Journal of Organic Chemistry Research
Vol.
10
No.
01
(
2022
), Article ID:
49907
,
13
pages
10.12677/JOCR.2022.101003
非均相无溶剂Amberlyst-15催化一锅法 高效合成1,4-二氢吡啶
雷兹然,俞锦航,吕新*,谢云龙*
浙江师范大学化学与生命科学学院先进催化材料教育部重点实验室,浙江 金华
收稿日期:2022年3月3日;录用日期:2022年3月25日;发布日期:2022年3月31日

摘要
本文主要研究了在无溶剂体系及非均相催化剂离子交换树脂Amberlyst 15催化下,高效的合成了1,4-二氢吡啶。该反应体系该具有原料廉价、底物适用范围广、产率高、可放大量制备、催化剂可回收利用、环境友好、操作简便等优良特点。
关键词
1,4-二氢吡啶,无溶剂合成,一锅法,非均相,Amberlyst 15

Heterogeneous Solvent-Free Amberlyst-15-Catalyzed Efficient One-Pot Synthesis of 1,4-Dihydropyridine
Ziran Lei, Jinhang Yu, Xin Lyu*, Yunlong Xie*
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Life Science, Zhejiang Normal University, Jinhua Zhejiang
Received: Mar. 3rd, 2022; accepted: Mar. 25th, 2022; published: Mar. 31st, 2022

ABSTRACT
This paper mainly studies the efficient synthesis of 1,4-dihydropyridine in a solvent-free system and a heterogeneous catalyst catalyzed by ion exchange resin Amberlyst-15. This process has many merits, such as commercial available catalysts with low cost, broad substrate scope, high yields, large-scale preparation, easy recovery, environmental friendliness, and simple work-up procedure.
Keywords:1,4-Dihydropyridines, Solvent-Free Synthesis, One-Pot, Heterogeneous, Amberlyst 15

Copyright © 2022 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
1882年,化学家Arthur Hantzsch首次合成了1,4-二氢吡啶(1,4-DHP) [1]。随后,1,4-二氢吡啶化合物被证明为一类重要的杂环化合物,该类化合物具有很好的生理活性,在生物、医药等方面有广泛应用。如,钙离子通道阻滞剂 [2] [3] [4],抗肿瘤 [5],消炎 [6],镇痛 [7],抗血小板凝聚 [8],抗糖尿病活性 [9] [10],也作为帕金森疾病的抗脑萎缩剂 [8] 和肿瘤治疗的化疗剂 [5] [11]。目前,有十几个重要的1,4-二氢吡啶化合物,如非洛地平,氨氯地平,尼莫地平,作为药物在全球内生产和使用 [12] [13] [14],如图1所示。
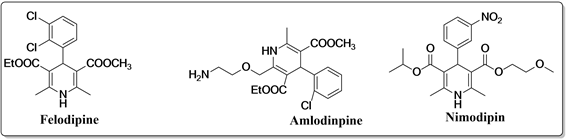
Figure 1. Several 1,4-dihydropyridine compounds with surface properties
图1. 几个的具有表性的1,4-二氢吡啶化合物
由于1,4-二氢吡啶化合物的重要性,国内外对该类化合物的合成十分活跃。目前,合成该类化合物的策略和方法有微波法 [15]、超声波法 [16] [17]、离子液体法 [18]、相转移催化剂法 [19]、布朗斯酸法 [20] [21] [22] [23]、无溶剂法 [24] [25]、路易斯酸法 [26] [27] 等。然而,大多数方法存在许多不足,如反应时间长、产率低、后处理繁琐、大量使用有机溶剂、催化剂无法回收利用、大批量生产工业化比较困难等。最近,国内化学家张国林 [28]、刘建利 [29] 等课题组在合成1,4-二氢吡啶也取得了较好的结果。但是,发展一种简单、高效的1,4-二氢吡啶合成方法仍然是十分必要的。
本文提供一种以Amberlyst 15离子交换树脂作为催化剂,无溶剂下高效的合成1,4-二氢吡啶的方法。该方法还有几个显著特点:1) 催化剂可以回收利用;2) 反应可以放大,对收率影响不大;3) 后处理简单,可实验工业化生产。
2. 实验部分
2.1. 仪器与试剂
WRS-1B数字熔点仪(温度计未经校正);Bruker Avance 400型或600型核磁共振波谱仪(DMSO或CDCl3为溶剂,TMS为基准物质);有机反应用薄层硅胶板(TLC)跟踪监测。德国Bruker高分辨质谱仪(BioTOFⅢQ)。
试剂:苯甲醛;对甲基苯甲醛;对甲氧基苯甲醛;对氯苯甲醛;间氯苯甲醛;邻氯苯甲醛;对硝基苯甲醛;间硝基苯甲醛;2-呋喃甲醛;2-噻吩甲醛;对溴苯甲醛;邻羟基苯甲醛;5-氯-2-羟基苯甲醛;5-溴-2-羟基苯甲醛;5-甲基-2-羟基苯甲醛;乙酰乙酸乙酯;2,4-戊二酮;1,3-环己二酮;醋酸铵;Amberlyst 15等。所用试剂均为市售分析纯。
2.2. 1,4-二氢吡啶的一般合成步骤及产物结构分析
在25毫升的烧瓶里,加入芳香醛(10 mmol、乙酰乙酸乙酯(2.6 g, 20 mmol)和醋酸铵(1.0 g, 13 mmol),以及100毫克的Amberlyst 15,搅拌下加热到90℃反应1 h,待反应完成后,趁热滤弃催化剂,并用少量乙醇洗涤。将滤液密封好,冷冻,即析出大量晶体,过滤,乙醇洗涤,干燥即得到纯的产物。滤液可以进一步浓缩,继续析出晶体,或者母液浓缩后柱层析(石油醚:乙酸乙酯 = 4:1),将所得的产物合并,可以得到较好的收率。目标产物3的表征数据如下:
Diethyl 2,6-dimethyl-4-phenyl-1,4-dihydropyridine-3,5-dicarboxylate, 3a [30]: Yield. 91%; Pale yellow solid; m.p. 154˚C~156˚C (EtOH) (lit. m.p. 158˚C~160˚C). 1H NMR (600 MHz, CDCl3) δ 7.32 − 7.25 (m, 2H), 7.20 (t, J = 7.6 Hz, 2H), 7.14 − 7.10 (m, 1H), 5.58 (s, 1H), 4.99 (s, 1H), 4.13 − 4.04 (m, 4H), 2.33 (s, 6H), 1.22 (t, J = 7.1 Hz, 6H). 13C NMR (150 MHz, CDCl3) δ 167.7, 147.8, 143.9, 128.0, 127.8, 126.1, 104.1, 59.7, 39.6, 19.6, 14.3; IR v 3342.3, 1687.8, 1651.4, 1488.5, 1453.3, 703.1 cm−1; UVmax = 238.22 nm; HRMS (ESI-TOF) calcd for C19H24NO4 (M+H)+ 330.1700, found 330.1691.
