Hans Journal of Food and Nutrition Science
Vol.
08
No.
01
(
2019
), Article ID:
28551
,
-2
pages
10.12677/HJFNS.2019.81004
Advances in the Application Effects of 25-Hydroxyvitamin D3 in Pig Diets
Lianhua Zhang, Xiangshu Piao*
State Key Laboratory of Animal Nutrition, College of Animal Science and Technology, China Agricultural University, Beijing

Received: Jan. 3rd, 2019; accepted: Jan. 14th, 2019; published: Jan. 21st, 2019

ABSTRACT
25-Hydroxyvitamin D3 (25-OH-D3), the first active metabolite of vitamin D3 in the liver, is the primary form of vitamin D3 in the blood. Previous studies showed that dietary 25-OH-D3 supplementation can improve production performance and vitamin D status of pigs. From the perspective of the nutritional functions and advantages of 25-OH-D3, this paper mainly reviews the application effects of 25-OH-D3 in pig diets, and provides theoretical basis for practical production.
Keywords:25-Hydroxyvitamin D3, Application Effect, Diets, Pig
25-羟基维生素D3在猪日粮上应用效果 的研究进展
张连华,朴香淑*
中国农业大学动物科学技术学院,动物营养学国家重点实验室,北京

收稿日期:2019年1月3日;录用日期:2019年1月14日;发布日期:2019年1月21日

摘 要
25-羟基维生素D3 (25-OH-D3)作为维生素D3在肝脏中的第一个活性代谢产物,是维生素D3在血液循环中的主要形式。研究表明,在猪的日粮中添加25-OH-D3能够显著提高生产性能,改善机体维生素D营养状况。本文从25-OH-D3的营养功能与优势的角度出发,主要总结25-OH-D3在猪日粮上应用效果的研究进展,为实际生产提供理论基础。
关键词 :25-羟基维生素D3,应用效果,日粮,猪

Copyright © 2019 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
在现代集约化养殖条件下,由于缺乏户外活动和紫外线照射,畜禽易出现维生素D缺乏状况,进而导致畜禽骨骼软弱和生产性能下降。因此饲料中通常添加维生素D3 (VD3)及其衍生物来调节畜禽体内钙磷的代谢,改善骨骼性能和维持机体正常生长发育。25-羟基维生素D3 (25-OH-D3)作为维生素D3代谢链中的第一个羟基化代谢产物,能够参与小肠粘膜上皮细胞中钙转运蛋白的生成。虽然VD3也能促进细胞间钙离子的流动,但是普通的脂溶性VD3易受脂肪、胆汁、肠道炎症或损伤及原料中高铜、高铁等氧化因素的影响。因此,25-OH-D3在刺激肠道对钙磷的吸收、肾脏对钙磷的重吸收、促进骨骼发育以及提高机体免疫力等方面比普通维生素D3具有更高的应用价值。外源添加25-OH-D3能以即用的活性形式被畜禽直接利用,这就意味着畜禽饲养过程中添加25-OH-D3可以快速改善畜禽维生素D的营养状况,有助于解决畜禽健康问题和改善动物生产性能。
2. 25-OH-D3的概述
