Journal of Organic Chemistry Research
Vol.
11
No.
01
(
2023
), Article ID:
63037
,
9
pages
10.12677/JOCR.2023.111003
主–客体化学中大环主体分子的研究进展
原焱焱
浙江师范大学化学与材料科学学院,浙江 金华
收稿日期:2023年2月13日;录用日期:2023年3月15日;发布日期:2023年3月24日

摘要
超分子化学专注于通过弱非共价相互作用,包括π-π相互作用、氢键相互作用、疏水相互作用、主–客体相互作用等,对各种结构单元进行分子识别和自组装。非共价相互作用、主客体识别和刺激响应自组装结构之间的关系具有重要意义,在各种非共价相互作用的驱动下,基于主客体相互作用的超分子体系由于大环主体的引入而表现出迷人的性质。本文研究了冠醚、环糊精、柱芳烃以及葫芦脲等大环主体分子的进展,同时近年来,合成了新的大环主体,极大地促进了超分子化学的发展。
关键词
超分子化学,非共价相互作用,主–客体相互作用,大环主体分子

Research Progress of Macrocyclic Molecules in Host-Guest Chemistry
Yanyan Yuan
College of Chemistry and Materials Sciences, Zhejiang Normal University, Jinhua Zhejiang
Received: Feb. 13th, 2023; accepted: Mar. 15th, 2023; published: Mar. 24th, 2023

ABSTRACT
Supramolecular chemistry focuses on molecular recognition and self-assembly of various structural units through weak non-covalent interactions, including π-π interactions, hydrogen bond interactions, hydrophobic interactions, and host-guest interactions. The relationship between non-covalent interactions, host-guest recognition, and stimulus-response self-assembly structures are of great significance. Driven by various non-covalent interactions, supramolecular systems based on host-guest interactions exhibit fascinating properties due to the introduction of macrocyclic agents. In this paper, the progress of macrocyclic main molecules such as crown ether, cyclodextrin, column aromatics and cucurbit[n]urils has been studied. In recent years, new macrocyclic main molecules have been synthesized, which has greatly promoted the development of supramolecular chemistry.
Keywords:Supramolecular Chemistry, Non-Covalent Interactions, Host-Guest Interactions, Macrocyclic Host Molecules

Copyright © 2023 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
超分子化学(Supramolecular Chemistry)作为化学领域的新兴交叉学科,发展至今已有半个多世纪 [1]。自1987年Charles Pedersen [2] 、Donald Cram [3] 和Lehn [4] 获得诺贝尔化学奖以来,超分子化学的重要性和意义已经显现出来,此后,该领域备受瞩目,因为它促进和加速了许多新概念的发展,如分子自组装、分子识别、主–客体化学和动态共价化学 [5]。
主–客体化学属于超分子化学的一个分支学科,它的研究对象是主–客体复合过程中涉及到的主体、客体以及主–客体复合物(Host-Guest Complex)。主–客体复合物由两个或以上的分子或离子、以特定的结构关系、通过非共价相互作用结合而成。Donald J. Cram进一步阐明“在主客体化学中,主体是两者尺寸上更大的一方,而客体则是更小的一方,主体必须能够识别那些在排列和空间上匹配的客体 [6] ”。此外,主–客体相互作用(Host-Guest Interaction)这一术语也被广泛接纳和采用,通常,主客系统的形成涉及一种以上的非共价相互作用,图1中如疏水缔合、氢键、静电相互作用、金属配位、范德华力和π-π等叠加相互作用 [7]。
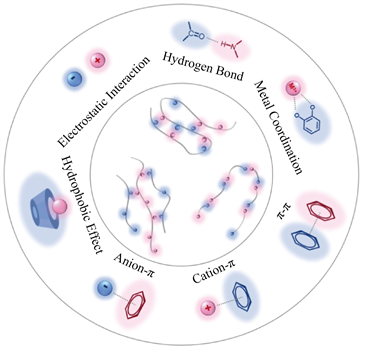
Figure 1. Typical intramolecular and intermolecular noncovalent interactions