Diethyl 2,6-dimethyl-4-(p-tolyl)-1,4-dihydropyridine-3,5-dicarboxylate, 3b [31]: Yield. 77%;Yellow solid; m.p. 131˚C~133˚C (EtOH) (lit. m.p. 136˚C~137˚C). 1H NMR (600 MHz, CDCl3) δ 7.19 (d, J = 8.0 Hz, 2H), 7.04 (d, J = 7.9 Hz, 2H), 5.75 (s, 1H), 4.97 (s, 1H), 4.15 − 4.07 (m, 4H), 2.34 (s, 6H), 2.30 (s, 3H), 1.26 (t, J = 7.1 Hz, 6H). 13C NMR (151 MHz, CDCl3) δ 167.7, 144.8, 143.8, 135.5, 128.5, 127.8, 104.2, 59.7, 39.1, 21.0, 19.6, 14.2; IR v 3342.5, 1688.7, 1650.2, 1487.8, 142.3, 701.8; UVmax = 231.27 nm; HRMS (ESI-TOF) calcd for C20H26NO4 (M+H)+ 344.1856, found 344.1851.
Diethyl 4-(4-methoxyphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, 3c [32]: Yield. 72%; Yellow solid; m.p. 156˚C~158˚C (EtOH) (lit. m.p. 161˚C~163˚C). 1H NMR (600 MHz, CDCl3) δ 7.23 − 7.17 (m, 2H), 6.77 − 6.72 (m, 2H), 5.58 (bs, 1H), 4.93 (s, 1H), 4.14 − 4.03 (m, 4H), 3.75 (s, 3H), 2.32 (s, 6H), 1.23 (t, J = 7.1 Hz, 6H). 13C NMR (150 MHz, CDCl3) δ 167.7, 157.9, 143.5, 140.3, 129.0, 113.2, 104.4, 59.7, 55.1, 38.7, 19.6, 14.3; IR v 3342.8, 1689.8, 1650.5, 1609.5, 1500.8, 834.4 cm−1; UVmax = 222.34 nm; HRMS (ESI-TOF) calcd for C20H26NO5 (M+H)+ 360.1805, found 360.1801.
Diethyl 4-(4-chlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, 3d [33]: Yield. 93%; Yellow solid; m.p. 150˚C~152˚C (EtOH) (lit. m.p. 150˚C). 1H NMR (600 MHz, CDCl3) δ 7.24 (d, J = 8.5 Hz, 2H), 7.19 (d, J = 8.5 Hz, 2H), 5.65 (bs, 1H), 4.98 (s, 1H), 4.18 − 4.01 (m, 4H), 2.35 (s, 6H), 1.24 (t, J = 7.1 Hz, 6H); 13C NMR (150 MHz, CDCl3) δ 167.4, 146.3, 143.9, 131.7, 129.4, 127.9 103.9, 59.8, 39.3, 19.6, 14.3; IR v 3357.8, 1696.1, 1651.2, 1487.5, 1461.6, 830.4 cm−1; UVmax = 238.56 nm; HRMS (ESI-TOF) calcd for C19H23ClNO4 (M+H)+ 364.1310, found 364.1291.
Diethyl 4-(3-chlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, 3e [34]: Yield. 90%; Yellow solid; m.p. 140˚C~142˚C (EtOH) (lit. m.p. 141˚C). 1H NMR (600 MHz, CDCl3) δ7.26 (t, J = 1.7 Hz, 1H), 7.20 (dt, J = 7.5, 1.4 Hz, 1H), 7.16 (t, J = 7.6 Hz, 1H), 7.14 − 7.10 (m, 1H), 5.64 (bs, 1H), 4.99 (s, 1H), 4.21 − 4.03 (m, 4H), 2.37 (s, 6H), 1.25 (t, J = 7.1 Hz, 6H); 13C NMR (150 MHz, CDCl3) δ 167.3, 149.7, 144.1, 133.6, 129.0, 128.2, 126.3, 126.2, 103.7, 59.8, 39.7, 19.6, 14.2; IR v 3321.8, 1703.0, 1650.2, 1488.6 cm−1; UVmax = 237.85 nm; HRMS (ESI-TOF) calcd for C19H23ClNO4 (M+H)+ 364.1310, found 364.1304.
Diethyl 4-(2-chlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, 3f [35]: Yield. 88%; Yellow solid; m.p. 142˚C~144˚C (EtOH) (lit. m.p. 146˚C~147˚C). 1H NMR (600 MHz, CDCl3) δ 7.40 (dd, J = 7.8, 1.6 Hz, 1H), 7.25 (dd, J = 7.9, 1.1 Hz, 1H), 7.14 (td, J = 7.6, 1.1 Hz, 1H), 7.06 (td, J = 7.8, 1.6 Hz, 1H), 5.79 (s, 1H), 5.42 (s, 1H), 4.14 – 4.06 (m, 4H), 2.32 (s, 6H), 1.22 (t, J = 7.1 Hz, 6H). 13C NMR (151 MHz, CDCl3) δ 167.7, 145.6, 143.9, 132.4, 131.6, 129.2, 127.3, 126.7, 103.8, 59.7, 37.5, 19.5, 14.3; IR v 3354.51, 1701.2, 1648.5, 1491.4 cm−1; UVmax = 238.74 nm; HRMS (ESI-TOF) calcd for C19H24NO4 (M+H)+ 364.1310, found 364.1309.
Diethyl 4-(4-bromophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, 3g [36]: Yield. 88%; Yellow solid; m.p. 162˚C~163˚C (EtOH) (lit. m.p. 162˚C~164˚C). 1H NMR (600 MHz, CDCl3) δ7.32 (d, J = 8.3 Hz, 2H), 7.16 (d, J = 8.3 Hz, 2H), 5.60 (bs, 1H), 4.94 (s, 1H), 4.14 − 4.04 (m, 4H), 2.33 (s, 6H), 1.22 (t, J = 7.1 Hz, 6H); 13C NMR (150 MHz, CDCl3) δ 167.4, 146.8, 143.9, 130.9, 129.8, 119.9, 103.9, 59.8, 39.3, 19.6, 14.3; IR v 3360.6, 1693.2, 1650.4, 1625.5, 1485.8 cm−1; UVmax = 238.65 nm; HRMS (ESI-TOF) calcd for C19H23BrNO4 (M+H)+ 408.0805, found 408.0768.
Diethyl 2,6-dimethyl-4-(4-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate, 3h [33]: Yield. 90%; Yellow solid; m.p. 130˚C~131˚C (EtOH) (lit. m.p. 132˚C). 1H NMR (600 MHz, CDCl3) δ1H NMR (600 MHz, CDCl3) δ 8.11 (d, J = 8.7 Hz, 2H), 7.47 (d, J = 8.7 Hz, 2H), 5.73 (bs, 1H), 5.12 (s, 1H), 4.16 − 4.06 (m, 4H), 2.38 (s, 6H), 1.24 (t, J = 7.1 Hz, 6H); 13C NMR (150 MHz, CDCl3) δ 167.0, 155.1, 146.4, 144.5, 128.9, 123.3, 103.3, 60.0, 40.1, 19.7, 14.3; IR v 3320.4 , 1682.2, 1647.9, 1518.2, 1469.0 cm−1; UVmax = 234.56 nm; HRMS (ESI-TOF) calcd for C19H23N2O6 (M+H)+ 375.1551, found 375.1543.
Diethyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate, 3i [37]: Yield. 91%; Yellow solid; m.p. 156˚C~158˚C (EtOH) (lit. m.p. 159˚C~161˚C). 1H NMR (600 MHz, CDCl3) δ 8.14 (s, 1H), 8.04 − 8.00 (m, 1H), 7.66 (d, J = 7.6 Hz, 1H), 7.39 (t, J = 7.9 Hz, 1H), 5.98 (s, 1H), 5.11 (s, 1H), 4.18 − 4.02 (m, 4H), 2.38 (s, 6H), 1.24 (t, J = 7.1 Hz, 6H). 13C NMR (151 MHz, CDCl3) δ 167.16, 149.95, 148.14, 144.81, 134.55, 128.61, 123.13, 121.34, 103.30, 60.02, 39.97, 19.61, 14.24; IR v 3319.3, 1680.5, 1645.4, 1517.8, 1465.7 cm−1; UVmax = 228.42 nm; HRMS (ESI-TOF) calcd for C19H24NO4 (M+H)+ 375.1551, found 375.1549.
Diethyl 4-(furan-2-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, 3j [38]: Yield. 88%; Yellow solid; m.p. 160˚C~161˚C (EtOH) (lit. m.p. 159˚C~162˚C). 1H NMR (600 MHz, CDCl3) δ7.25 − 7.23 (dd, J = 1.7, 0.8 Hz, 1H), 6.24 (dd, J = 3.1, 1.8 Hz, 1H), 5.97 (d, J = 3.1 Hz, 1H), 5.72 (bs, 1H), 5.22 (s, 1H), 4.29 − 4.09 (m, 4H), 2.36 (s, 6H), 1.29 (t, J = 7.1 Hz, 6H); 13C NMR (150 MHz, CDCl3) δ 167.4, 158.6, 144.9, 140.9, 110.0, 104.4, 100.8, 59.8, 33.4, 19.6, 14.3; IR v 3346.3, 1698.8, 1650.3 cm−1; UVmax = 227.68 nm; HRMS (ESI-TOF) calcd for C17H22NO5 (M+H)+ 320.1492, found 320.1490.
Diethyl 2,6-dimethyl-4-(thiophen-2-yl)-1,4-dihydropyridine-3,5-dicarboxylate, 3k [38]: Yield. 90%; Yellow solid; m.p. 172˚C~174˚C (EtOH) (lit. m.p. 170˚C~172˚C). 1H NMR (600 MHz, CDCl3) δ7.07 (d, J = 5.0 Hz, 1H), 6.89 − 6.84 (m, 1H), 6.83 – 6.81 (m, 1H), 6.07 (bs, 1H), 5.37 (s, 1H), 4.27 − 4.11 (m, 4H), 2.35 (s, 6H), 1.29 (t, J = 7.1 Hz, 6H); 13C NMR (150 MHz, CDCl3) δ 167.4, 151.6, 144.6, 126.3, 123.2, 123.1, 103.6, 103.5, 59.9, 34.4, 19.4, 14.3; IR v 3344.5, 1655.2, 1636.2 cm−1; UVmax = 230.66 nm; HRMS (ESI-TOF) calcd for C17H22NO4S (M+H)+ 336.1264, found 336.1267.
1,1'-(2,6-Dimethyl-4-phenylpyridine-3,5-diyl)diethanone, 3l [39]: Yield. 95%; colorless solid; m.p. 131˚C~132˚C (EtOH) (lit. m.p. 135˚C~136˚C). 1H NMR (600 MHz, CDCl3) δ 7.58 − 7.55 (m, 2H), 7.48 − 7.46 (m, 3H), 2.59 (s, 3H), 2.57 (s, 3H), 2.23 (s, 3H), 2.00 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 206.1, 205.9, 154.6, 152.5, 139.5, 139.1, 136.3, 134.6, 129.5, 128.9, 32.3, 32.2, 22.7, 21.4, 16.2; IR v 3171.6, 1636.2, 1603.6, 1483.2 cm−1; UVmax =247.39 nm; HRMS (ESI-TOF) calcd for C17H18NO2 (M+H)+ 268.1332, found 268.1327.
1,1'-(2,6-Dimethyl-4-(p-tolyl)pyridine-3,5-diyl)diethanone, 3m [39]: Yield. 91%; Yellow solid; m.p. 86˚C~88˚C (EtOH) (lit. m.p. 90˚C~91˚C). 1H NMR (600 MHz, CDCl3) δ 7.46 (d, J = 8.1 Hz, 2H), 7.27 (d, J = 7.9 Hz, 2H), 2.58 (s, 3H), 2.55 (s, 3H), 2.42 (s, 3H), 2.21 (s, 3H), 2.01 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 206.1, 205.9, 154.6, 152.5 139.5, 139.1, 136.3, 134.6, 129.5, 128.9, 32.3, 32.2, 22.7, 21.4, 16.2; IR v 3172.4, 1637.5, 1601.3, 140.2 cm−1; UVmax =238.92 nm; HRMS (ESI-TOF) calcd for C18H20NO2 (M+H)+ 282.1489, found 282.1473.
1,1'-(4-(4-Chlorophenyl)-2,6-dimethylpyridine-3,5-diyl)diethanone, 3n [40]: Yield. 90%; Pale-yellow solid; m.p. 138˚C~140˚C (EtOH) (lit. m.p. 137˚C~139˚C). 1H NMR (600 MHz, CDCl3) δ 7.54 − 7.50 (m, 2H), 7.45 (d, J = 8.4 Hz, 2H), 2.58 (s, 3H), 2.56 (s, 3H), 2.22 (s, 3H), 2.05 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 205.8, 205.5, 153.1, 152.7, 139.3, 137.6, 136.8, 135.7, 130.9, 130.4, 129.4, 129.1, 32.3, 22.7, 16.2; IR v 3173.4, 1639.3, 1607.4, 1484.6 cm−1; UVmax =251.63 nm; HRMS (ESI-TOF) calcd for C17H17ClNO2 (M+H)+ 302.0942, found 302.0936.
9-Phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, 3o [41]: Yield. 88%; Yellow solid; m.p. 275˚C~277˚C (EtOH) (lit. m.p. 279˚C~281˚C). 1H NMR (600 MHz, DMSO-d6) δ9.45 (s, 1H), 7.21 − 7.13 (m, 4H), 7.08 − 7.00 (m, 1H), 4.92 (s, 1H), 2.56 − 2.50 (m, 4H), 2.22 − 2.19 (m, 4H), 1.94 − 1.90 (m, 2H),1.81 − 1.77 (m, 2H); 13C NMR (150 MHz, DMSO-d6) δ194.9, 151.4, 147.4, 127.8, 127.6, 125.5, 112.5, 36.8, 32.2, 26.4, 20.9; IR v 3171.6, 1636.2, 1603.6, 1483.2 cm−1; UVmax =247.39 nm; HRMS (ESI-TOF) calcd for C19H20NO2 (M+H)+ 294.1489, found 294.1493.