Lund和DeLuca (1966) [1] 发现,在服用维生素D3后,维生素D3本身会消失,但会出现几种代谢物,这些代谢物具有更强的治疗佝偻病活性。第一种代谢物是25-OH-D3,它是在肝脏中发现的,在正常情况下该物质是维生素D在体内的循环形式。
2.1. 25-OH-D3的结构与性质
25-OH-D3是脂溶性维生素D3的一种次级代谢产物,分子式为C22H44O2∙H2O,化学结构如图1所示,相对分子质量为418.7 (1999年国际相对原子质量)。从分子结构上讲,25-OH-D3是在VD3的第25位碳原子上添加了一个羟基。与普通维生素D3相比,25-OH-D3亲水性更好,其生物学活性更高 [2] 。研究表明,以生长性能和骨骼矿化指标来评价25-OH-D3的生物学效价约为VD3的1.81倍 [3] 。而且,25-OH-D3与VD3生物学效价在添加水平较低情况下差异更加明显 [4] 。通过饲料直接供给激素形式的1,25-(OH)2-D3超越了机体自身对该成分的调控,如饲料中钙含量过高,容易导致产生毒性效应,因此不宜直接使用。25-OH-D3半衰期较长,在机体中比较稳定且浓度较高,能反映机体摄入和自身合成的VD3情况,因此25-OH-D3被认为是衡量机体内VD3营养状态的最佳指标 [5] 。目前,25-OH-D3作为一种新型的维生素D来源已经被应用于饲料添加剂 [4] [6] 。
2.2. 25-OH-D3的代谢与功能
自然情况下,皮肤内产生的7-脱氢胆固醇和日粮的提供是动物体内VD3的主要供给来源 [7] 。VD3经肠道吸收进入血液循环后被运输到肝脏,首先在线粒体和微粒体25-羟化酶作用下发生第一次羟化反应形成25-OH-D3,25-OH-D3再经血液输送至肾脏,在肾脏线粒体1α-羟化酶作用下发生第二次羟化反应,转化为最终活性形式1,25-(OH)2-D3 [8] [9] 。与普通VD3相比,25-OH-D3由于增加了一个亲水性的羟基,其吸收机制与脂溶性的VD3不同。如图2所示,25-OH-D3吸收不依赖胆汁的分泌和脂肪吸收形成的微团结构,不易受胆肠道炎症或损伤及饲料原料中霉菌毒素、高铜以及高铁等因素的影响,且与色素、胡萝卜素以及其他脂溶性维生素无竞争吸收 [10] 。25-OH-D3被吸收后能够绕过肝脏的羟化过程,简化了VD3在动物机体内的代谢过程(见图3),避免因肝脏损伤或肝功能失调引起的VD3功能紊乱,可提供更有效的并且比VD3释放更多的活性代谢物。1,25-(OH)2-D3通过血液进入肠道上皮细胞后,与两种特异性维生素D受体即肠道上皮细胞核受体(nVDR)和细胞膜受体(mVDR)结合后发挥生物学功能,调节肠道对钙、磷的吸收和肾脏对钙、磷的重吸收,提高血液中钙、磷的浓度,维持机体钙、磷稳态 [11] [12] [13] 。1,25-(OH)2-D3与靶细胞核上的nVDR结后合,通过基因组途径对靶基因进行转录和翻译调控,其具体作用途径为1,25-(OH)2-D3进入细胞后,可迅速转移至细胞核,与细胞核上的nVDR结合,引起nVDR的构象变化和磷酸化并激活nVDR,同时与视黄醛X受体(RXR)结合形成异质二聚体,启动对相关基因的转录和翻译调控 [14] 。1,25-(OH)2-D3与靶细胞膜上的mVDR结后合,通过非基因组途径对靶细胞进行调控,其过程为1,25-(OH)2-D3与靶细胞膜上的mVDR结合后,诱导细胞膜L型钙离子通道开放,使胞外钙离子内流 [15],同时贮存于内质网内的钙离子释放到胞内 [16],导致细胞内钙离子浓度迅速增加,其信号传导途径通过由蛋白激酶C (PKC)介导的信号通路实现 [17] 。试验研究表明,日粮中用25-OH-D3代替VD3可改善骨骼的矿化、促进肠道发育和提高免疫力 [18] [19] 。总之,25-OH-D3不仅具有普通VD3的功能与营养价值,同时还具有VD3所不及的优势。
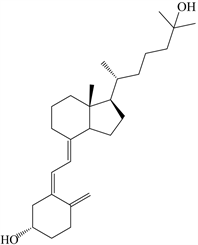
Figure 1. The chemical structure of 25-OH-D3
图1. 25-OH-D3的化学结构

Figure 2. The absorption advantage of 25-OH-D3 [10]
