World Journal of Cancer Research
Vol.
08
No.
04
(
2018
), Article ID:
27148
,
6
pages
10.12677/WJCR.2018.84023
Expression of SNAT3 in Colorectal Cancer Tissues and Its Relationship with Clinicopathological Biological Features
Yu He, Yujun Yu, Hanzhang Huang, Chen Wang, Guoyu Huang, Shaoliang Han*
Department of Gastrointestinal Surgery, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou Zhejiang

Received: Sep. 27th, 2018; accepted: Oct. 11th, 2018; published: Oct. 18th, 2018

ABSTRACT
Objective: To investigate the expression of Na + coupled neutral amino acid protein A3 (SNAT3) protein in colorectal cancer tissues and its clinical significance. Methods: The expression of SNAT3 protein in 96 cases of colorectal cancer was detected by immunohistochemical staining, and the relationship between clinicopathological characteristics and colorectal cancer was analyzed. Results: The over-expression rate of SNAT3 protein in colorectal cancer was 56.3% (54/96 cases), including the over-expression rate of SNAT3 in depth of T3 + T4 patients was 74.1% (40/57 cases) was significantly higher that (25.9%, 14/39 cases) in T1 + T2 patients (P < 0.05), over-expression rate of SNAT3 (88.9%, 16/18 cases) in poorly-differentiated cancer patients was significantly higher than that (48.7%, 38/78 cases) in well-differentiated cancer patients (P < 0.05), over-expression rate of SNAT3 (64.8%, 35/48 cases) in patients with lymph node involvement was significantly higher than that (35.2%, 19/48 cases) in patients without (P < 0.05). Compared with I + II patients, the expression rate of SNAT3 protein in patients with stage III + IV increased significantly (75.9%, 41/58 cases vs. 24.1%, 13/38 cases, P < 0.01). Moreover, the expression rate of SNAT3 protein (81.8%, 18/22 cases) in patients with stage recurrence and metastasis was significantly higher that (48.6%, 36/74cases) P < 0.01). In this group, 93 patients had complete followed up data except 3 missing cases, the 1, 3, 5-year survival rate were 89.2% (83/93 cases), 38.7% (36/93 cases) and The 1, 3, 5 survival rate of SNAT3 positive patients was significantly