9-(p-Tolyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, 3p [42]: Yield. 85%; Yellow solid; m.p. 293˚C~295˚C (EtOH) (lit. m.p. 300˚C). 1H NMR (600 MHz, DMSO) δ 9.43 (s, 1H), 7.03 (d, J = 8.0 Hz, 2H), 6.95 (d, J = 7.9 Hz, 2H), 4.86 (s, 1H), 2.51 (s, 5H), 2.24 − 2.16 (m, 7H), 1.91 (dt, J = 13.0, 4.9 Hz, 2H), 1.83 − 1.72 (m, 2H). 13C NMR (151 MHz, DMSO) δ 195.2, 151.6, 145.0, 134.8, 128.8, 127.9, 113.1, 37.3, 32.1, 26.8, 21.3, 21.0; IR v 3169.3, 1635.3, 1601.0, 1480.7 cm−1; UVmax =242.62 nm; HRMS (ESI-TOF) calcd for C20H22NO2 (M+H)+ 308.1645, found 308.1647.
9-(4-Methoxyphenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, 3q [41]: Yield. 89%; Yellow solid; m.p. 301˚C~303˚C (EtOH) (lit. m.p. 306˚C~308˚C). 1H NMR (600 MHz, DMSO) δ 9.43 (s, 1H), 7.05 (d, J = 8.5 Hz, 2H), 6.72 (d, J = 8.5 Hz, 2H), 4.85 (s, 1H), 3.67 (s, 3H), 2.50 (d, J = 13.7 Hz, 5H), 2.25 − 2.14 (m, 4H), 1.95 − 1.87 (m, 2H), 1.83 − 1.72 (m, 2H). 13C NMR (151 MHz, DMSO) δ 195.3, 157.6, 151.5, 140.2, 128.9, 113.6, 113.2, 55.3, 37.3, 31.6, 26.8, 21.3; IR v 3167.3, 1631.3, 1600.1, 1480.7 cm−1; UVmax =242.63 nm; HRMS (ESI-TOF) calcd for C20H22NO3 (M+H)+ 324.1594, found 324.1591.
9-(4-Chlorophenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, 3r [41]: Yield. 96%; Yellow solid; m.p.292˚C~294˚C (EtOH) (lit. m.p. 298˚C~299˚C). 1H NMR (600 MHz, DMSO) δ 9.52 (s, 1H), 7.22 (d, J = 8.4 Hz, 2H), 7.16 (d, J = 8.5 Hz, 2H), 4.88 (s, 1H), 2.51 (d, J = 1.5 Hz, 6H), 2.25 − 2.16 (m, 4H), 1.91 (dt, J = 13.1, 4.8 Hz, 2H), 1.79 (dt, J = 18.3, 7.9 Hz, 2H). 13C NMR (151 MHz, DMSO) δ 195.3, 152.0, 146.7, 130.5, 129.9, 128.2, 112.5, 37.2, 32.4, 26.8, 21.2; IR v 3172.1, 1639.7, 1607.3, 1485.5 cm−1; UVmax =252.21nm; HRMS (ESI-TOF) calcd for C19H19ClNO2 (M+H)+ 328.1099, found 328.1107.
9-(4-Bromophenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, 3s [43]: Yield. 93%; Yellow solid; m.p. 305˚C~307˚C (EtOH) (lit. m.p. 311˚C~312˚C). 1H NMR (600 MHz, DMSO) δ 9.52 (s, 1H), 7.35 (d, J = 8.3 Hz, 2H), 7.11 (d, J = 8.3 Hz, 2H), 4.87 (s, 1H), 2.51 (d, J = 7.8 Hz, 4H), 2.25 − 2.16 (m, 4H), 1.95 – 1.86 (m, 2H), 1.84 − 1.72 (m, 2H). 13C NMR (151 MHz, DMSO) δ 195.3, 152.0, 147.2, 131.1, 130.3, 119.0, 112.5, 37.2, 32.5, 26.8, 21.2; IR v 3169.1, 1634.8, 1602.7, 1482.7 cm−1; UVmax =249.23 nm; HRMS (ESI-TOF) calcd for C19H19BrNO2 (M+H)+ 372.0594, found 372.0597.
9-(2-Hydroxyphenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, 3t [44]: Yield. 68%; Yellow solid; m.p. 304˚C~306˚C (EtOH) (lit. m.p. 302˚C~304˚C). 1H NMR (600 MHz, DMSO) δ 9.86 (s, 1H), 9.72 (s, 1H), 6.96 (t, J = 7.5 Hz, 1H), 6.83 (d, J = 7.0 Hz, 1H), 6.69 (dd, J = 11.7, 7.7 Hz, 2H), 4.88 (s, 1H), 2.70 − 2.53 (m, 4H), 2.35 − 2.21 (m, 4H), 1.98 − 1.89 (m, 2H), 1.82 (dt, J = 23.5, 9.1 Hz, 2H).13C NMR (151 MHz, DMSO) δ 197.2, 153.7, 134.43 (s), 128.4, 127.6, 120.4, 117.6, 112.8, 36.8, 26.9, 26.7, 21.0, 14.6; IR v 3182.3, 1672.4, 1642.2, 1504.8 cm−1; UVmax =232.62 nm; HRMS (ESI-TOF) calcd for C19H20NO3 (M+H)+ 310.1438, found 310.1431.
9-(5-Chloro-2-hydroxyphenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, 3u [21]: Yield. 75%; Yellow solid; m.p. 289˚C~291˚C (EtOH) (lit. m.p. 295˚C~297˚C). 1H NMR (600 MHz, DMSO) δ 9.92 (s, 1H), 9.89 (s, 1H), 7.00 (dd, J = 8.5, 2.7 Hz, 1H), 6.76 (d, J = 2.6 Hz, 1H), 6.71 (d, J = 8.6 Hz, 1H), 4.85 (s, 1H), 2.62 (dd, J = 13.4, 8.7 Hz, 2H), 2.59 − 2.52 (m, 2H), 2.34 − 2.24 (m, 4H), 1.95 (dt, J = 13.1, 4.9 Hz, 2H), 1.87 − 1.75 (m, 2H).13C NMR (151 MHz, DMSO) δ 197.1, 153.9, 153.1, 136.4, 128.1, 127.5, 123.7, 119.4, 112.1, 100.0, 36.8, 27.6, 26.8, 21.1; IR: 3185.3, 1673.4, 1642.2, 1490.3 cm−1; UVmax = 233.21 nm; HRMS (ESI-TOF) calcd for C19H19ClNO3 (M+H)+ 344.1048, found 344.1051.
