Studies in Synthetic Chemistry
Vol.
07
No.
02
(
2019
), Article ID:
30816
,
5
pages
10.12677/SSC.2019.72004
Synthesis of 2H-Pyranes Catalyzed by 2-Dimethylaminopyridine
Wanle Yang, Yifan Liu, Chenjiang Liu*
The Key Laboratory of Oil and Gas Fine Chemicals of Ministry of Education & Xinjiang Uygur Autonomous Region, College of Chemistry and Chemical Engineering, Xinjiang University, Urumqi Xinjiang

Received: May 29th, 2019; accepted: Jun. 11th, 2019; published: Jun. 18th, 2019

ABSTRACT
In this paper, a series of 2H-pyrane compounds were synthesized by the reaction of α-bromocinnamaldehydes with 1,3-dicarbonyl compounds in dichloromethane using 2-dimethylaminopyridine as an organic catalyst. The method is environment friendly and easy to operate.
Keywords:2-Dimethylaminopyridine, 2H-Pyrane, Catalyze, Synthesis
2-二甲氨基吡啶催化合成2H-吡喃类化合物
杨万乐,刘议璠,刘晨江*
新疆大学化学化工学院,石油天然气精细化工教育部&自治区重点实验室,新疆 乌鲁木齐

收稿日期:2019年5月29日;录用日期:2019年6月11日;发布日期:2019年6月18日

摘 要
本文以2-二甲氨基吡啶作为一种有机催化剂,催化α-溴代肉桂醛、1,3-二羰基化合物在二氯甲烷中反应合成了一系列2H-吡喃类化合物。该方法具有环境友好、操作简单的特点。
关键词 :2-二甲氨基吡啶,2H-吡喃,催化,合成

Copyright © 2019 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
吡喃不仅具有抗菌、抗癌、抗病毒、抗炎、抗氧化剂等作用,而且可作用于中枢神经系统的受体,在医药和农药领域应用广泛 [1] - [7] 。因此,该类化合物的合成研究受到人们的关注。
近年来,路易斯酸 [8] [9] 、布朗斯特酸 [10] [11] 、酶 [12] 、手性二芳基脯氨醇硅醚 [13] 等已被应用于吡喃衍生物的催化合成,这些方法具有产率高,底物普适性好,催化剂可循环使用的优点,遗憾的是有的方法存在使用过渡金属催化剂或反应条件苛刻等不足。寻找一种绿色、操作简单的方法构建吡喃化合物仍然是值得深入研究的课题之一。因此,本文提出了一种无金属条件下2-二甲氨基吡啶(2-DMAP)催化α-溴代肉桂醛和1,3环己二酮合成2H-吡喃类化合物的方法(反应式如式1所示),考察了溶剂的种类、催化剂种类及氧化剂种类等因素对反应的影响,同时对反应底物的普适性进行了研究。
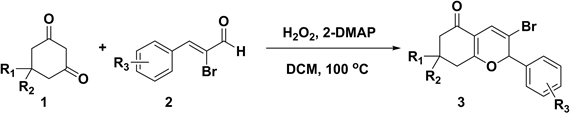
Scheme 1. Synthesis of 2H-pyrane derivatives
式1. 2H-吡喃衍生物的合成
2. 实验部分
2.1. 仪器与试剂
美国Varian inova-400型核磁共振仪(400 MHz, TMS);美国Thermo Fisher Scientific Q-Exactive高分辨质谱仪;瑞士Büchi B-560型熔点仪;上海顾村电光仪器厂ZF-I型四用紫外仪。2-二甲氨基吡啶(北京百灵威科技有限公司),所用药品及试剂均为市售分析纯,用前未经处理。α-溴代肉桂醛的合成参照文献 [14] 。