图1. 典型的分子内和分子间非共价相互作用
在超分子化学的发展中,各种人工大环分子的设计与合成在对客体分子的选择性识别中有着不可忽视的意义。大环主体里冠醚、环糊精、杯芳烃、柱芳烃以及葫芦脲 [8] [9] [10] [11] 等较为经典,一直受众多研究学者的青睐。目前,越来越多的功能性超分子组装体系被不断地探索和研究,应用也越来越广泛,如分子马达、纳米医药 [12] 、仿生结构与功能 [13] 、传感 [14] 和细胞成像 [15] 等。
基于上述认知,化学家们利用有机化学的合成工具,发展了许多结构不同、形态多样的主体,如图2所示,它们的共同点是拥有可容纳分子或离子的空腔(各种大环名称) [16]。
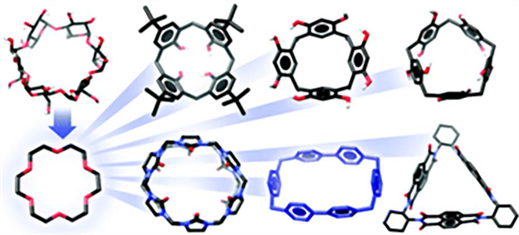
Figure 2. The main species expanding from crown ether to calixarene, cyclophanium, cucurbazide and other molecules or ions
图2. 主体种类从冠醚拓展到杯芳烃、环蕃、葫芦脲等分子或离子的示意图
由于主–客体相互作用具有可逆性、可修饰性以及刺激响应性等特点,因此基于主–客体作用构筑的超分子组装体,对外界刺激往往表现出优异的响应行为,这加快了智能新型材料的发展。目前,常见的外界刺激有光、热、电、pH以及氧化还原等 [17]。其中,光照由于其非侵入性、清洁无污染以及时空可控等独特的性质而得到越来越广泛的关注。在众多光致异构分子(如螺吡喃 [18] 、二芳基乙烯 [19] 、香豆素 [20] 、偶氮苯 [21] )中,偶氮苯结构简单、合成容易、异构可控性强且具有良好的往复性。当暴露于特定波长的紫外光时,反式变为顺式,而当暴露于可见光或加热时,顺式又回到反式。近年来,偶氮苯类衍生物因其独特的光响应特性和顺反构型的差异,在染料行业、生物探针、智能材料以及超分子领域表现出巨大的应用潜力。
光源具有成本低、强度与波长易调节、能够对目标物质实现空间与时间的远程调控等诸多优点,是最理想的刺激源 [22]。同时,非共价相互作用的引入能够很大程度上增强了聚合物的光响应特性。
2. 主–客体体系
2.1. 冠醚
冠醚(Crown Ether)是超分子化学的第一代主体化合物。它是以乙氧基(-CH2CH2O-)为重复单元的环状分子,在超分子化学的发展中占有举足轻重的地位。1967年,C. J. Pedersen [23] 教授首次报道了60多冠醚化合物,并对金属离子的选择性键合行为进行了详细的研究。1969年,J.-M. Lehn [24] 教授报道了第一个穴醚化合物,并对其键合能力进行了深入研究。1973年,D. J. Cram [25] 合成了第一个球醚,并提出了“主–客化学”的概念。通过对冠醚早期研究工作的总结,1978年,J.-M. Lehn提出了超分子化学的概念——“研究分子间键和分子组装的化学”。在90年代以前,对于冠醚的研究主要集中在冠醚的衍生化和对金属离子的识别行为上。在过去的几十年中,合成了各种不同大小环的冠醚,如图3 [26]。
冠醚作为一种大环化合物,已经被证明可以结合适当大小的有机阳离子。由于冠醚易于修饰,不同的官能团也可以很容易地被安装到冠醚的边缘,这使得冠醚成为构建互锁结构的优秀构件。由于主客体相互作用的刺激响应性和二硫键的动态性质,线性CSP具有pH-、离子-、氧化还原-和光响应特性 [27]。
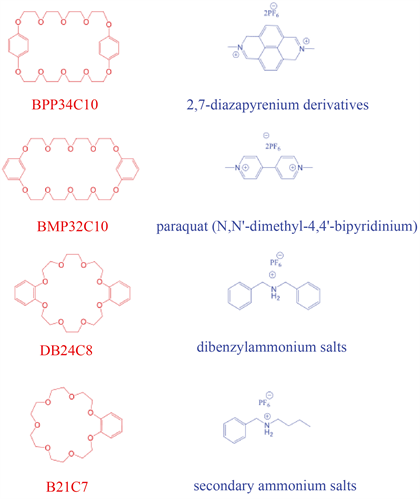
Figure 3. Chemical structure of crown ether and its complementary guest parts
图3. 冠醚及其互补客体部分的化学结构
2.2. 环糊精
如图4环糊精的结构所示 [28],环糊精(CD)由1,4-糖苷键连接,是淀粉酶水解淀粉产生的一种环状低聚糖。根据CD中葡萄糖单位的数量,市场上的CD主要包括α-、β-和γ-CD。
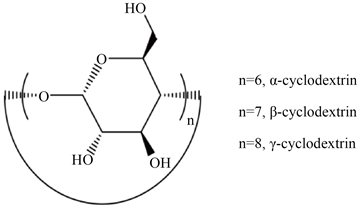
Figure 4. The structure of CDs
图4. 环糊精的结构
CDs作为传统的药物载体,也被广泛应用于可根据pH值、光照、酶等进行分类的智能刺激反应性药物载体 [29]。因为光可以在空间或时间上被控制,所以它可以作为一种特殊的外部刺激施加。目前已陆续研制出各种光敏药物载体材料。将这些药物载体按照特定波长的光(紫外光、可见光、近红外) [30] 进行切割或破坏,使药物成功释放达到相应的治疗目的。如图5所示,Harada课题组利用α-CD与偶氮苯之间的主客体相互作用构建了光驱动的丙烯酰胺水凝胶 [31]。该水凝胶驱动器的光驱动行为源于通过紫外光或可见光照射下偶氮苯与α-CD主客体络合物的可逆包结–解离过程,作者通过以环糊精为主体分子,偶氮苯衍生物为光敏客体分子,设计了一种可控的光敏超分子水凝胶。

Figure 5. Experimental devices and the size of αCD-Azo gel(2, 2) in water
图5. 基于α-环糊精与偶氮苯主客体相互作用的光驱动超分子水凝胶
2.3. 柱芳烃和杯芳烃
柱芳烃由Ogoshi于2008年首次报道 [31],是一个新的大环芳烃主体家族。柱芳烃是一类由对二酚醚或者苯二酚通过亚甲基桥在苯环的对位链接成的环状低聚物,柱状芳烃具有高度对称的刚性圆柱结构、富电子空腔和多个修饰位点 [32]。如图6,柱芳烃的化学结构与杯芳烃非常相似,杯[n]芳烃与柱[n]芳烃的重要区别在于连接各单元的亚甲基桥的位置,柱形[n]芳烃中,酚单位在对位通过亚甲基桥连接,杯形[n]芳烃中酚单位在对位通过甲基桥连接,亚甲基桥体位置的不同导致杯芳烃和柱芳烃形状的不同。其杯