lower than that of negative cases (86.5% vs. 92.7%, 32.7% vs. 46.3% and 25.0% vs. 36.6%) (P < 0.05). Conclusions: SNAT3 protein was highly expressed in colorectal cancer tissues, and its high expression may be closely related to invasion and metastasis of colorectal cancer, and may play an important role in the metastasis of colorectal cancer.
Keywords:Colorectal Neoplasm, SNAT3 Gene, Metastasis, Prognosis
结直肠癌组织中SNAT3 表达水平与临床病理 生物学的关系
何宇,余育俊,黄瀚章,王琛,黄国裕,韩少良*
温州医科大学附属第一医院胃肠外科,浙江 温州

收稿日期:2018年9月27日;录用日期:2018年10月11日;发布日期:2018年10月18日

摘 要
目的:探讨钠耦合中性氨基酸转运蛋白A3 (SNAT3)在结直肠癌组织中的表达及其临床意义。方法:采用免疫组化检测96例结直肠癌组织中SNAT3蛋白的表达情况,分析其与结直肠癌患者临床病理指标之间的关系。结果:本组结直肠癌SNAT3蛋白高表达率为56.3% (54/96例),其中癌浸润深度T3 + T4病例的SNAT3蛋白表达率(74.1%、40/57例)显著高于T1 + T2病例(25.9%、14/39例) (P < 0.05);低分化型癌SNAT3蛋白表达率(88.9%、16/18例)显著高于分化型癌(48.7%、38/78例) (P < 0.05),淋巴结转移病例SNAT3表达水平(64.8%、35/48例)显著高于无淋巴结转移(35.2%、19/48例) (P < 0.05);与I + II期患者相比,III +IV患者的SNAT3蛋白表达率显著升高(75.9%、41/58例vs. 24.1%、13/38例) (P < 0.01);且复发转移结直肠癌的SNAT3表达率(81.8%、18/22例)显著高于无转移患者(48.6%、36/74例) (P < 0.01)。本组除3例失访外,其余93例随访资料完整,1、3、5年生存率分别为89.2% (83/93例)、38.7% (36/93例)及30.1% (28/93例),且SNAT3阳性患者的1、3、5年生存率显著低于阴性病例(86.5% vs. 92.7%、32.7% vs. 46.3%及25.0% vs. 36.6%) (P < 0.05)。结论:SNAT3蛋白在结直肠癌组织中高表达,其高表达可能与结直肠癌的侵袭和转移密切相关,且可能在结直肠癌转移中发挥重要作用。
关键词 :结直肠肿瘤,SNAT3基因,转移,预后

Copyright © 2018 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
肿瘤的发生发展是多种因素相互作用的结果,如癌基因激活及抑癌基因失活、免疫抑制及免疫逃逸、肿瘤细胞增殖和转移等,这些均与氨基酸等代谢异常密切相关 [1] [2] 。近年来研究表明钠耦联的中性氨基酸转动蛋白A3 (SNAT3)主要负责小分子脂肪族氨基酸的转运,且在多种实体瘤组织中呈高表达,但有关SNAT3在结直肠癌表达情况及其发生、发展的机制还不明确 [2] [3] [4] [5] [6] 。本研究采用免疫组化方法检测SNAT3蛋白在结直肠癌中的表达情况,并分析其与临床病理指标及预后之间的关系。
2. 资料与方法
2.1. 一般资料
回顾性收集2015年1月至2016年2月于温州医科大学附属第一医院行手术治疗96例结直肠癌患者的临床资料及术后新鲜标本,其中男58例,女38例,年龄38~78岁(中位数59.6岁)。患者术前均未进行放化疗,术前均经肠镜检查活检病理确诊为腺癌,且均实施结直肠癌根治术。术后即刻切取肿瘤组织以及距离肿瘤边缘5 cm以上的结直肠黏膜组织。所有病例资料均完整,均经术后病理检查确诊。排除家族性腺瘤性息肉病(FAP)和遗传性非息肉性结肠癌(HNPCC)患者。采用国际抗癌协会(UICC) 2016年第8版TNM分期 [7] 系统进行分期:I期6例,II期30例,III期33例,IV期27例。本研究获温州医科大学附属第一医院伦理委员会的许可。
2.2. 免疫组化染色
兔抗SNAT3购自美国Abcam公司,DAB染色试剂盒及免疫组化染色试剂盒购自上海蓝基因生物科技有限公司。按照免疫组化试剂盒的操作说明进行操作:① 每个组织蜡块取4 μm厚连续切片,经脱蜡、热修复、过氧化氢封闭消除内源性过氧化物酶;② 滴加SNAT3一抗(Proteintech公司、稀释比例1:200)孵育4℃冰箱过夜;③ 滴加二抗