图2. 25-OH-D3的吸收优势 [10]

Figure 3. The metabolic pathway of 25-OH-D3 [9]
图3. 25-OH-D3的代谢途径 [9]
2.3. 25-OH-D3的毒性
肉鸡上的安全性评价试验结果显示,VD3添加量达到基础日粮添加水平(按69 μg/kg计) 50倍时,肾脏出现钙化症状;25-OH-D3添加量达到基础日粮添加水平(按69 μg/kg计) 10倍时,肉鸡肾脏已出现钙化症状 [20] 。因此,25-OH-D3的毒性要高于VD3。而von Rosenberg等(2016) [21] 发现,仔猪日粮中25-OH-D3添加量达到5到10倍的推荐量水平时对一般机体健康指标如血液和尿液特征、骨骼参数、器官重量、肾脏干物质、灰分和钙含量等均无不利影响。
3. 猪的日粮中25-OH-D3应用效果的研究
在饲料行业中,VD3通常作为维生素类饲料添加剂,用来提高动物的骨骼矿化和生长性能,促进动物肠道和肾脏对钙、磷的吸收,但VD3的生物学效价较低。鉴于此,国内外许多公司加大了对VD3衍生物研发的人力与资金投入,以寻求VD3的替代物。因此,25-OH-D3成为普通VD3的主要衍生替代产品之一。近年来,25-OH-D3获得美国食品药品管理局认证,成为一般公认安全级的维生素D3衍生物。2014年2月1日,我国将25-OH-D3列入《饲料添加剂品种目录(2013)》(农业部公告第2045号),可以作为猪和家禽饲料添加剂使用,属于维生素类添加剂。
3.1. 妊娠和哺乳母猪
妊娠和哺乳母猪是养猪生产和管理过程的关键环节。妊娠和哺乳母猪由于舍内限位栏饲养和特殊的生理需求,容易导致骨钙流失和骨骼性能下降,最终导致繁殖性能降低。在妊娠期间,25-OH-D3通过胎盘从母体转运到胎儿,出生时脐带血中25-OH-D3的浓度约为母体血液浓度的80% [22] [23] 。因此,胎儿与新生仔猪体内血液25-OH-D3浓度与母体血液维生素D营养状况息息相关。在妊娠和哺乳期间,母体的VD3代谢过程受到胎儿生长和产奶过程对钙需求量不断增长的影响 [24],这一特殊需求势必会造成母体骨钙的动员和流失。然而,母体骨钙在生殖期间已被大量消耗,导致母体骨骼的弱化、跛行甚至骨折 [25] 。目前,集约化商业养殖条件下,骨骼系统问题是母猪淘汰的主要原因之一,比例可达10%~15%,最高可达40% [26] 。
目前,NRC (2012)在妊娠哺乳母猪上维生素D推荐量为800 IU/kg,但这仅能保证正常生理条件下动物机体不出现维生素D缺乏症,并不能满足动物的最佳生长、骨骼强度和特殊生理功能(如免疫)。因此,饲料生产中维生素D3的添加量有不断提高的趋势,而且日粮中添加25-OH-D3效果更优。日粮添加25-OH-D3对妊娠和泌乳母猪繁殖性能的影响见表1。目前,关于妊娠和泌乳母猪日粮中25-OH-D3应用的研究结果相对较少。Coffey等(2012) [27] 发现,玉米–豆粕基础日粮添加50 μg/kg 25-OH-D3能改善后备母体和新生仔猪血液VD营养状况,提高配种受胎率和窝产仔数。给第一次怀孕母猪饲喂25-OHD3能提高胎儿肌纤维含量 [28] 。Zhou等(2017) [29] 在玉米–豆粕基础日粮添加50 μg/kg 25-OH-D3,结果发现能有效提高初产母猪繁殖性能和第14天常乳乳蛋白和乳糖含量,加快母猪骨骼周转代谢以及改善新生仔猪骨质量。本课题组研究发现,玉米–豆粕基础日粮添加50 μg/kg 25-OH-D3能显著提高仔猪断奶窝重和窝增重,同时有降低母猪体重损失的趋势。
Table 1. Effect of 25-OH-D3 supplementation to diets on reproductive performance of sows
表1. 日粮添加25-OH-D3对母猪繁殖性能的影响
注:“+”代表有显著影响,“−”代表无显著影响。
3.2. 断奶仔猪