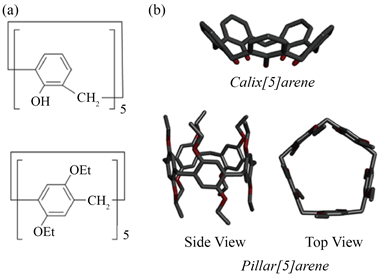
Figure 6. Shape and chemical structure chemical (a) and crystal (b) structures of calix[5]arene and pillar[5]arene
图6. 杯状[5]芳烃和柱状[5]芳烃的化学结构(a)和晶体结构(b)
芳烃具有不对称的杯形结构,相比之下,柱状芳烃具有高度对称的柱状结构 [32]。
杯芳烃是一类对位烷基苯酚与甲醛缩合的寡聚大环化合物,该化合物的分子模型表明它的形状象一个杯子或花瓶,故称之为杯芳烃 [33]。通过调节苯基取代基的数量,可以构建新型的大环芳烃,同时研究发现,对柱状[n]芳烃环上的基团进行修饰和衍生,可以赋予其更多的功能,使其具有多刺激反应性、两亲性、荧光性和液晶性等特性 [34] [35] [36] [37] [38]。
柱芳烃及其衍生物最重要的性质之一是它们的主–客体相互作用。Zhou [39] 等通过引入一个具有动态共价键的客体分子,即蒽的可逆光环加成和季铵基团的客体部分,构建了一个巯基取代基5[5]的柱状[5]芳烃修饰Au NPs的光激活可逆组装。
2.4. 葫芦脲
葫芦脲(Cucurbit[n]uril)是超分子化学中继冠醚、环糊精、柱芳烃等之后一类备受关注的有机笼状大环分子。它是在酸性条件下由甘脲和甲醛通过一步缩合反应得到的,如图7是各CB同系物CB[5]、CB[6]、CB[7]和CB[8]的结构表征 [40],因为其形状酷似葫芦,因而被命名为葫芦脲 [41]。虽然葫芦[6]脲早在1905年就被合成出来,但是直到1981年才通过单晶X-射线衍射解析出其结构 [42]。此后,科学家又相继报道了一系列具有不同空腔尺寸的葫芦脲同系物(CB[5]、CB[7]、CB[8]、CB[10]等) [43] [44]。随着甘脲数目的增加,其直径和空腔尺寸不断增加。葫芦脲同系物都是由羰基组成的亲水端口和内部的疏水空腔组成。由于这种独特的结构特征,葫芦脲分子对多种金属离子和有机阳离子都有很强的键合能力,被广泛地应用于各个领域 [5] [11] [45]。
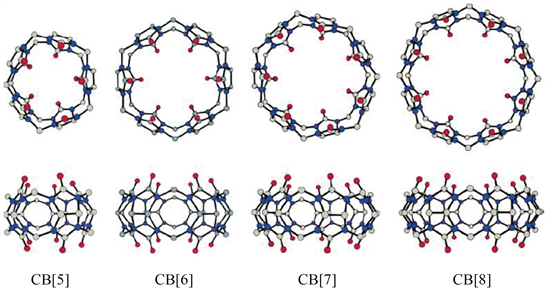
Figure 7. Crystall structures of Cucurbit[n]uril (n = 5~8)
图7. 葫芦脲的晶体结构(n = 5~8)
3. 展望
超分子化学基于不同结构单元通过非共价相互作用许多体系中协同共存,形成具有方向性和选择性的强相互作用,成为超分子识别和自组装的驱动力。在各种非共价相互作用的驱动下,基于主客体相互作用的超分子体系由于大环主体的引入而表现出迷人的性质。开发新的大环并研究它们的分子识别/组装行为是超分子化学重要的研究课题,近年来,新的大环主体被合成出来,极大地促进了超分子化学的发展,目前对超分子大环体系的研究还处于起步阶段,其在化学、生命科学、分子机器和材料科学等领域有着巨大的研究价值和应用前景。
文章引用
原焱焱. 主–客体化学中大环主体分子的研究进展
Research Progress of Macrocyclic Molecules in Host-Guest Chemistry[J]. 有机化学研究, 2023, 11(01): 17-25. https://doi.org/10.12677/JOCR.2023.111003
参考文献
- 1. Lehn, J.M. (1978) Cryptates: Inclusion Complexes of Macropolycyclic Receptor Molecules. Pure and Applied Chemistry, 50, 871-892. https://doi.org/10.1351/pac197850090871
- 2. Pedersen, C.J. (1988) The Discovery of Crown Ethers (Noble Lecture). Angewandte Chemie International Edition in English, 27, 1021-1027. https://doi.org/10.1002/anie.198810211
- 3. Cram, D.J. (1988) The Design of Molecular Hosts, Guests, and Their Complexes (Nobel Lecture). Angewandte Chemie International Edition in English, 27, 1009-1020. https://doi.org/10.1002/anie.198810093
- 4. Lehn, J.-M. (1988) Supramolecular Chemistry—Scope and Perspectives Molecules, Supermolecules, and Molecular Devices (Nobel Lecture). Angewandte Chemie International Edition in English, 27, 89-112. https://doi.org/10.1002/anie.198800891
