLD的独立危险因素(P < 0.05),随着R2*值及BMI值的增加,脂肪肝的风险增加。见表3。
Table 3. Multivariate logistic regression analysis of influencing factors of NAFLD
表3. 多因素logistic回归分析NAFLD的影响因素
3.4. 脂肪肝组肝脏脂肪含量与铁含量的关系
Spearman相关分析显示脂肪肝组肝脏脂肪含量与铁含量之间呈低度正相关(rs = 0.307, P < 0.05)。如图5。

Figure 5. Scatter diagram of correlation between liver fat content and iron content of subjects in the fatty liver group (n = 48)
图5. 脂肪肝组研究对象肝脏脂肪含量与铁含量的相关性散点图(n = 48)
4. 讨论
目前,肝脏病理活检仍是精确定量肝脏脂肪含量及铁含量的金标准,但活检存在感染或出血等风险,难以在临床上推广。超声和CT检查是筛查肝脂肪变性的常规手段,但对轻度脂肪肝的诊断效能均较差。目前,超声及CT检查对于肝脏铁沉积定量评估的准确性仍需进一步研究。磁共振成像被认为是目前最佳的可精确定量评估肝脏脂肪变性的影像学方法,包括化学位移成像技术及磁共振波谱(Magnetic resonance spectroscopy, MRS)等。既往MRS被认为是多脏器脂肪浸润无创性定量的金标准 [9],但MRS检测对于设备及仪器的要求较高,扫描时间长且后处理繁琐,以及无法全脏器进行检查,这些因素在一定程度上也限制了其在临床上的应用 [10]。传统的化学位移成像法,即双回波技术对于磁场的均匀性要求高,水脂难以完全分离,且其对重度脂肪肝的诊断准确性较差,肝脏内铁沉积也容易影响测量的结果。随着技术的发展,基于梯度回波序列的复合化学位移编码MRI技术产生,其采集三个及以上的回波时间信号,可实现水脂信号的完全分离 [11]。
本研究采用的3D mDIXON-Quant技术采集6回波并结合7峰值脂肪模型自动生成高质量FF图和R2* mapping图,通过后处理软件,在FF图及R2* mapping图上直接勾画ROI即可获得FF值及R2*值,即脂肪含量及铁含量,操作简单,且均具有极高的可重复性及可信度,国内外已有相关报道 [12] [13] [14]。洪居陆 [12] 等研究显示不同医师、同一医师采取不同的ROI以及与肝脏包膜不同的距离所测得的FF值均无统计学差异。许桂晓 [13] 等研究显示应用R2*值评估肝内铁沉积准确性明显高于肝脏/肌肉信号强度对比噪声比(ROC曲线下面积分别为0.94、0.69)。本研究中两位医师测量的肝脏FF值及R2*值的alpha系数及ICC均大于0.9,亦说明了mDIXON-Quant用于定量评估肝脏脂肪含量和铁含量可信度及可重复性极高。
目前NAFLD与铁过载之间的病理生理机制尚不清楚。近年来研究发现,铁过载可能是NAFLD由单纯性脂肪肝向非酒精性脂肪性肝炎或肝纤维化进展的危险因素 [15]。铁作为人体必需的微量元素之一,亦是一种强催化剂,通过Fenton反应催化氧化反应生成活性氧中间产物促进氧化应激反应。氧化应激是IR的核心机制,氧化应激和IR是NAFLD的主要致病因素,与不伴有铁超载的NAFLD相比,存在铁过载的NAFLD氧化应激标志物水平增高 [16] [17]。铁含量过高可在肝脏中沉积,导致肝脏铁过载,促使NAFLD发展至NASH或肝纤维化。而脂质过氧化、氧化应激、胰岛素抵抗亦可能通过正反馈机制导致肝脏进一步铁过载,导致更多的氧化应激和炎症,对肝脏进行再次打击,肝细胞损害更加严重 [18] [19]。受损后再生的肝细胞,转铁蛋白受体表达水平增高,导致肝脏铁沉积进一步加重 [20]。目前的研究表明去铁治疗可改善胰岛素敏感性 [21] [22],这也为临床使用去铁治疗NAFLD提供了依据。
本研究应用mDIXON-Quant技术探讨NAFLD患者肝脏脂肪含量与肝脏铁含量的关系,结果显示NAFLD患者肝脏铁含量高于正常人,且肝脏脂肪含量与铁含量呈正相关,这与既往国内外报道基本一致 [23] [24] [25]。高琪 [23] 等研究显示随着肝脏病理脂肪变分级的增加,肝脏R2*值也不断增加,且肝脏病理脂肪变程度与肝脏R2*值亦呈低度正相关(r = 0.382, P < 0.05)。
本研究的不足之处在于:1) 本研究未能与肝脏病理结果做比照分析,且对于肝脏脂肪含量和铁含量的测量应用的是临床研究常用的ROI画取法,近年来有研究用半自动分割方法测量整个胰腺脂肪含量的 [26],其应用于肝脏是否更能代表肝脏整体的脂肪含量及铁含量,仍需进一步做相关方面的研究。2) 本研究只分了脂肪肝组及正常组,未对NAFLD患者肝脏脂肪变性程度进行轻、中、重度分级,肝脏铁含量与脂肪肝病程、脂肪肝分级是否存在相关性,后续仍需进一步研究。