2.2. 2H-吡喃类化合物的合成
将装有α-溴代肉桂醛(0.3 mmol)、1,3-二羰基化合物(0.6 mmol)、35%过氧化氢水溶液(0.6 mmol)、催化剂2-二甲氨基吡啶(0.06 mmol)和2 mL二氯甲烷的耐压管在100℃下磁力搅拌反应16 h,冷却至室温,用乙酸乙酯(3 × 15 mL)萃取,合并有机相,无水硫酸钠干燥,抽滤除去干燥剂,将所得滤液减压旋除得残余物,经柱层析分离(V石油醚:V乙酸乙酯 = 20:1)得到目标产物。
3. 结果讨论
3.1. 反应条件的优化
以α-溴代肉桂醛、1,3-环己二酮为模型反应,考察了溶剂种类、催化剂种类、氧化剂种类等条件对反应的影响,实验结果见表1。首先,不同溶剂对该反应影响的结果表明(表1,entries 1-4),二氯甲烷(DCM)作为溶剂时产物产率可以达到62%,因此二氯甲烷为最优溶剂。其次,探究了2-二甲氨基吡啶和4-二甲氨基吡啶两种催化剂对反应的影响(表1,entry 3和entry 5),发现2-二甲氨基吡啶(2-DMAP)的催化效果较好,反应产率可达78%。接着,研究了氧化剂的种类对反应的影响,当氧化剂分别为双氧水(H2O2),过氧化苯甲酸叔丁酯(TBPB),二叔丁基过氧化物(DTBP),过硫酸铵((NH4)2S2O8),过硫酸钾(K2S2O8),产物产率分别为90%,39%,43%,82%,41% (表1,entries 6-10),其中氧化剂为H2O2时反应效果最佳。综上,该反应的最佳反应条件为:20 mol% 2-DMAP为催化剂,H2O2(2 equiv.)为氧化剂,在二氯甲烷溶剂中100℃下磁力搅拌反应16 h。
Table 1. Optimization of reaction conditionsa
表1. 反应条件的优化a
a反应条件:α-溴代肉桂醛(0.3 mmol),1,3-环己二酮(0.6 mmol),100℃,16 h;b分离产率。
3.2. 底物普适性研究
在最优条件下,研究了反应底物的普适性,结果见表2。从中可以看出,当1,3-环己二酮5位上无取代基时,以90%的产率得到目标化合物3a;当其5位的取代基分别是的-CH3和-C6H5时,以89%和85%的产率得到相应的产物3b和3c;此外,5位上连有两个甲基时反应也能顺利发生,产物3d的产率为89%。当α-溴代肉桂醛的苯环上取代基为F、Cl、NO2等吸电子基团时都能顺利地得到目标产物3e-3h,产率为45-86%。当取代基NO2分别在α-溴代肉桂醛苯环上的2-位和4-位时,相应产物3g和3h的产率分别为45%和60%,说明空间位阻效应对该反应有一定的影响。总体而言,该反应的底物普适性较好。化合物结构经1H NMR,13C NMR,HRMS表征。
Table 2. Research of substrate scopea
表2. 底物的普适性研究a
a反应条件:α-溴代肉桂醛(0.3 mmol),1,3-环己二酮(0.6 mmol),2-DMAP (0.06 mmol),100℃,16 h;b分离产率。
未被报道的化合物结构表征如下:
化合物3a: 3-bromo-2-phenyl-2,6,7,8-tetrahydro-5H-chromen-5-one, 白色固体,1H NMR (400 MHz, CDCl3) δ (ppm) = 7.43~7.39 (m, 5H), 7.09 (s, 1H), 5.90 (s, 1H), 2.45~2.23 (m, 4H), 1.99~1.92 (m, 2H); 13C NMR (101 MHz, CDCl3) δ (ppm) = 193.56, 169.49, 136.74, 129.55, 128.81, 127.80, 120.18, 111.23, 110.24, 82.76, 35.99, 27.95, 20.24; HRMS (ESI) m/z[M+H]+calcd for C15H13BrO2: 305.01717, found: 305.01669.