- 5. Barrow, S.J., Kasera, S., Rowland, M.J., del Barrio, J. and Scherman, O.A. (2015) Cucurbituril-Based Molecular Recognition. Chemical Reviews, 115, 12320-12406. https://doi.org/10.1021/acs.chemrev.5b00341
- 6. Cram, D.J. and Cram, J.M. (1974) Host-Guest Chemistry: Complexes Between Organic Compounds Simulate the Substrate Selectivity of Enzymes. Science, 183, 803-809. https://doi.org/10.1126/science.183.4127.803
- 7. Chen, J., Peng, Q., Peng, X., Zhang, H. and Zeng, H. (2022) Probing and Manipulating Noncovalent Interactions in Functional Polymeric Systems. Chemical Reviews, 122, 14594-14678. https://doi.org/10.1021/acs.chemrev.2c00215
- 8. Dong, S., Zheng, B., Wang, F. and Huang, F. (2014) Supramolecular Polymers Constructed from Macrocycle-Based Host-Guest Molecular Recognition Motifs. Accounts of Chemical Research, 47, 1982-1994. https://doi.org/10.1021/ar5000456
- 9. Li, Z.-Y., Zhang, Y., Zhang, C.-W., Chen, L.-J., Wang, C., Tan, H., Yu, Y., Li, X. and Yang, H.-B. (2014) Cross- Linked Supramolecular Polymer Gels Constructed from Discrete Multi-Pillararene Metallacycles and Their Multiple Stimuli-Responsive Behavior. Journal of the American Chemical Society, 136, 8577-8589. https://doi.org/10.1021/ja413047r
- 10. Zhang, C.-W., Ou, B., Jiang, S.-T., Yin, G.-Q., Chen, L.-J., Xu, L., Li, X. and Yang, H.-B. (2018) Cross-Linked AIE Supramolecular Polymer Gels with Multiple Stimuli-Responsive Behaviours Constructed by Hierarchical Self-Assembly. Polymer Chemistry, 9, 2021-2030. https://doi.org/10.1039/C8PY00226F
- 11. Yang, H., Yuan, B., Zhang, X. and Scherman, O.A. (2014) Supramolecular Chemistry at Interfaces: Host-Guest Interactions for Fabricating Multifunctional Biointerfaces. Accounts of Chemical Research, 47, 2106-2115. https://doi.org/10.1021/ar500105t
- 12. Zheng, Z., Geng, W.-C., Xu, Z. and Guo, D.-S. (2019) Macrocyclic Amphiphiles for Drug Delivery. Israel Journal of Chemistry, 59, 913-927. https://doi.org/10.1002/ijch.201900032
- 13. Tu, Y., Peng, F., Adawy, A., Men, Y., Abdelmohsen, L.K. and Wilson, D.A. (2016) Mimicking the Cell: Bio-Inspired Functions of Supramolecular Assemblies. Chemical Reviews, 116, 2023-2078. https://doi.org/10.1021/acs.chemrev.5b00344
- 14. You, L., Zha, D. and Anslyn, E.V. (2015) Recent Advances in Supramolecular Analytical Chemistry Using Optical Sensing. Chemical Reviews, 115, 7840-7892. https://doi.org/10.1021/cr5005524