2.3. 结果判断
采用半定量记数的方法,即由二位病理医生在光镜下实施免疫染色结果判定。每个标本随机抽取10个高倍视野(400×)进行观察,阳性细胞数 > 10%为阳性,≤10%为阴性。
2.4. 随访
随访采取信访、电话随访或门诊随访形式进行随访,失访病例按死亡计算。末次随访时间为2016年2月。生存时间从手术日开始算起至死亡日或最后随访日,以月为单位计算。
2.5. 统计学方法
统计学分析应用SPSS 20.0软件进行,SNAT3表达差异分析用卡方或fisher检验;生存时间用Kaplar-Meier法统计及Log-rank检验;多因素分析采用Cox比例风险模型,P < 0.05为差异有统计学意义。
3. 结果
3.1. SNAT3蛋白在结直肠癌及癌旁组织中的表达
免疫组化染色显示结直肠癌及癌旁组织中均有SNAT3蛋白表达,其阳性表达定位于细胞质中、呈棕褐色颗粒(图1),而对应的正常黏膜无SNAT3蛋白表达,且肿瘤组织中SNAT3蛋白表达水平明显高于转移病灶(P < 0.05)。96例结直肠癌组织中结直肠癌SNAT3蛋白高表达有54例,高表达率为56.3%。
3.2. 结直肠癌组织中SNAT3表达水平与临床病理生物学指标的关系
结直肠癌浸润深度T3 + T4病例的SNAT3蛋白表达率(74.1%、40/57例)显著高于T1 + T2病例(25.9%、14/39例) (P < 0.05),说明随着癌浸润深度增加而蛋白表达增高;低分化型癌SNAT3蛋白表达率(88.9%、16/18例)显著高于分化型癌(48.7%、38/78例) (P < 0.05),说明与癌去分化程度呈正相关;淋巴结转移患者SNAT3表达水平(64.8%、35/48例)显著高于无淋巴结转移(35.2%、19/48例) (P < 0.05);与I + II期患者相比,III + IV患者的SNAT3蛋白表达率显著升高(75.9%、41/58例vs. 24.1%、13/38例) (P < 0.01);且复发转移结直肠癌的SNAT3表达率(81.8%、18/22例)显著高于无转移患者(48.6%、36/74例) (P < 0.01),但与年龄、性别及肿瘤大小无关。上述结果,提示SNAT3蛋白阳性表达率与反应结直肠癌恶性度的分化程度、浸润深度、淋巴结转移、临床病期及肿瘤复发转移密切相关(见表1)。
3.3. 结直肠癌SNAT3的表达与预后的关系
本组除3例失访外(包括阳性表达2例及阴性1例),其余93例随访资料完整,1、3、5年生存率分别为89.2% (83/93例)、38.7% (36/93例)及30.1% (28/93例),且SNAT3阳性患者的1、3、5年生存率分
Table 1. Relationship between SNAT3 gene expression and clinicopathological factors in colorectal cancer
表1. 结直肠癌组织SNAT3基因表达水平与临床病理因素关系
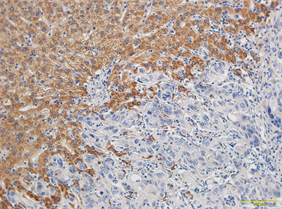
Figure 1. SLC38A3 positive expression was located in cytoplasm of colorectal cancer and showed brown granules (×200)
图1. SLC38A3阳性表达定位于结直肠癌细胞质中、呈棕褐色颗粒(×200)
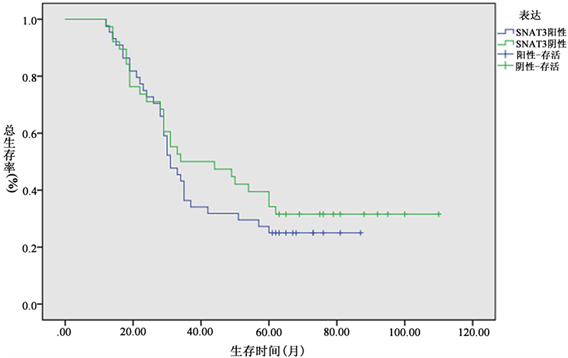
Figure 2. The 1, 3, and 5-year survival rates of SNAT3 positive colorectal cancer patients were significantly lower than those of negative colorectal cancer patients (86.5% vs. 92.7%, 32.7% vs. 46.3% and 25.0% vs. 36.6%) (P < 0.05)
图2. SNAT3阳性结直肠癌患者的1、3、5年生存率显著低于阴性病例(86.5% vs. 92.7%、32.7% vs. 46.3%及25.0% vs. 36.6%) (P < 0.05)
别为86.5% (45/52例)、32.7% (17/52例)及25.0% (13/52例),显著低于阴性病例的92.7% (38/41例)、46.3% (19/41例)及36.6% (15/41例) (P < 0.05,图2)。单因素分析表明结直肠癌预后不佳因素包括癌浸润深度、淋巴结转移、临床病期、SNAT3表达阳性及肿瘤复发转移,多因素分析显示淋巴结转移及临床分期是结直肠癌预后的独立影响因素(风险度为3.043、回归系数为1.121、Wald统计量为14.185、P < 0.001),且与预后呈负相关,但SNAT3表达水平不是影响预后的独立因素。