综上所述,mDIXON-Quant可用于非酒精性脂肪肝患者肝脏铁含量的定量评估,且可信度及重复度高。非酒精性脂肪肝患者肝脏存在一定的铁沉积,且肝脏脂肪含量与铁含量呈低度正相关。BMI及肝脏铁含量是非酒精性脂肪肝的独立危险因素。
文章引用
朱文妹,李 晖. 非酒精性脂肪性肝病患者肝脏脂肪含量与铁含量的相关性
Correlation between Liver Fat Content and Iron Content in Patients with Non Alcoholic Fatty Liver Disease[J]. 临床医学进展, 2023, 13(03): 4711-4718. https://doi.org/10.12677/ACM.2023.133675
参考文献
- 1. Reimer, K.C., Wree, A., Roderburg, C., et al. (2020) New Drugs for NAFLD: Lessons from Basic Models to the Clinic. Hepatology International, 14, 8-23. https://doi.org/10.1007/s12072-019-10001-4
- 2. Zhou, F., Zhou, J.H., Wang, W.X., et al. (2019) Unexpected Rapid Increase in the Burden of NAFLD in China from 2008 to 2018: A Systematic Re-view and Meta-Analysis. Hepatology, 70, 1119-1133. https://doi.org/10.1002/hep.30702
- 3. 向辉, 张晓晶, 李红良, 等. 影响非酒精性脂肪肝病发生发展的主要危险因素[J]. 武汉大学学报(医学版), 2022, 43(6): 1020-1024.
- 4. 谢春晓. 铁过载与2型糖尿病非酒精性脂肪肝病严重程度的关系探讨[D]: [硕士学位论文]. 天津: 天津医科大学, 2016.
- 5. Dang, Y.X., Shi, K.N., Wang, X.M., et al. (2017) Early Changes in Glutamate Metabolism and Perfusion in Basal Ganglia Following Hypox-ia-Ischemia in Neonatal Piglets: A Multi-Sequence 3.0T MR Study. Frontiers in Physiology, 8, 237-245. https://doi.org/10.3389/fphys.2017.00237
- 6. 陈冉, 刘阳. 铁死亡的机制及其与非酒精性脂肪肝病的关系[J]. 湖北民族大学学报(医学版), 2020, 37(3): 80-83.
- 7. 王庆玲, 岳伟, 张欣欣, 陈立. 铁代谢异常与非酒精性脂肪性肝病的关系[J]. 中华健康管理学杂志, 2020, 14(6): 587-591.
- 8. 非酒精性脂肪性肝病防治指南(2018年更新版) [J]. 临床肝胆病杂志, 2018, 34(5): 947-957.
- 9. Szczepaniak, L.S., Nurenberg, P., Leonard, D., et al. (2005) Magnetic Resonance Spectroscopy to Measure Hepatic Triglyceride Content: Prevalence of Hepatic Steatosis in Thegen-eral Population. American Journal of Physiology-En- docrinology and Metabolism, 288, 462-468. https://doi.org/10.1152/ajpendo.00064.2004
- 10. Krishan, S., Jain, D., Bathina, Y., et al. (2016) Non-Invasive Quantification of Hepatic Steatosis in Living, Related Liver Donors Using Dual-Echo Dixon Imaging and Single-Voxel Proton Spectroscopy. Clinical Radiology, 71, 58-63. https://doi.org/10.1016/j.crad.2015.10.002
- 11. 宋彬, 叶铮, 魏毅, 等. 肝脏脂肪变性的磁共振影像定量分析: 新技术方法与临床应用[J]. 西部医学, 2020, 32(4): 489-491+495.
- 12. 洪居陆, 贺小红, 李慧, 等. 医师资历和ROI设置对IDEAL-IQ评价肝脏脂肪含量的影响[J]. 国际放射医学核医学杂志, 2020, 44(6): 359-364.