断奶应激是影响仔猪健康生长的重要原因。仔猪断奶后由于机体免疫功能低下、消化道发育不健全,易发生肠道疾病和腹泻等一系列问题 [34] [35] [36] 。因此,如何通过改变营养手段来提高仔猪机体抗病能力,促进仔猪健康生长成为动物营养学研究的热点问题。VD3的激素活性形式1,25-(OH)2-D3不仅能够维持机体钙磷稳态,而且在调节机体的免疫应答方面也发挥着重要作用 [37] [38] 。例如,1,25-(OH)2-D3能调节巨噬细胞、T细胞和B细胞的成熟和分化,同时促进细胞因子和免疫球蛋白的分泌 [39] [40] 。25-OH-D3作为饲料中的一种新型维生素添加剂,与普通VD3相比在吸收和转运方面具有明显的优势 [2] 。因此,在断奶仔猪日粮中添加25-OH-D3能否更好地发挥VD3的促进机体健康生长和免疫调节功能值得研究。
迄今为止,在断奶仔猪日粮中25-OH-D3的应用研究也相对较少。廖波等(2011) [38] 在玉米 + 豆粕基础日粮中添加2200 IU/kg 25-OH-D3,结果发现可以提高轮状病毒攻毒和未攻毒组断奶仔猪血清及肠内容物RV-Ab水平,降低促炎因子白细胞介素-6 (IL-6)和干扰素-γ (IFN-γ)的分泌,促进抗炎因子白细胞介素-4 (IL-4)的生成,表现出提高断奶仔猪免疫功能的作用。小麦 + 豆粕基础日粮添加50 μg/kg 25-OH-D3提高仔猪血清中白细胞的存活能力和吞噬能力 [41] 。Sugiyama等(2013) [42] 发现,日粮中添加50 μg/kg 25-OH-D3对断奶仔猪6~30 kg阶段的平均日采食量、平均日增重和饲料转化效率均无显著影响。Jefferies等(2002) [43] 发现,日粮中添加0.1 mg/kg 25-OH-D3对软骨病和骨关节病的发病率及严重程度均无影响。
3.3. 生长猪
腿部骨骼软弱是影响生长猪快速生长的一个重要问题,它会增加繁殖动物的淘汰率并影响育肥猪的健康生长 [44] 。然而,日粮中达到生长猪最大生长速率的钙磷水平不一定满足骨骼矿化的需求 [45] 。研究表明,日粮钙磷水平在满足生长猪最大生长速率的基础上至少要高出1 g/kg才满足骨骼矿化的需求 [46] 。在维持体重方面,25-OH-D3比VD3具有更强的单位代谢能力 [4] 。Duffy等(2018) [47] 发现,低磷日粮中添加50 μg/kg 25-OH-D3显著提高磷、氮素和灰分的全肠道表观消化率,分别可达15.75%,5.0%和15.3%,但对平均日采食量、平均日增重和饲料转化效率均无显著影响。大麦 + 小麦 + 豆粕日粮中用25 μg/kg 25-OH-D3替代50% VD3显著提高全期平均日采食量和钙的消化率,分别可达3.2%和5.2% [48] 。Regassa等(2015) [49] 也发现,玉米 + 豆粕日粮中添加50或100 μg/kg 25-OH-D3对平均日采食量、平均日增重和饲料转化效率均无显著影响,但显著提高钠依赖性磷酸转运蛋白1 (SLC34A1)基因的表达,降低粪便中磷的排泄量。
4. 小结
25-OH-D3作为一种新型维生素类添加剂,因其独特的吸收特色优势,在猪营养中发挥重要的生理功能。目前,关于25-OH-D3在猪营养上应用研究相对较少,但已取得一定的进展。从现阶段研究成果来看,在猪的日粮中可以使用一定量的25-OH-D3来提高生产性能,改善机体维生素D营养状况。然而,将25-OH-D3广泛应用于猪的日粮中还需要对其适宜添加量和作用机理进行深入研究,从而更好地发挥替代普通VD3功能的作用,提高猪的生产性能。这些措施有利于推广25-OH-D3替代普通维生素D3在畜禽生产上的实际应用,提高畜禽生产效率,在实现畜牧业的安全健康发展中具有重要作用。
基金项目
感谢国家自然科学基金(批准号:31772612)项目资助。
文章引用
张连华,朴香淑. 25-羟基维生素D3在猪日粮上应用效果的研究进展
Advances in the Application Effects of 25-Hydroxyvitamin D3 in Pig Diets[J]. 食品与营养科学, 2019, 08(01): 37-34. https://doi.org/10.12677/HJFNS.2019.81004
参考文献
- 1. Lund, J. and DeLuca, H.F. (1966) Biologically Active Metabolite of Vitamin D3 from Bone, Liver, and Blood Serum. Journal of Lipid Research, 7, 739-744.