4. 讨论
众多研究表明氨基酸、尤其是非必需氨基酸的谷氨酰胺,是构成肌肉组织的氮储备及不同组织器官之间的氮运输载体,参与细胞内许多重要的代谢途径,具有重要的生理作用 [6] [7] [8] [9] [10] 。新近研究显示氨基酸代谢异常是肿瘤的一个重要特征、且与肿瘤的发生发展互为因果,即原癌基因激活、抑癌基因失活以及信号通路的异常活化在转录和翻译后修饰等多个水平上调节代谢酶或代谢调控蛋白,通过影响其活性、亚细胞定位、稳定性、自噬等多种机制引起细胞代谢流的改变 [2] [3] [4] [5] [6] 。哺乳动物氨基酸的跨膜运输由多种氨基酸转运载体蛋白介导、即氨基酸转运子,其中SLC38A3/SNAT3基因家族编码钠耦合中性氨基酸转运蛋白(SNAT),分为两类A型和N型氨基酸转运子,主要转运丙氨酸、丝氨酸和谷氨酸等小分子脂肪族氨基酸的转运,且其活性与细胞生长相关 [2] [3] [4] [5] 。多项研究证实谷氨酰胺代谢能够促进胰腺癌、前列腺癌及乳腺癌等多种实体瘤进展的作用 [2] [3] [4] [5] [6] 。近年研究显示在肝癌、胆管癌和乳腺癌中A型氨基酸转运子SNAT1表达明显升高,且和肿瘤进展相关,且N型氨基酸转运子的SNAT3在胶质瘤中表达也上调。
Wang等 [2] 报道SLC38A3基因在转移性非小细胞肺癌(NSCLC)组织中呈高表达。Kondoh等 [11] 比较肝细胞癌组织与非癌组织中氨基酰转运蛋白mRNA水平,发现与非癌组织相比,肝癌组织中SLC38A1 mRNA水平显著升高,并在肝癌组织中显著活化、并在癌前病变的肝硬化组织明显升高。Sidoryk等 [6] 报道神经胶质瘤细胞组织中SNATA3 mRNA水平显著高于转移性肿瘤或对照组织,高达3~4倍。王新军等 [12] 报道89例胶质瘤SNAT1表达明显高于瘤周组织,且其表达与胶质瘤直径,病理级别有关(P < 0.05)。本组结直肠癌SNAT3蛋白高表达率为56.3% (54/96例),其中癌浸润深度T3 + T4病例的SNAT3蛋白表达率(74.1%、40/57例)显著高于T1 + T2病例(25.9%、14/39例) (P < 0.05);低分化型癌SNAT3蛋白表达率(88.9%、16/18例)显著高于分化型癌(48.7%、38/78例) (P < 0.05),淋巴结转移患者SNAT3表达水平(64.8%、35/48例)显著高于无淋巴结转移(35.2%、19/48例) (P < 0.05);与I + II期患者相比,III + IV患者的SNAT3蛋白表达率显著升高(75.9%、41/58例vs. 24.1%、13/38例) (P < 0.01);且复发转移结直肠癌的SNAT3表达率(81.8%、18/22例)显著高于无转移患者(48.6%、36/74例) (P < 0.01),提示SNAT3蛋白阳性表达率与反应结直肠癌恶性度的分化程度、浸润深度、淋巴结转移、临床病期及肿瘤复发转移密切相关。
Wang等 [2] 报道转移性非小细胞肺癌(NSCLC)组织中SLC38A3高表达水平与NSCLC病人预后密切相关。王新军等 [12] 观察89例胶质瘤与SNAT1表达的关系,发现Cox回归模型显示肿瘤病理级别、SNAT1蛋白表达水平为影响胶质瘤患者预后的独立危险因素。本组除3例失访外,其余93例随访资料完整,1、3、5年生存率分别为89.2% (83/93例)、38.7% (36/93例)及30.1% (28/93例),且SNAT3阳性患者的1、3、5年生存率显著低于阴性病例(86.5% vs. 92.7%、32.7% vs. 46.3%及25.0% vs. 36.6%) (P < 0.05)。多因素分析显示淋巴结转移及临床分期是影响结直肠癌预后的独立因素,但SNAT3表达水平不是。
综上所述,本研究结果表明SNAT3蛋白在结直肠癌组织中高表达,其高表达可能与结直肠癌的侵袭和转移密切相关,且可能在结直肠癌转移中发挥重要作用。
文章引用
何 宇,余育俊,黄瀚章,王 琛,黄国裕,韩少良. 结直肠癌组织中SNAT3表达水平与临床病理生物学的关系
Expression of SNAT3 in Colorectal Cancer Tissues and Its Relationship with Clinicopathological Biological Features[J]. 世界肿瘤研究, 2018, 08(04): 144-149. https://doi.org/10.12677/WJCR.2018.84023
参考文献
- 1. Yang, K., Yan, J., Peng, L., et al. (2016) Effect of PLCε Gene Silencing on Inhibiting the Cancerous Transformation of Ulcerative Colitis. Experimental and Therapeutic Medicine, 12, 422-426.