9-(5-Bromo-2-hydroxyphenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, 3v [45]: Yield. 70%; Yellow solid; m.p. 268˚C~270˚C (EtOH) (lit. m.p. 272˚C~274˚C). 1H NMR (600 MHz, DMSO) δ 9.94 (s, 1H), 9.89 (s, 1H), 7.12 (dd, J = 8.5, 2.2 Hz, 1H), 6.88 (d, J = 2.2 Hz, 1H), 6.66 (d, J = 8.5 Hz, 1H), 4.84 (s, 1H), 2.62 (dd, J = 13.0, 8.7 Hz, 2H), 2.57 (dd, J = 9.9, 4.7 Hz, 2H), 2.29 (d, J = 13.5 Hz, 4H), 1.99 − 1.89 (m, 2H), 1.87 − 1.74 (m, 2H).13C NMR (151 MHz, DMSO) δ 211.7, 197.0, 153.9, 153.6, 136.8, 131.1, 130.4, 119.9, 112.1, 111.5, 36.8, 27.7, 26.9, 21.1, 14.6; IR v 3180.9, 1674.4, 1641.0, 1488.4 cm−1; UVmax = 233.64 nm; HRMS (ESI-TOF) calcd for C19H19BrNO3 (M+H)+ 388.0543, found 388.0535.
9-(2-Hydroxy-5-methylphenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, 3w: Yield. 53%; Yellow solid; m.p. 253˚C~254˚C (EtOH), 1H NMR (600 MHz, DMSO) δ 9.82 (s, 1H), 9.53 (s, 1H), 6.76 (dd, J = 8.1, 1.9 Hz, 1H), 6.58 (d, J = 8.1 Hz, 2H), 4.85 (s, 1H), 2.66 − 2.59 (m, 2H), 2.59 − 2.53 (m, 2H), 2.34 − 2.23 (m, 4H), 2.11 (s, 3H), 1.95 (dt, J = 13.1, 4.8 Hz, 2H), 1.87 − 1.77 (m, 2H).13C NMR (151 MHz, DMSO) δ 197.2, 153.6, 151.5, 134.2, 128.7, 128.1, 117.5, 112.8, 36.9, 26.9, 26.7, 21.0; IR v 3179.2, 1672.4, 1635.8, 1485.1 cm−1; UVmax =232.54 nm; HRMS (ESI-TOF) calcd for C20H22NO3 (M+H)+ 324.1594, found 324.1597.
2.3. 催化剂回收利用研究
在25毫升的烧瓶里,加入苯甲醛(50 mmol、乙酰乙酸乙酯(100 mmol)和醋酸铵(65 mmol),搅拌下加热到90℃反应1 h,待反应完成后,趁热过滤,少量乙醇洗涤催化剂,直接用于下一次反应。
2.4. 放大反应研究
在250毫升的烧瓶里,加入苯甲醛(300 mmol、乙酰乙酸乙酯(600 mmol)和醋酸铵(390 mmol),搅拌下加热到90℃反应1 h,待反应完成后,趁热过滤,滤液冷却后,置于冰箱冷冻,析出晶体,过滤,得到目标产物。
3. 结果与讨论
3.1. 反应条件的优化
经过文献调研,我们发现利用酸,如硫酸 [30]、负载硫酸 [31]、负载高氯酸 [32] 作为催化剂,可以催化合成1,4-二氢吡啶的合成。然而,他们所利用的催化剂存在不能回收利用或者催化剂合成困难,成本高,不利工业化生产。Amberlyst 15离子交换树脂是一种固体酸催化剂,广泛应用于酯化、烷基化、酰化、醚化、缩合、水合等化学合成的反应,它不溶于溶剂,反应后可以通过简单的过滤回收利用。本文直接采用商业化的Amberlyst 15离子交换树脂作为催化剂,考察了他们在不同温度及不同介质的反应情况(表1)。如表1所示,在Amberlyst 15离子交换树脂(200 mg)催化下,苯甲醛(10 mmol)、乙酰乙酸乙酯(20 mmol)、醋酸铵(13 mmol)的摩尔比为1:2:1.3进行条件筛选。以水为溶剂在70℃反应1个小时,收率仅为52% (表1,entry 1),而在无溶剂下反应收率达到72% (表1,entry 2)。我们也尝试在相同条件下,将反应置于水热反应釜中进行反应,但是收率明显低于在常压搅拌下的反应(表1,entries 3-4),原因可能是在水热反应釜无法搅拌,反应物无法进行充分接触反应。通过温度筛选,反应在90℃及无溶剂条件下,1小时后,苯甲醛完全消失,收率可以达到92% (表1,entry 5)。接着,降低催化剂的用量,我们发现,1小时后,苯甲醛同样消失,分离收率没有多大的变化(表1,entry 6, 91% yield)。
Table 1. Reaction condition optimization resultsa
表1. 反应条件优化结果a
a除非另有说明,将苯甲醛1a (10 mmol)、乙酰乙酸乙酯2a (20 mmol)、乙酸铵(12 mmol)和H2O (5 mL)的混合物在Amberlyst-15 (200 mg)存在下搅拌60分钟。b孤立产量。c使用Amberlyst-15 (100 mg)。d加入50 mg Amberlyst 15。e30分钟。
3.2. 反应底物的拓展
在优化的条件(无溶剂,Amberlyst 15离子交换树脂为催化剂,90℃下反应)下,考察了芳香醛上取代基电子效应对该反应的影响(图2)。从图2中可以看出,1) 反应的适用范围比较广。所有的反应在60 分钟内都可以反应完全,收率为72~95%。2) 带有供电子基团(CH3, -OCH3)的,反应进行了60分钟后,收率只有72% (3b, 3c);反之,带有拉电子基团的芳香醛,在该反应体系可以获得较为满意的结果,收率最高可以达到93% (3c-3i)。3) 对于带有杂环的醛,如2-呋喃甲醛及2-噻吩甲醛,在该反应条件下,15分钟内就产生大量固体,反应完全,分离收率分别为88%和90% (3j-3k)。4) 芳环上位阻效应不明显,如芳环上取代基是氯时,分别在邻位(3f)、间位(3e)和对位(3d),收率都非常高(88%~93%)。
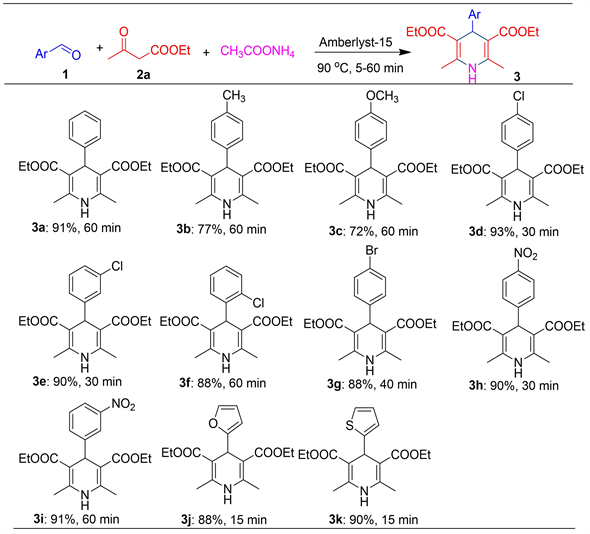