- 15. Cheng, H.B., Li, Y., Tang, B.Z. and Yoon, J. (2020) Assembly Strategies of Organic-Based Imaging Agents for Fluorescence and Photoacoustic Bioimaging Applications. Chemical Society Reviews, 49, 21-31. https://doi.org/10.1039/C9CS00326F
- 16. Liu, Z., Nalluri, S.K.M. and Stoddart, J.F. (2017) Surveying Macrocyclic Chemistry: From Flexible Crown Ethers to Rigid Cyclophanes. Chemical Society Reviews, 46, 2459-2478. https://doi.org/10.1039/C7CS00185A
- 17. Mohamed, M. A., Fallahi, A., El-Sokkary, A. M., et al. (2019) Stimuli-Responsive Hydrogels for Manipulation of Cell Microenvironment: From Chemistry to Biofabrication Technology. Progress in Polymer Science, 98, Article ID: 101147. https://doi.org/10.1016/j.progpolymsci.2019.101147
- 18. Suda, M., Takashina, N., Namuangruk, S., Kungwan, N., Sakurai, H. and Yamamoto, H.M. (2017) N-Type Superconductivity in an Organic Mott Insulator Induced by Light-Driven Electron-Doping. Advanced Materials, 29, Article ID: 1606833. https://doi.org/10.1002/adma.201606833
- 19. Yun, C., You, J., Kim, J., Huh, J. and Kim, E. (2009) Photochromic Fluorescence Switching From Diarylethenes and Its Applications. Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 10, 111-129. https://doi.org/10.1016/j.jphotochemrev.2009.05.002
- 20. Wang, F., Ji, W., Yang, P. and Feng, C.L. (2019) Inversion of Circularly Polarized Luminescence of Nanofibrous Hydrogels through Co-assembly with Achiral Coumarin Derivatives. ACS Nano, 13, 7281-7290. https://doi.org/10.1021/acsnano.9b03255
- 21. Vapaavuori, J., Bazuin, C. G. and Priimagi, A. (2018) Supramolecular Design Principles for Efficient Photoresponsive Polymer-Azobenzene Complexes. Journal of Materials Chemistry C, 6, 2168-2188. https://doi.org/10.1039/C7TC05005D
- 22. Boelke, J. and Hecht, S. (2019) Designing Molecular Photoswitches for Soft Materials Applications. Advanced Optical Materials, 7, Article ID: 1900404. https://doi.org/10.1002/adom.201900404
- 23. Pedersen, C.J. (1967) Cyclic Polyethers and Their Complexes with Metal Salts. Journal of the American Chemical Society, 89, 2495-2496. https://doi.org/10.1021/ja00986a052
- 24. Dietrich, B., Lehn, J.M. and Sauvage, J.P. (1969) Diaza-Polyoxa-Macrocycles et Macrobicycles. Tetrahedron Letters, 10, 2885-2888. https://doi.org/10.1016/S0040-4039(01)88299-X
- 25. Helgeson, R.C., Koga, K., Timko, J.M. and Cram, D.J. (1973) Complete Optical Resolution by Differential Complexation in Solution Between a Chiral Cyclic Polyether and an .Alpha.-Amino Acid. Journal of the American Chemical Society, 95, 3021-3023. https://doi.org/10.1021/ja00790a052