- 2. Bar, A., Sharvit, M., Noff, D., et al. (1980) Absorption and Excretion of Cholecalciferol and of 25-Hydroxycholecalciferol and Metabolites in Birds. Journal of Nutrition, 110, 1930-1940. https://doi.org/10.1093/jn/110.10.1930
- 3. 瞿红侠, 王建国, 陈冠华, 等. 肉鸡日粮中25-羟基维生素D3与维生素D3生物学效价比较[J]. 中国饲料, 2015(20): 25-28.
- 4. Fritts, C.A. and Waldroup, P.W. (2003) Effect of Source and Level of Vitamin D on Live Performance and Bone Development in Growing Broilers. Journal of Applied Poultry Research, 12, 45-52. https://doi.org/10.1093/japr/12.1.45
- 5. Jakobsen, J., Maribo, H., Bysted, A., et al. (2007) 25-Hydroxyvitamin D3 Affects Vitamin D Status Similar to Vitamin D3 in Pigs—But the Meat Produced Has a Lower Content of Vitamin D. British Journal of Nutrition, 98, 908-913. https://doi.org/10.1017/S0007114507756933
- 6. Lauridsen, C. (2014) Triennial Growth Sympo-sium-Establishment of the 2012 Vitamin D Requirements in Swine with Focus on Dietary Forms and Levels of Vitamin D. Journal of Animal Science, 92, 910-916. https://doi.org/10.2527/jas.2013-7201
- 7. Jones, G. (2012) Metabolism and Biomarkers of Vitamin D. Scandinavian Journal of Clinical and Laboratory Investigation, 72, 7-13.
- 8. Christakos, S. (2017) In Search of Regulatory Circuits That Control the Biological Activity of Vitamin D. Journal of Biological Chemistry, 292, 17559-17560. https://doi.org/10.1074/jbc.H117.806901
- 9. Haussler, M.R., Whitfield, G.K., Kaneko, I., et al. (2013) Molecular Mechanisms of Vitamin D Action. Calcified Tissue International, 92, 77-98. https://doi.org/10.1007/s00223-012-9619-0
- 10. 国内首个自主研发“25-羟基维生素D3”上市[J]. 家禽科学, 2014(11): 61.
- 11. 赵成毅, 刘少喻, 李青, 等. 1,25-羟基维生素D3通过其核受体与膜受体协同调节骨代谢机制的研究进展[J]. 中华骨科杂志, 2012, 32(8): 788-791.