Figure 2. Expansion of reaction substrate aldehydes
图2. 反应底物醛的扩展
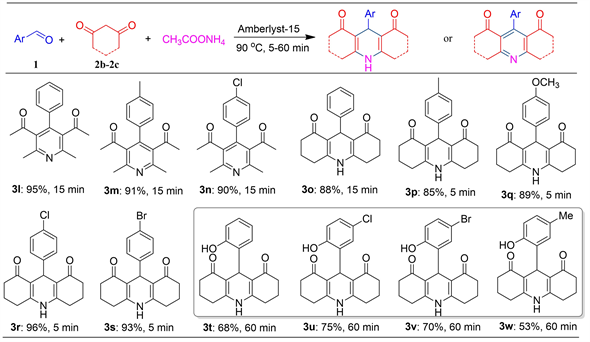
Figure 3. Further study on the scope of the substrates
图3. 底物范围的进一步研究
在获得了一系列含有两个酯基的1,4-二氢吡啶后,我们把目光转向合成含有其他官能团,例如,以1,3-二酮代替乙酰乙酸乙酯,则可以合成含有二酮基的1,4-二氢吡啶,如图3所示。首先,以2,4-戊二酮为反应底物,反应得以顺利进行,有趣的是,主要产物并不是1,4-二氢吡啶衍生物,而是直接被氧化得到了吡啶衍生物,这些反应效率均很高(3l-3n, 90%~95%)。以环状的1,3-环己二酮为底物时,则可以顺利获得三并环结构的1,4-二氢吡啶产物,收率也很高,但芳香醛上芳环上有拉电子基团要比有供电子基团的活性高,在相同时间收率也比较高,产物一般可直接通过乙醇洗涤、二氯甲烷洗涤的简单方法得到纯度为95%左右的产物(3o-3s)。当芳香醛为水杨醛时,反应也能顺利进行,但是收率相对稍低(3t-3w),此时获得4-邻羟苯基取代的1,4-二氢喹啉衍生物。
3.3. 催化剂的回收利用研究
由于Amberlyst 15离子交换树脂不溶于反应体系,我们对它的回收利用进行了研究(图4)。将反应在优化条件下进行反应,当反应完毕后,趁热过滤,少量乙醇洗涤,回收得到催化剂,不需要进一步处理,直接进行下一次反应,经过6次使用后,该催化剂还保留这高的催化活性,转化率可以达到100%,收率变化不大,始终介于85%~90%之间。
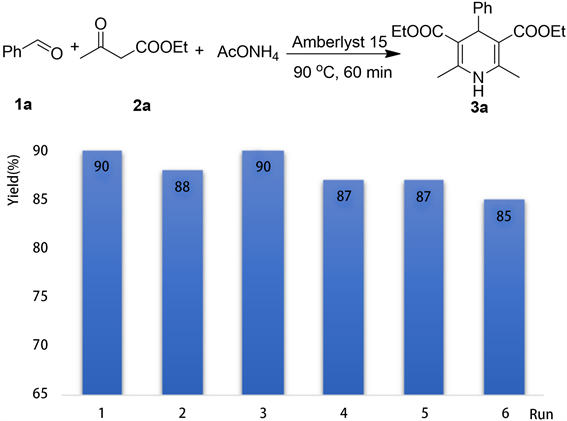
Figure 4. Catalyst recycling
图4. 催化剂的回收利用
3.4. 放大反应研究
最后,我们还对反应进行了放大量的研究。如下方程式(1)所示,将反应放大30倍,可以一次获得约83克目标产物,收率为84% (图5)。
3.5. 反应机理推测
基于上述实验结果及相关文献报道,我们对反应机理进行了推测。如图5所示,在酸性条件下,苯甲醛质子化得到活化,并促进乙酰乙酸乙酯I与其烯醇式II之间的互变,后者进攻活化的苯甲醛,接着

Figure 5. Amplified synthesis of 1,4-dihydropyridine
图5. 1,4-二氢吡啶放大量合成研究
失去一分子水得到Knoevenagel产物III,随后质子化得到中间体IV。乙酰乙酸乙酯与醋酸铵发生反应得到烯胺V,烯胺进攻中间体IV得到中间体VI,最后失去一分子水得到目标产物3 (图6)。Amberlyst-15在这里可能起到了优异的杂相质子转移催化剂和良好的脱水剂的重要作用。
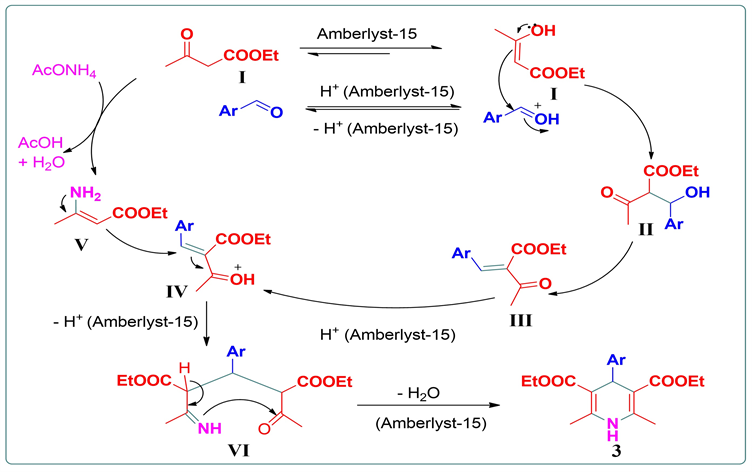
Figure 6. A probable mechanism
图6. 可能的反应机理
4. 结论
在无溶剂及非均相条件下,Amberlyst 15 离子交换树脂为催化剂,以芳香醛、1,3-二羰基化合物、醋酸铵于90℃缩合,高收率地得到1,4-二氢吡啶。该方法具有无需溶剂,原料便宜易得、反应产率高、反应时间短以及后处理简单等优点。由于Amberlyst 15离子交换树脂在反应体系不溶,可以很好的回收利用,回收利用可以达到6次,且产率没有明显变化。此外,反应规模可以放大至一次性合成80克以上的产物,收率良好,操作简便。
基金项目
感谢浙江师范大学“先进催化材料”教育部重点实验室和浙江省“固体表面反应化学”重点实验室开放课题基金项目(KLMEACM201908)资助。
文章引用
雷兹然,俞锦航,吕 新,谢云龙. 非均相无溶剂Amberlyst-15催化一锅法高效合成1,4-二氢吡啶
Heterogeneous Solvent-Free Amberlyst-15-Catalyzed Efficient One-Pot Synthesis of 1,4-Dihydropyridine[J]. 有机化学研究, 2022, 10(01): 26-38. https://doi.org/10.12677/JOCR.2022.101003
参考文献
- 1. Hantzsch, A. (1882) Ueber die Synthese pyridinartiger Verbindungen aus Acetessigäther und Aldehydammoniak. Justus Liebigs Annalen der Chemie, 215, 1-82. https://doi.org/10.1002/jlac.18822150102
- 2. Zamponi, G.W., Stotz, S.C., Staples, R.J., Andro, T.M., Nelson, J.K., Hulubei, V., Blumenfeld, A. and Natale, N.R. (2003) Unique Structure-Activity Relationship for 4-Isoxazolyl-1,4-dihydropyridines. Journal of Medicinal Chemistry, 46, 87-96. https://doi.org/10.1021/jm020354w
- 3. Visentin, S., Rolando, B., Distlio, A., Frutterro, R., Novara, M., Carbone, E., Roussel, C., Vanthuyne, N. and Gasco, A. (2004) New 1,4-Dihydropyridines Endowed with NO-Donor and Calcium Channel Agonist Properties. Journal of Medicinal Chemistry, 47, 2688-2693. https://doi.org/10.1021/jm031109v
- 4. David, J.T. (2007) Calcium Channel Antagonists: Clinical Uses—Past, Present and Future. Biochemical Pharmacology, 74, 1-9. https://doi.org/10.1016/j.bcp.2007.01.016
- 5. Boer, R. and Gekeler, V. (1995) Chemosensitizer in Tumor Therapy: New Compounds Promise Better Efficacy. Drugs of the Future, 20, 499-509.