- 13. 许桂晓, 何浩强, 彭康强, 等. 应用磁共振IDEAL-IQ技术评估肝脏铁沉积[J]. 临床放射学杂志, 2019, 38(11): 2200-2204.
- 14. Caussy, C., Alquiraish, M.H., Nguyen, P., et al. (2018) Optimal Threshold of Controlled Attenuation Parameter with MRI-PDFF as the Gold Standard for the Detection of Hepatic Steatosis. Hepatology, 67, 1348-1359. https://doi.org/10.1002/hep.29639
- 15. Lin, S.C., Ang, B., Hernandez, C., et al. (2016) Cardiovascular Risk As-sessment in the Treatment of Nonalcoholic Steatohepatitis: A Secondary Analysis of the MOZART Trial. Therapeutic Advances in Gastroenterology, 9, 152-161. https://doi.org/10.1177/1756283X15621232
- 16. Tan, T.C., Crawford, D.H., Jaskowski, L.A., et al. (2013) Excess Iron Modulates Endoplasmic Reticulum Stress-Associated Pathways in a Mouse Model of Alcohol and High-Fat Di-et-Induced Liver Injury. Laboratory Investigation, 93, 1295-1312. https://doi.org/10.1038/labinvest.2013.121
- 17. Maliken, B.D., Nelson, J.E., Klintworth, H.M., et al. (2013) He-patic Reticuloendothelial System Cell Iron Deposition Is Associated with Increased Apoptosis in Nonalcoholic Fatty Liv-er Disease. Hepatology, 57, 1806-1813. https://doi.org/10.1002/hep.26238
- 18. Jou, J., Choi, S.S. and Diehl, A.M. (2008) Mechanisms of Disease Pro-gression in Nonalcoholic Fatty Liver Disease. Seminars in Liver Disease, 28, 370-379. https://doi.org/10.1055/s-0028-1091981
- 19. Diehl, A.M. (2005) Lessons from Animal Models of NASH. Hepa-tology Research, 33, 138-144. https://doi.org/10.1016/j.hepres.2005.09.022
- 20. Lee, A.W., Oates, P.S. and Trinder, D. (2003) Effects of Cell Proliferation on the Uptake of Transferrin-Bound Iron by Human Hepatoma Cells. Hepatology, 38, 967-977. https://doi.org/10.1002/hep.1840380423
- 21. Valenti, L., Fracanzani, A.L., Dongiovanni, P., et al. (2007) Iron De-pletion by Phlebotomy Improves Insulin Resistance in Patients with Nonalcoholic Fatty Liver Disease and Hyperfer-ritinemia: Evidence from a Case-Control Study. The American Journal of Gastroenterology, 102, 1251-1258. https://doi.org/10.1111/j.1572-0241.2007.01192.x
- 22. Facchini, F.S., Hua, N.W., Stoohs, R.A., et al. (2002) Ef-fect of Iron Depletion in Carbohydrate-Intolerant Patients with Clinical Evidence of Nonalcoholic Fatty Liver Disease. Gastroenterology, 122, 931-939. https://doi.org/10.1053/gast.2002.32403
- 23. 高琪, 丁建平, 蒋迪华, 章建华. T2*校正的多回波Dixon成像序列检查评估非酒精性脂肪性肝病脂肪变和铁沉积的价值[J]. 现代实用医学, 2022, 34(5): 682-684.
- 24. Imajo, K., Kessoku, T., Honda, Y., et al. (2021) MRI-Based Quantitative R2(*) Mapping at 3 Tesla Reflects Hepatic Iron Overload and Pathogenesis in Nonalcoholic Fatty Liver Disease Patients. Journal of Magnetic Resonance Imaging, 55, 111-125. https://doi.org/10.1002/jmri.27810
- 25. Kim, T.H., Jeong, C.W., Jun, H.Y., et al. (2019) Noninvasive Differential Diagnosis of Liver Iron Contents in Nonalcoholic Steatohepatitis and Simple Steatosis Using Multiecho Dixon Magnetic Resonance Imaging. Academic Radiology, 26, 766-774. https://doi.org/10.1016/j.acra.2018.06.022
- 26. 尤亚茹, 张钦和, 刘爱连, 梁超, 王家正, 林良杰, 陈丽华, 宋清伟. 半自动分割技术对全胰腺脂肪定量的可行性: 与传统ROI方法的比较性研究[J]. 磁共振成像, 2020, 11(12): 1124-1128.
NOTES
*通讯作者。