- 12. Nemere, I., Farach-Carson, M.C., Rohe, B., et al. (2004) Ribozyme Knockdown Functionally Links a 1,25(OH)2D3 Membrane Binding Protein (1,25D3-MARRS) and Phos-phate Uptake in Intestinal Cells. Proceedings of the National Academy of Sciences, 101, 7392-7397. https://doi.org/10.1073/pnas.0402207101
- 13. Tunsophon, S. and Nemere, I. (2010) Protein Kinase C Isotypes in Signal Transduction for the 1,25D3-MARRS Receptor (ERp57/PDIA3) in Steroid Hormone-Stimulated Phosphate Uptake. Steroids, 75, 307-313. https://doi.org/10.1016/j.steroids.2010.01.004
- 14. Takeyama, K. and Kato, S. (2011) The Vitamin D3 1alpha-Hydroxylase Gene and Its Regulation by Active Vitamin D3. Bioscience, Biotechnology, and Biochemistry, 75, 208-213. https://doi.org/10.1271/bbb.100684
- 15. Zanello, L.P. and Norman, A.W. (2004) Electrical Responses to 1α,25(OH)2-Vitamin D3 and Their Physiological Significance in Osteoblasts. Steroids, 69, 561-565. https://doi.org/10.1016/j.steroids.2004.05.003
- 16. Baran, D.T., Sorensen, A.M., Shalhoub, V., et al. (1991) 1α,25-Dihydroxyvitamin D3 Rapidly Increases Cytosolic Calcium in Clonal Rat Osteosarcoma Cells Lacking the Vitamin D Receptor. Journal of Bone and Mineral Research, 6, 1269-1275. https://doi.org/10.1002/jbmr.5650061202
- 17. Nemere, I., Larsson, D. and Sundell, K. (2000) A Specific Binding Moiety for 1,25-Dihydroxyvitamin D3 in Basal Lateral Membranes of Carp Enterocytes. American Journal of Physiology-Endocrinology and Metabolism, 279, E614-E621. https://doi.org/10.1152/ajpendo.2000.279.3.E614
- 18. Gabriela, G.V., Rene, M.L. and Ernesto, A.G. (2013) Use of 25-Hydroxycholecalciferolin Diets of Broiler Chickens: Effects on Growth Performance, Immunity and Bone Calcification. Journal of Poultry Science, 50, 60-64. https://doi.org/10.2141/jpsa.0120071
- 19. Janocha, A., Osek, M., Klocek, B., et al. (2009) Effect of Adding 25-Hydroxycholecalciferol in Plant Diets with and without Fish Meal on Rearing Results and Bones of Broiler Chickens. Annals of Animal Science, 9, 415-423.
- 20. Yarger, J.G., Quarles, C.L., Hollis, B.W., et al. (1995) Safety of 25-Hydroxycholecalciferol as a Source of Cholecalciferol in Poultry Rations. Poultry Science, 74, 1437-1446. https://doi.org/10.3382/ps.0741437
- 21. von Rosenberg, S.J., Weber, G.M., Erhardt, A., et al. (2016) Tolerance Evaluation of Overdosed Dietary Levels of 25-Hydroxyvitamin D3 in Growing Piglets. Journal of Animal Physiology and Animal Nutrition, 100, 371-380. https://doi.org/10.1111/jpn.12355
- 22. Kimball, S., Fuleihan, G. and Vieth, R. (2008) Vitamin D: A Growing Perspective. Critical Reviews in Clinical Laboratory Sciences, 45, 339-414. https://doi.org/10.1080/10408360802165295
- 23. Petersen, S.B., Olsen, S.F., Mølgaard, C., et al. (2014) Maternal Vitamin D Status and Offspring Bone Fractures: Prospective Study over Two Decades in Aarhus City, Denmark. PLoS ONE, 9, e114334. https://doi.org/10.1371/journal.pone.0114334