- 6. Bahekar, S. and Shinde, D. (2002) Synthesis and Anti-Inflammatory Activity of 1-4-Dihydropyridines. Acta Pharmaceutica, 52, 281-287.
- 7. Gullapalli, S. and Ramarao, P. (2002) L-Type Ca2+ Channel Modulation by Dihydropyridines Potentiates Kappa-Opioid Receptor Agonist Induced Acute Analgesia and Inhibits Development of Tolerance in Rats. Neuropharmacology, 42, 467-475. https://doi.org/10.1016/S0028-3908(01)00200-3
- 8. Bretzel, R.G., Bollen, C.C., Maeser, E. and Federlin, K.F. (1992) Nephroprotective Effects of Nitrendipine in Hypertensive Tune I and Type II Diabetic Patients. American Journal of Kidney Diseases, 21, S53-S64. https://doi.org/10.1016/0272-6386(93)70125-I
- 9. Mcormack, J.G., Westergaard, N., Kristiansen, M., Brand, C.L. and Lau, J. (2001) Pharmacological Approaches to Inhibit Endogenous Glucose Production as a Means of Anti-Diabetic Therapy. Current Pharmaceutical Design, 7, 1451-1474. https://doi.org/10.2174/1381612013397393
- 10. Ogawa, A.K., Willoughby, C.A., Raynald, B., Ellsworth, K.P., Geissler, W.M., Myer, R.W., Yao, J., Georgianna, H. and Chapman, K.T. (2003) Glucose-Lowering in a db/db Mouse Model by Dihydropyridine Diacid Glycogen Phosphorylase Inhibitors. Bioorganic & Medicinal Chemistry Letters, 13, 3405-3408. https://doi.org/10.1016/S0960-894X(03)00798-4
- 11. Sabitha, G., Reddy, G.S.K.K., Reddy, C.S. and Yadav, J.S. (2003) A Novel TMSI-Mediated Synthesis of Hantzsch 1,4-Dihydropyridines at Ambient Temperature. Tetrahedron Letters, 44, 4129-4131. https://doi.org/10.1016/S0040-4039(03)00813-X
- 12. Bossert, F., Mayer, H. and Wehinger, E. (1981) 4-Aryldihydropyridines, a New Class of Highly Active Calcium Antagonists. Angewandte Chemie International Edition, 20, 762-769. https://doi.org/10.1002/anie.198107621
- 13. Gilpin, P.K. and Pachla, L.A. (1999) Pharmaceuticals and Related Drugs. Analytical Chemistry, 71, 3755-3770. https://doi.org/10.1021/ac050580o
- 14. Cosconati, S., Marinelli, L., Lavecchia, A. and Novellino, E. (2007) Characterizing the 1,4-Dihydropyridines Binding Interactions in the L-Type Ca2+ Channel: Model Construction and Docking Calculations. Journal of Medicinal Chemistry, 50, 1504-1513. https://doi.org/10.1021/jm061245a
- 15. Anniyappan, M., Muralidharan, D. and Perumal, P.T. (2002) Synthesis of Hantzsch 1,4-Dihydropyridines under Microwave Irradiation. Synthetic Communications, 32, 659-663. https://doi.org/10.1081/SCC-120002415
- 16. Shaabani, A., Rezayan, A.H., Rahmati, A. and Sharifi, M. (2008) A Mild and Efficient Approach for the Selective Deprotection of Benzyl and Phenyl Trimethylsilyl Ethers in 1-Butyl-3-Methyl-Imidazolium Chloride. Monatshefte für Chemie, 139, Article No. 1471. https://doi.org/10.1007/s00706-008-0960-y
- 17. Yadav, J.S., Reddy, B.V.S., Basak, A.K. and Narsaiah, A.V. (2003) Three-Component Coupling Reactions in Ionic Liquids: An Improved Protocol for the Synthesis of 1,4-Dihydropyridines. Green Chemistry, 5, 60-63. https://doi.org/10.1039/B210017G
- 18. Xia, J.J. and Wang G.W. (2005) One-Pot Synthesis and Aromatization of 1,4-Dihydropyridines in Refluxing Water. ChemInform, 37, 2379. https://doi.org/10.1002/chin.200604136
- 19. Deshayes, S., Liagre, M., Loupy, A., Luche, J.L. and Petit, A. (1999) Microwave Activation in Phase Transfer Catalysis. Tetrahedron, 55, 10851-10870. https://doi.org/10.1016/S0040-4020(99)00601-8
- 20. Correa, W.H. and Scott, J.L. (2001) Solvent-Free, Two-Step Synthesis of Some Unsymmetrical 4-Aryl-1,4-Dihydro- pyridines. Green Chemistry, 3, 296-301. https://doi.org/10.1039/B106397A
- 21. Sapkal, S.B., Shelke, K.F., Shingate, B.B. and Shingare, M.S. (2009) 1-Butyl-3-Methyl Imidazolium Hydrogen Sulphate Promoted One-Pot Three-Component Synthesis of Amidoalkyl Naphthols. Bulletin of the Korean Chemical Society, 30, 2887-2889. https://doi.org/10.5012/bkcs.2009.30.12.2887
- 22. Bridgwood, K.L., Veitch, G.E. and Ley, S.V. (2008) Magnesium Nitride as a Convenient Source of Ammonia: Preparation of Dihydropyridines. Organic Letters, 10, 3627-3629. https://doi.org/10.1021/ol801399w
- 23. Kumar, A. and Maurya, R.A. (2008) Efficient Synthesis of Hantzsch Esters and Polyhydroquinoline Derivatives in Aqueous Micelles. Synlett, 6, 883-885. https://doi.org/10.1055/s-2008-1042908
- 24. Varma, R.S. (1999) Solvent-Free Organic Syntheses. Using Supported Reagents and Microwave Irradiation. Green Chemistry, 1, 43-55. https://doi.org/10.1039/A808223E
- 25. Loupy, A., Petit, A., Hamelin, J., Texier-Boullet, F.P. and Jacquault, Mathe, D. (1998) New Solvent Free Organic Synthesis Using Focused Microwaves. ChemInform, 29, 1213. https://doi.org/10.1002/chin.199846308
- 26. Sharma, S.D., Hazarika, P. and Konwar, D. (2008) A Simple, Green and One-Pot Four-Component Synthesis of 1,4-Dihydropyridines and Their Aromatization. Catalysis Communications, 9, 709-714. https://doi.org/10.1016/j.catcom.2007.08.008
- 27. Wang, L., Sheng, J., Zhang, L., Han, J.W., Fan, Z.Y., Tian, H. and Qian, C.T. (2005) Facial Yb(OTf)3 Promoted One-Pot Synthesis of Polyhydroquinoline Derivatives Through Hantzsch Reaction. Tetranhedron, 61, 1539-1543. https://doi.org/10.1016/j.tet.2004.11.079
- 28. 蔡小华, 张国林. 无溶剂一锅法合成1,4-二氢吡啶[J]. 有机化学, 2005, 25(8): 930-933. https://doi.org/10.3321/j.issn:0253-2786.2005.08.007
- 29. 刘竹兰, 王翠玲, 令亚萍, 王玉英, 刘建利. 无溶剂研磨法合成1,4-二氢吡啶衍生物[J]. 化学通报 2008, 71(9): 718-720.