https://doi.org/10.3892/etm.2016.3257 - 2. Wang, Y., Fu, L., Cui, M., et al. (2017) Amino Acid Transporter SLC38A3 Promotes Metastasis of Non-Small Cell LUNG Cancer Cells by Activating PDK1. Cancer Letters, 393, 8-15.
https://doi.org/10.1016/j.canlet.2017.01.036 - 3. Rubioaliaga I, Wagner C A. Regulation and function of the SLC38A3/SNAT3 glutamine transporter. Channels. 1933, 10(6):440.
https://doi.org/10.1080/19336950.2016.1207024 - 4. Wang, K., Cao, F., Fang, W., et al. (2013) Activation of SNAT1/SLC38A1 in Human Breast Cancer: Correlation with p-Akt Overexpression. BMC Cancer, 13, 343.
https://doi.org/10.1186/1471-2407-13-343 - 5. Yu, W.L., Cong, W.M., Zhang, Y., et al. (2011) Overexpression of ATA1/SLC38A1 Predicts Future Recurrence and Death in Chinese Patients with Hilar Cholangiocarcinoma. Journal of Surgical Research, 171, 663-668.
https://doi.org/10.1016/j.jss.2010.03.049 - 6. Sidoryk, M., Matyja, E., Dybel, A., et al. (2004) Increased Expression of a Glu-tamine Transporter SNAT3 Is a Marker of Malignant Gliomas. NeuroReport, 15, 575-578.
https://doi.org/10.1097/00001756-200403220-00001 - 7. Bertero, L., Massa, F., Metovic, J., et al. (2018) Eighth Edition of the UICC Classification of Malignant Tumors: An Overview of the Changes in the Pathological TNM Classification Criteria-What Has Changed and Why? Virchows Arch., 472, 519-531.
- 8. Jenstad, M. and Chaudhry, F.A. (2013) The Amino Acid Transporters of the Glutamate/GABA-Glutamine Cycle and Their Impact on Insulin and Glucagon Secretion. Frontiers in Endocrinology, 4, 199.
https://doi.org/10.3389/fendo.2013.00199 - 9. Bürki, R., Mohebbi, N., Bettoni, C., et al. (2015) Impaired Expression of Key Molecules of Ammoniagenesis Underlies Renal Acidosis in a Rat Model of Chronic Kidney Disease. Nephrology Dialysis Trans-plantation, 30, 770-781.
https://doi.org/10.1093/ndt/gfu384 - 10. Mackenzie, B. and Erickson, J.D. (2004) Sodium-Coupled Neutral Amino Acid (System N/A) Transporters of the SLC38 Gene Family. Pflügers Archiv, 447, 784-795.
https://doi.org/10.1007/s00424-003-1117-9 - 11. Kondoh, N., Imazeki, N., Arai, M., et al. (2007) Activation of a System A Amino Acid Transporter, ATA1/SLC38A1, in Human Hepatocellular Carcinoma and Preneoplastic Liver Tissues. International Journal of Oncology, 31, 81-87.
- 12. 王新军, 杨卓, 杨如意, 袁小威, 孙利坤, 牛晓. 脑胶质瘤组织SNAT1蛋白表达及其意义[J]. 中国神经精神疾病杂志, 2016, 42(7): 426-430.
NOTES
*通讯作者。