- 24. Halloran, B.P., Barthell, E.N. and DeLuca, H.F. (1979) Vitamin D Metabolism during Pregnancy and Lactation in the Rat. Proceedings of the National Academy of Sciences, 76, 5549-5553. https://doi.org/10.1073/pnas.76.11.5549
- 25. Giesemann, M.A., Lewis, A.J., Miller, P.S., et al. (1998) Effects of the Reproductive Cycle and Age on Calcium and Phosphorus Metabolism and Bone Integrity of Sows. Journal of Animal Science, 76, 796-807. https://doi.org/10.2527/1998.763796x
- 26. Kirk, R.K., Svensmark, B., Ellegaard, L.P., et al. (2005) Locomotive Disorders Associated with Sow Mortality in Danish Pig Herds. Journal of Veterinary Medicine, 52, 423-428. https://doi.org/10.1111/j.1439-0442.2005.00747.x
- 27. Coffey, J.D., Hines, E.A., Starkey, J.D., et al. (2012) Feeding 25-Hydroxycholecalciferol Improves Gilt Reproductive Performance and Fetal Vitamin D Status. Journal of Animal Science, 90, 3783-3788. https://doi.org/10.2527/jas.2011-5023
- 28. Hines, E.A., Coffey, J.D., Starkey, C.W., et al. (2013) Improvement of Maternal Vitamin D Status with 25-Hydroxycholecalciferol Positively Impacts Porcine Fetal Skeletal Muscle Development and Myoblast Activity. Journal of Animal Science, 91, 4116-4122. https://doi.org/10.2527/jas.2013-6565
- 29. Zhou, H., Chen, Y., Zhuo, Y., et al. (2017) Effects of 25-Hydroxycholecalciferol Supplementation in Maternal Diets on Milk Quality and Serum Bone Status Markers of Sows and Bone Quality of Piglets. Animal Science Journal, 88, 476-483. https://doi.org/10.1111/asj.12638
- 30. Weber, G.M., Witschi, A.K., Wenk, C., et al. (2014) Triennial Growth Symposium-Effects of Dietary 25-Hydroxycholecalciferol and Cholecalciferol on Blood Vitamin D and Mineral Status, Bone Turnover, Milk Composition, and Reproductive Performance of Sows. Journal of Animal Science, 92, 899-909. https://doi.org/10.2527/jas.2013-7209
- 31. Witschi, A.K., Liesegang, A., Gebert, S., et al. (2011) Effect of Source and Quantity of Dietary Vitamin D in Maternal and Creep Diets on Bone Metabolism and Growth in Piglets. Journal of Animal Science, 89, 1844-1852. https://doi.org/10.2527/jas.2010-3787
- 32. Lauridsen, C., Halekoh, U., Larsen, T., et al. (2010) Reproductive Performance and Bone Status Markers of Gilts and Lactating Sows Supplemented with Two Different Forms of Vitamin D. Journal of Animal Science, 88, 202-213. https://doi.org/10.2527/jas.2009-1976
- 33. Flohr, J.R., Woodworth, J.C., Bergstrom, J.R., et al. (2016) Evaluating the Impact of Maternal Vitamin D Supplementation: I. Sow Performance, Serum Vitamin Metabolites, and Neonatal Muscle Characteristics. Journal of Animal Science, 94, 4629-4642. https://doi.org/10.2527/jas.2016-0409
- 34. Blecha, F., Pollmann, D.S. and Nichols, D.A. (1983) Weaning Pigs at an Early Age Decreases Cellular Immunity. Journal of Animal Science, 56, 396-400. https://doi.org/10.2527/jas1983.562396x
- 35. Hammerberg, C., Schurig, G.G. and Ochs, D.L. (1989) Immunodeficiency in Young Pigs. American Journal of Veterinary Research, 50, 868-874.