化合物3b: 3-bromo-7-methyl-2-phenyl-2,6,7,8-tetrahydro-5H-chromen-5-one, 黄色液体, 1H NMR (400 MHz, CDCl3)δ (ppm) = 7.41 (m, 5H), 7.08 (d,J = 5.5 Hz, 1H), 5.90 (s, 1H), 2.51~1.94 (m, 5H), 1.04 (t, J = 6.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ (ppm) = 193.66, 169.08, 136.64, 128.89, 127.82, 120.28, 111.09, 110.07, 82.84, 44.51, 36.23, 28.37, 20.94; HRMS (ESI) m/z[M+H]+calcd for C16H15BrO2 319.03282, found: 319.03235.
化合物3c: 3-bromo-2,7-diphenyl-2,6,7,8-tetrahydro-5H-chromen-5-one, 黄色固体,1H NMR (400 MHz, CDCl3) δ (ppm) = 7.47~7.41 (m, 5H), 7.31 (t, J = 7.3 Hz, 2H), 7.28~7.24 (m, 1H), 7.19 (t, J = 7.8 Hz, 2H), 7.13 (d, J = 9.7 Hz, 1H), 5.94 (s, 1H), 3.40~3.28 (m, 1H), 2.76~2.46 (m, 4H); 13C NMR (101 MHz, CDCl3) δ (ppm) = 192.68, 168.56, 142.02, 136.46, 129.69, 128.83, 127.84, 127.10, 126.55, 120.17, 111.30, 110.30, 82.97, 43.33, 38.74, 35.53; HRMS (ESI) m/z[M+H]+calcd forC21H17BrO2:347.06412, found: 347.06360.
化合物3d: 3-bromo-7,7-dimethyl-2-phenyl-2,6,7,8-tetrahydro-5H-chromen-5-one, 黄色液体,1H NMR (400 MHz, CDCl3) δ (ppm) = 7.44~7.39 (m, 5H), 7.08 (s, 1H), 5.90 (s, 1H), 2.27~2.13 (m, 4H), 1.05 (s, 3H), 1.02 (s, 3H); 13C NMR (101 MHz, CDCl3) δ (ppm) = 193.29, 168.08, 136.77, 129.53, 128.79, 127.73, 120.02, 110.13, 82.82, 77.22, 76.90, 76.58, 49.97, 41.72, 32.14, 28.90, 27.47; HRMS (ESI) m/z[M+H]+calcd for C17H17BrO2: 333.04847, found: 333.04770.
化合物3e: 3-bromo-2-(4-fluorophenyl)-2,6,7,8-tetrahydro-5H-chromen-5-one, 白色固体,1H NMR (400 MHz, CDCl3) δ (ppm) = 7.40 (dd, J = 8.7, 5.3 Hz, 2H), 7.09 (dd, J = 9.5, 7.7 Hz, 3H), 5.88 (s, 1H), 2.45~2.21 (m, 4H), 1.99~1.92 (m, 2H); 13C NMR (101 MHz, CDCl3) δ (ppm) = 193.50, 169.25, 164.54, 162.06, 132.60, 129.80, 120.41, 115.96, 115.74, 111.23, 109.96, 81.91, 35.97, 27.92, 20.22; HRMS (ESI) m/z[M+H]+calcd for C15H12BrFO2: 323.00775, found: 323.00708.
化合物3f: 3-bromo-2-(4-chlorophenyl)-2,6,7,8-tetrahydro-5H-chromen-5-one, 黄色液体,1H NMR (400 MHz, CDCl3) δ (ppm) = 7.42~7.31 (m, 4H), 7.09 (s, 1H), 5.87 (s, 1H), 2.44~2.21 (m, 4H), 1.95 (m, 2H); 13C NMR (101 MHz, CDCl3) δ (ppm) = 193.70, 169.50, 135.58, 135.12, 129.24, 129.08, 120.47, 111.25, 109.70, 81.88, 35.93, 27.92 , 20.20 (s); HRMS (ESI) m/z[M+H]+calcd for C15H12BrClO2: 338.97820, found: 338.97757.