- 26. Izatt, R.M., Bradshaw, J.S., Nielsen, S.A., Lamb, J.D., Christensen, J.J. and Sen, D. (1985) Thermodynamic and Kinetic Data for Cation-Macrocycle Interaction. Chemical Reviews, 85, 271-339. https://doi.org/10.1021/cr00068a003
- 27. Mai, Y., An, Z. and Liu, S. (2022) Self-Assembled Materials and Applications. Macromolecular Rapid Communications, 43, Article ID: 2200481. https://doi.org/10.1002/marc.202200481
- 28. Tian, B. and Liu, J. (2020) The Classification and Application of Cyclodextrin Polymers: A Review. New Journal of Chemistry, 44, 9137-9148. https://doi.org/10.1039/C9NJ05844C
- 29. Liu, Q., Zhou, Y., Lu, J. and Zhou, Y. (2020) Novel Cyclodextrin-Based Adsorbents for Removing Pollutants from Wastewater: A Critical Review. Chemosphere, 241, Article ID: 125043. https://doi.org/10.1016/j.chemosphere.2019.125043
- 30. Tian, B., Liu, Y. and Liu, J. (2021) Smart Stimuli-Responsive Drug Delivery Systems Based on Cyclodextrin: A Review. Carbohydrate Polymers, 251, Article ID: 116871. https://doi.org/10.1016/j.carbpol.2020.116871
- 31. Takashima, Y., Hatanaka, S., Otsubo, M., Nakahata, M., Kakuta, T., Hashidzume, A., Yamaguchi, H. and Harada. A. (2012) Expansion-Contraction of Photoresponsive Artificial Muscle Regulated by Host-Guest Interactions. Nature Communications, 3, Article No. 1270. https://doi.org/10.1038/ncomms2280
- 32. Ogoshi, T., Kanai, S., Fujinami, S., Yamagishi, T. and Nakamoto, Y. (2008) J. Para-Bridged Symmetrical Pillararenes: Their Lewis Acid Catalyzed Synthesis and Host-Guest Property. Journal of the American Chemical Society, 130, 5022-5023. https://doi.org/10.1021/ja711260m
- 33. Song, N., Kakuta, T., Yamagishi, T.A., Yang, Y.W. and Ogoshi, T. (2018) Molecular-Scale Porous Materials Based on Pillar[n]arenes. Chem, 4, 2029-2053. https://doi.org/10.1016/j.chempr.2018.05.015
- 34. Akiba, U., Minaki, D. and Anzai, J.-I. (2018) Host-Guest Chemistry in Layer-by-Layer Assemblies Containing Calix[n]arenes and Cucurbit[n]urils: A Review. Polymers, 10, Article No. 130. https://doi.org/10.3390/polym10020130
- 35. Chang, J.-X., Zhao, Q.-H., Kang, L., Li, H.-M., Xie, M.-R. and Liao, X.-J. (2016) Multiresponsive Supramolecular Gel Based on Pillararene-Containing Polymers. Macromolecules, 49, 2814-2820. https://doi.org/10.1021/acs.macromol.6b00270