- 36. Stokes, C.R., Bailey, M., Haverson, K., et al. (2004) Postnatal Development of Intestinal Immune System in Piglets: Implications for the Process of Weaning. Animal Research, 53, 325-334. https://doi.org/10.1051/animres:2004020
- 37. Cantorna, M.T., Zhu, Y., Froicu, M., et al. (2004) Vitamin D Status, 1,25-Dihydroxyvitamin D3, and the Immune System. American Journal of Clinical Nutrition, 80, 1717S-1720S. https://doi.org/10.1093/ajcn/80.6.1717S
- 38. 廖波, 张克英, 丁雪梅, 等. 饲粮添加25-羟基维生素D3对轮状病毒攻毒和未攻毒断奶仔猪血清和肠内容物抗体和细胞因子水平的影响[J]. 动物营养学报, 2011, 23(1): 34-42.
- 39. Bouillon, R., Garmyn, M., Verstuyf, A., et al. (1995) Paracrine Role for Calcitriol in the Immune System and Skin Creates New Therapeutic Possibilities for Vitamin D Analogs. European Journal of Endocrinology, 133, 7-16. https://doi.org/10.1530/eje.0.1330007
- 40. Hewison, M. (1992) Vitamin D and the Immune System. Journal of Endocrinology, 132, 173-175. https://doi.org/10.1677/joe.0.1320173
- 41. Konowalchuk, J.D., Rieger, A.M., Kiemele, M.D., et al. (2013) Modulation of Weanling Pig Cellular Immunity in Response to Diet Supplementation with 25-Hydroxyvitamin D3. Veterinary Immunology and Immunopathology, 155, 57-66. https://doi.org/10.1016/j.vetimm.2013.06.002
- 42. Sugiyama, T., Kusuhara, S., Chung, T.K., et al. (2013) Effects of 25-Hydroxy-Cholecalciferol on the Development of Osteochondrosis in Swine. Animal Science Journal, 84, 341-349. https://doi.org/10.1111/asj.12000
- 43. Jefferies, D., Farquharson, C., Thomson, J., et al. (2002) Differences in Metabolic Parameters and Gene Expression Related to Osteochondrosis/Osteoarthrosis in Pigs Fed 25-Hydroxyvitamin D3. Veterinary Research, 33, 383-396. https://doi.org/10.1051/vetres:2002024
- 44. Jørgensen, B. (1995) Effect of Different Energy and Protein Levels on Leg Weakness and Osteochondrosis in Pigs. Livestock Production Science, 41, 171-181. https://doi.org/10.1016/0301-6226(94)00048-C
- 45. Stern, S., Lundeheim, N., Johansson, K., et al. (1995) Osteochondrosis and Leg Weakness in Pigs Selected for Lean Tissue Growth Rate. Livestock Production Science, 44, 45-52. https://doi.org/10.1016/0301-6226(95)00056-Q
- 46. Cromwell, G.L., Hays, V.W., Chaney, C.H., et al. (1970) Effects of Dietary Phosphorus and Calcium Level on Performance, Bone Mineralization and Carcass Characteristics of Swine. Journal of Animal Science, 30, 519-525. https://doi.org/10.2527/jas1970.304519x
- 47. Duffy, S.K., Kelly, A.K., Rajauria, G., et al. (2018) The Effect of 25-Hydroxyvitamin D3 and Phytase Inclusion on Pig Performance, Bone Parameters and Pork Quality in Finisher Pigs. Journal of Animal Physiology and Animal Nutrition, 102, 1296-1305. https://doi.org/10.1111/jpn.12939
- 48. O’Doherty, J.V., Gahan, D.A., O’Shea, C., et al. (2010) Effects of Phytase and 25-Hydroxyvitamin D3 Inclusions on the Performance, Mineral Balance and Bone Parameters of Grower-Finisher Pigs Fed Low-Phosphorus Diets. Animal, 4, 1634-1640. https://doi.org/10.1017/S1751731110000807
- 49. Regassa, A., Adhikari, R., Nyachoti, C.M., et al. (2015) Effects of 25-(OH)D3 on Fecal Ca and P Excretion, Bone Mineralization, Ca and P Transporter mRNA Expression and Performance in Growing Female Pigs. Journal of Environmental Science and Health, 50, 293-299. https://doi.org/10.1080/03601234.2015.999612
NOTES
*通讯作者。