化合物3g: 3-bromo-2-(4-nitrophenyl)-2,6,7,8-tetrahydro-5H-chromen-5-one, 黄色固体,1H NMR (400 MHz, CDCl3) δ (ppm) = 8.28~8.24 (m, 2H), 7.63~7.59 (m, 2H), 7.12 (s, 1H), 5.98 (s, 1H), 2.49~2.24 (m, 5H), 2.01~1.94 (m, 2H); 13C NMR (101 MHz, CDCl3) δ (ppm) = 193.41, 169.11, 148.54, 143.44, 128.83, 124.10, 121.08, 111.45, 108.87, 81.23, 35.98, 27.84, 20.21; HRMS (ESI) m/z[M+H]+calcd for C15H12BrNO4: 350.00225, found: 350.00156.
化合物3h: 3-bromo-2-(2-nitrophenyl)-2,6,7,8-tetrahydro-5H-chromen-5-one, 黄色固体,1H NMR (400 MHz, CDCl3) δ (ppm) = 7.98 (dd, J = 8.0, 0.9 Hz, 1H), 7.69~7.62 (m, 2H), 7.57 (m, 1H), 7.18 (s, 1H), 6.79 (s, 1H), 2.46~2.16 (m, 4H), 1.97~1.89 (m, 2H); 13C NMR (101 MHz, CDCl3) δ (ppm) = 193.59, 169.43, 148.92, 133.33, 130.45 ,130.37, 129.66, 125.14, 122.17, 111.66, 108.10, 76.53, 35.99, 27.69, 20.17; HRMS (ESI) m/z [M+H]+calcd for C15H12BrNO4: 350.00225, found: 350.00146。
4. 结论
本文提出了一种无金属条件下,2-二甲氨基吡啶催化1,3环己二酮和α-溴代肉桂醛合成2H-吡喃类化合物的方法。该方法具有操作简单、原子经济性好和产率高等优点,丰富和发展了2H-吡喃类化合物的合成策略。
基金项目
国家自然科学基金(No. 21572195)。
文章引用
杨万乐,刘议璠,刘晨江. 2-二甲氨基吡啶催化合成2H-吡喃类化合物
Synthesis of 2H-Pyranes Catalyzed by 2-Dimethylaminopyridine[J]. 合成化学研究, 2019, 07(02): 18-22. https://doi.org/10.12677/SSC.2019.72004
参考文献
- 1. Costa, M., Dias, T.A., Brito, A. and Proenca, F. (2016) Biological Importance of Structurally Diversified Chromenes. European Journal of Medicinal Chemistry, 123, 487-507. https://doi.org/10.1016/j.ejmech.2016.07.057
- 2. Pratap, R. and Ram, V.J. (2014) Natural and Synthetic Chromenes, Fused Chromenes, and Versatility of Dihydrobenzo[h]Chromenes in Organic Synthesis. Chemical Reviews, 114, 10476-10526. https://doi.org/10.1021/cr500075s
- 3. Parmar, N.J., Pansuriya, B.R., Labana, B.M., Sutariya, T.R., Kant, R. and Gupta, V.K. (2012) Access to Some Angular Aminochromeno[2,3-c]Pyrazole Precursors by a Domino Knoevenagel-hetero-Diels-Alder Reaction. European Journal Organic Chemistry, 30, 5953-5964. https://doi.org/10.1002/ejoc.201200751
- 4. Radi, M., Bernardo, V., Bechi, B., Castagnolo, D., Pagano, M. and Botta, M. (2009) Microwave-assisted Organocatalytic Multicomponent Knoevenagel/Hetero Diels-Alder Reaction for the Synthesis of 2,3-Dihydropyran[2,3-c]Pyrazoles. Tetrahedron Letters, 50, 6572-6575. https://doi.org/10.1016/j.tetlet.2009.09.047
- 5. Maddila, S.N., Maddila, S., Khumalo, M., Bhaskaruni, S.V.H.S. and Jonnalagadda, S.B. (2019) An Eco-friendly Approach for Synthesis of Novel Substituted 4H-Chromenes in Aqueous Ethanol under Ultra-Sonication with 94% Atom Economy. Journal of Molecular Structure, 1185, 357-360. https://doi.org/10.1016/j.molstruc.2019.03.006