- 36. Li, Z., Song, N. and Yang, Y.-W. (2019) Stimuli-Responsive Drug-Delivery Systems Based on Supramolecular Nanovalves. Matter, 1, 345-368. https://doi.org/10.1016/j.matt.2019.05.019
- 37. Zhu, H., Shangguan, L., Shi, B., Yu, G. and Huang, F. (2018) Recent Progress in Macrocyclic Amphiphiles and Macrocyclic Host-Based Supra-Amphiphiles. Materials Chemistry Frontiers, 2, 2152-2174. https://doi.org/10.1039/C8QM00314A
- 38. Bojtár, M., Kozma, J., Szakács, Z., Hessz, D., Kubinyi, M. and Bitter, I. (2017) Pillararene-Based Fluorescent Indicator Displacement Assay for the Selective Recognition of ATP. Sensors and Actuators B: Chemical, 248, 305-310. https://doi.org/10.1016/j.snb.2017.03.163
- 39. Zhou, Q., Zhang, B., Han, D., Chen, R., Qiu, F.,Wu, J. and Jiang, H. (2015) Photo-Responsive Reversible Assembly of Gold Nanoparticles Coated with Pillararenes. Chemical Communications, 51, 3124-3126. https://doi.org/10.1039/C4CC09778E
- 40. Kim, J., Jung, I.-S., Kim, S.-Y., Lee, E., Kang, J.-K., Sakamoto, S., Yamaguchi, K. and Kim, K. (2000) New Cucurbituril Homologues: Syntheses, Isolation, Characterization, and X-Ray Crystal Structures of Cucurbit[n]uril (n = 5, 7, and 8). Journal of the American Chemical Society, 122, 540-541. https://doi.org/10.1021/ja993376p
- 41. Biedermann, F., Elmalem, E., Ghosh, I., Nau, W.M. and Scherman, O.A. (2012) Strongly Fluorescent, Switchable Perylene Bis(diimide) Host-Guest Complexes with Cucurbituril in Water. Angewandte Chemie, 124, 7859-7863. https://doi.org/10.1002/ange.201202385
- 42. Lagona, J., Wagner, B.D. and Isaacs, L. (2006) Molecular-Recognition Properties of a Water-Soluble Cucurbituril Analogue. The Journal of Organic Chemistry, 71, 1181-1190. https://doi.org/10.1021/jo052294i
- 43. Jiao, D., Geng, J., Loh, X.J., Das, D., Lee, T.-C. and Scherman, O.A. (2012) Supramolecular Peptide Amphiphile Vesicles through Host-Guest Complexation. Angewandte Chemie International Edition, 51, 9633-9637. https://doi.org/10.1002/anie.201202947
- 44. Cheng, X.-J., Liang, L.-L., Chen, K., Ji, N.-N., Xiao, X., Zhang, J.-X., Zhang, Y.-Q., Xue, S.-F., Zhu, Q.-J., Ni, X.-L. and Tao, Z. (2013) Twisted Cucurbituril. Angewandte Chemie International Edition, 52, 7252 -7255. https://doi.org/10.1002/anie.201210267
- 45. Liu, S., Zavalij, P.Y. and Isaacs, L. (2005) Cucurbituril. Journal of the American Chemical Society, 127, 16798- 16799. https://doi.org/10.1021/ja056287n