- 30. Bagley, M.C. and Lubinu, M.C. (2006) Microwave-Assisted Oxidative Aromatization of Hantzsch 1,4-Dihydropyridines Using Manganese Dioxide. Synthesis, No. 8, 1283-1288. https://doi.org/10.1055/s-2006-926407
- 31. Bandyopadhyay, D., Velazquez, J.M. and Banik, B.K. (2011) A Truly Green Synthesis of α-Aminonitriles via Strecker Reaction. Organic and Medicinal Chemistry Letters, 1, Article No. 11. https://doi.org/10.1186/2191-2858-1-11
- 32. Eynde, J.J.V., Delfosse, F., Mayence, A. and Van Haverbeke, Y. (1995) Old Reagents, New Results: Aromatization of Hantzsch 1,4-Dihydropyridines with Manganese Dioxide and 2,3-Dichloro-5,6-Dicyano-1,4-Benzoquinone. Tetrahedron, 51, 6511-6516. https://doi.org/10.1016/0040-4020(95)00318-3
- 33. Ghosh, S., Saikh, F., Das, J. and Pramanik, A.K. (2013) Hantzsch 1,4-Dihydropyridine Synthesis in Aqueous Ethanol by Visible Light. Tetrahedron Letters, 54, 58-62. https://doi.org/10.1016/j.tetlet.2012.10.079
- 34. Datta, B. and Pasha, M.A. (2011) Silica Sulfuric Acid: An Efficient Heterogeneous Catalyst for the One-Pot Synthesis of 1,4-Dihydropyridines under Mild and Solvent-Free Conditions. Chinese Journal of Catalysis, 32, 1180-1184. https://doi.org/10.1016/S1872-2067(10)60252-5
- 35. Zeynizadeh, B., Rahmani, S. and Eghbali, E. (2019) Anchored Sulfonic Acid on Silica-Layered NiFe2O4: A Magnetically Reusable Nanocatalyst for Hantzsch Synthesis of 1,4-Dihydropyridines. Polyhedron, 168, 57-66. https://doi.org/10.1016/j.poly.2019.04.035
- 36. Debache, A., Ghalem, W., Boulcina, R., Belfaitah, A., Rhouati, S. and Carboni, B. (2009) An Efficient One-Step Synthesis of 1,4-Dihydropyridines via a Triphenylphosphine-Catalyzed Three-Component Hantzsch Reaction under Mild Conditions. Tetrahedron Letters, 50, 5248-5250. https://doi.org/10.1016/j.tetlet.2009.07.018
- 37. Dhruva Kumar, S. and Sandhu, J.S. (2009) New Efficient Protocol for the Production of Hantzsch 1,4-Dihydropyridines Using RuCl3. Synthetic Communications, 39, 1957-1965. https://doi.org/10.1080/00397910802622762
- 38. Tajbakhsh, M., Alaee, E., Alinezhad, H., Khanian, M., Jahani, F., Khaksar, S., Rezaee, P. and Tajbakhsh, M. (2012) Titanium Dioxide Nanoparticles Catalyzed Synthesis of Hantzsch Esters and Polyhydroquinoline Derivatives. Chinese Journal of Catalysis, 33, 1517-1522. https://doi.org/10.1016/S1872-2067(11)60435-X
- 39. Böcker, R.H. and Guengerich, F.P. (1986) Oxidation of 4-Aryl- and 4-Alkyl-Substituted 2,6-Dimethyl-3,5-Bis(Al- koxycarbonyl)-1,4-Dihydropyridines by Human Liver Microsomes and Immunochemical Evidence for the Involvement of a Form of Cytochrome P-450. Journal of Medicinal Chemistry, 29, 1596-1603. https://doi.org/10.1021/jm00159a007
- 40. Chavan, S.P., Kharul, R.K., Kalkote, U.R. and Shivakumar, I. (2003) An Efficient Co(II) Catalyzed Auto Oxidation of 1,4-Dihydropyridines. Synthetic Communications, 33, 1333-1340. https://doi.org/10.1081/SCC-120018693
- 41. Seyyedhamzeh, M., Mirzaei, P. and Bazgir, A. (2008) Solvent-Free Synthesis of Aryl-14H-Dibenzo[a,j]Xanthenes and 1,8-Dioxo-Octahydro-Xanthenes Using Silica Sulfuric Acid as Catalyst. Dyes and Pigments, 76, 836-839. https://doi.org/10.1016/j.dyepig.2007.02.001
- 42. Zhu, A.L., Liu, R.X., Du, C.Y. and Li, L.J. (2017) Betainium-Based Ionic Liquids Catalyzed Multicomponent Hantzsch Reactions for the Efficient Synthesis of Acridinediones. RSC Advances, 7, 6679-6684. https://doi.org/10.1039/C6RA25709G
- 43. Kiani, M. and Mohammadipour, M. (2017) Fe3O4@SiO2-MoO3H Nanoparticles: A Magnetically Recyclable Nanocatalyst System for the Synthesis of 1,8-Dioxo-Decahydroacridine Derivatives. RSC Advances, 7, 997-1007. https://doi.org/10.1039/C6RA25571J
- 44. Kidwai, M. and Bhatnagar, D. (2010) Polyethylene Glycol-Mediated Synthesis of Decahydroacridine-1,8-Diones Catalyzed by Ceric Ammonium Nitrate. Chemical Papers, 64, 825-828. https://doi.org/10.2478/s11696-010-0070-2
- 45. Banothu, J., Bavantula, R. and Crooks, P.A. (2013) An Eco-Friendly Improved Protocol for the Synthesis of Bis(3-Indolyl)Methanes Using Poly(4-Vinylpyridinium)Hydrogen Sulfate as Efficient, Heterogeneous, and Recyclable Solid Acid Catalyst. International Scholarly Research Notices, 2013, Article ID: 616932. https://doi.org/10.1155/2013/616932