- 6. Singh, M., Kaur, M. and Silakar, O. (2014) Flavones: An Important Scaffold for Medicinal Chemistry. European Journal of Medicinal Chemistry, 84, 206-239. https://doi.org/10.1016/j.ejmech.2014.07.013
- 7. Keri, R.S., Budagumpi, S., Krishna P.R. and Balakrishna, R.G. (2014) Chromones as a Privileged Scaffold in Drug Discovery. European Journal of Medicinal Chemistry, 78, 340-374. https://doi.org/10.1016/j.ejmech.2014.03.047
- 8. Oshiro, P.B., Souzada, P., Gomes, L.S., Menezes, M.L. and Silva-Filho, L.C. (2015) Synthesis of 4H-Chromenes Promoted by NbCl5 Through Multicomponent Reaction. Tetrahedron Letters, 56, 4476-4479. https://doi.org/10.1016/j.tetlet.2015.05.099
- 9. Kurdyumov, A.V., Lin, N., Hsung, R.P., Gullickson, G.C., Cole, K.P., Syd-orenko, N. and Swidorski, J.J. (2006) A Lewis Acid-Catalyzed Formal [3+3] Cycloaddition of α,β-Unsaturated Aldehydes with 4-Hydroxy-2-Pyrone, Diketones, and Vinylogous Esters. Organic Letters, 8, 191-193. https://doi.org/10.1021/ol0523042
- 10. Hubert, C., Moreau, J., Batany, J., Duboc, A., Hurvois, J.-P. and Renaud, J.-L. (2008) Br?nsted Acid-Catalyzed Synthesis of Pyrans via a Formal [3+3] Cycloaddition. Advanced Synthesis & Catalysis, 350, 40-42.https://doi.org/10.1002/adsc.200700375
- 11. Moreau, J., Hubert, C., Batany, J., Toupet, L., Roisnel, T., Hurvois, J.-P. and Renaud, J.-L. (2009) Metal-Free Br?nsted Acid Catalyzed Formal [3+3] Annulation. Straightforward Synthesis of Dihy-dro-2H-Chromenones, Pyranones, and Tetrahydroquinolinones. Journal of Organic Chemistry, 74, 8963-8973. https://doi.org/10.1021/jo901238y
- 12. Yang, Q., Zhou, L.-H., Wu, W.-X., Zhang, W., Wang, N. and Yu, X.-Q. (2015) Li-pase-Catalyzed Regioselective Domino Reaction for the Synthesis of Chromenone Derivatives. RSC Advance, 5, 78927-78932. https://doi.org/10.1039/C5RA13267C
- 13. Rueping, M., Sugiono, E. and Merino, E. (2008) Asymmetric Organocatalysis: An Efficient Enantioselective Access to Benzopyranes and Chromenes. Chemistry-A European Journal, 14, 6329-6332. https://doi.org/10.1002/chem.200800836
- 14. Yao, B., Miao, T., Li, P.-H. and Wang, L. (2019) Direct Synthesis of Ben-zo[f]indazoles from Sulfonyl Hydrazines and 1,3-Enynes by Copper-Catalyzed Annulation. Organic Letters, 21, 124-128. https://doi.org/10.1021/acs.orglett.8b03564
NOTES
*通讯作者。
