Journal of Organic Chemistry Research
Vol.
09
No.
04
(
2021
), Article ID:
46972
,
9
pages
10.12677/JOCR.2021.94008
酰胺催化脱水制备腈的研究进展
王明慧
浙江师范大学化学与生命科学学院,浙江 金华
收稿日期:2021年10月19日;录用日期:2021年11月26日;发布日期:2021年12月6日

摘要
氰基官能团因广泛存在于药物、农用化学品、染料和精细化学品中,且可方便地转化为其他重要官能团如醛基、羧基、酰胺、伯胺、亚胺等或构建杂环体系而受到化学家的大量关注。传统的腈类制备方法如烷基、芳基(拟)卤代物与氰化物反应、酰胺或醛肟脱水等存在剧毒氰源的使用、剧烈反应条件和原子经济性差等问题。而温和条件下酰胺的催化脱水是制备腈类的理想途径。本文将重点讨论近年来酰胺催化脱水制备腈的研究进展。
关键词
腈,酰胺,催化脱水

Research Progress of Catalytic Dehydration of Amides to Nitriles
Minghui Wang
College of Chemistry and Life Sciences, Zhejiang Normal University, Jinhua Zhejiang
Received: Oct. 19th, 2021; accepted: Nov. 26th, 2021; published: Dec. 6th, 2021

ABSTRACT
The cyano functional group widely exists in pharmaceuticals, agrochemicals, dyes and fine chemicals. As it can be easily converted into other functional groups such as aldehyde, ester, amide, primary amine, and imine, its preparation has attracted numerous attention from organic chemists. Traditional methods for nitrile synthesis including reactions of alkyl- or aryl (pseudo)halides with cyanides and dehydration of amides or aldoximes often require highly toxic reagents, harsh conditions and suffer from poor atom economy. Catalytic amide dehydration under mild conditions is an ideal approach to nitriles. This article focuses on recent advances in catalytic dehydration of amides to nitriles.
Keywords:Nitrile, Amide, Catalytic Dehydration

Copyright © 2021 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
腈类化合物,天然存在于各种细菌、真菌和动植物中,并被广泛用作生产各种药物、农用化学品、聚合物和材料等的原料 [1] [2]。大量的药物分子结构中包含氰基 [3],如维格列汀(Vildagliptin,一种糖尿病药物) [4]、来曲唑(Letrozole) [5] 和阿那曲唑(Anastrozole,用于治疗乳腺癌的药物) [6] 等(图1(a))。此外,氰基官能团作为有机合成中的通用中间体,可以方便地转化为其他重要的官能团(如醛、羧酸、酯、酰胺、伯胺、亚胺等) [7] 或构建杂环体系(如咪唑、嘧啶、三唑等) [8] [9] [10] [11]。然而传统的腈类制备方法如烷基、芳基(拟)卤代物与氰化物反应、酰胺或醛肟脱水(图1(b))以及工业上常用的氨氧化、氢氰化反应等仍存在各种问题 [12],如剧毒氰源的使用、剧烈反应条件、原子经济性差等。相比之下,温和条件下金属或非金属催化的酰胺脱水是制备腈类的理想途径。本文将重点讨论近年来酰胺催化脱水的研究进展。
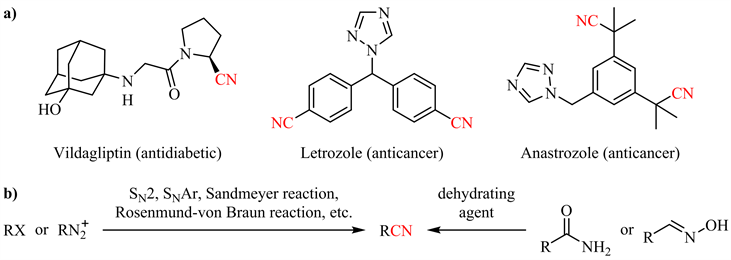
Figure 1. Representative cyano-containing pharmaceuticals (a) and traditional methods for nitrile synthesis (b)
图1. 代表性含氰基药物(a)与传统腈类合成方法(b)
2. 金属催化酰胺脱水
2.1. 高温下金属催化酰胺脱水
早期的金属催化酰胺脱水条件 [13] - [20] 如Yamamoto等人报道的高铼酸共沸去水体系(图2(a)) [18],Kaneda等人发展的钒氧化物/水滑石(Hydrotalcite, Mg6Al2(OH)16CO3)固相负载催化剂(图2(b)) [19],以及Bergman和Ruck报道的锆催化体系(图2(c)) [20] 等尽管在底物适用范围和官能团兼容性方面取得了一定进展,但普遍还需较高温度或在封管(高压釜)中进行。
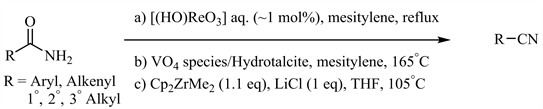
Figure 2. Metal-catalyzed amide dehydration at high temperature
图2. 高温下金属催化酰胺脱水
2.2. (取代)乙腈作为脱水剂的金属催化酰胺脱水
Marazzi小组于2005年首次报道了用乙腈作为脱水剂,在二氯化钯催化下酰胺脱水生成腈的可逆过程 [21]。随后,若干催化体系成功实现了酰胺向腈的这一完全转化 [22] [23] [24] [25]。其中,代明骥小组报道了二(三苯基膦基)二氯化钯/醋酸铜和氯化钯/醋酸银两个互补的催化体系 [23],脂肪族、芳香族伯酰胺以及α,β-不饱和酰胺特别是各种官能团取代的肉桂酰胺均取得了中等到优异的收率(图3)。尤其后一催化体系对敏感官能团如氯、溴、碘、硝基等表现出较好的兼容性。此外,Croatt小组发现Pd(OAc)2/Selectfluor体系同样可用于酰胺到腈的转化并取得较好的收率 [25],该体系对于大位阻叔烷基伯酰胺尤其有效(图4)。
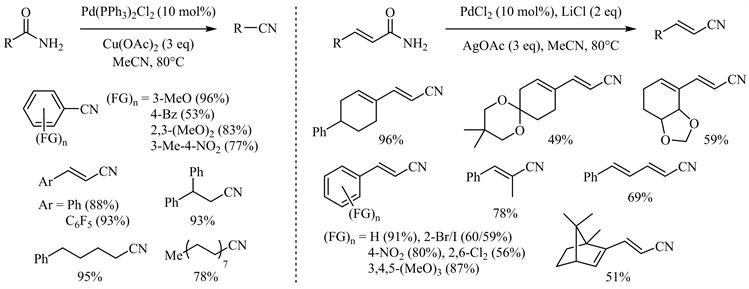
Figure 3. Cu(OAc)2/AgOAc participated palladium catalyzed amide dehydration
图3. 醋酸铜/醋酸银参与的钯催化酰胺脱水

Figure 4. Selectfluor participated palladium catalyzed amide dehydration
图4. Selectfluor参与的钯催化酰胺脱水
2019年,Naka等人发现改用二氯乙腈作为水分子受体对于水分子从酰胺到腈的转移过程在能量上更为有利 [26]。在三氟乙酸钯催化下,一系列天然氨基酸衍生的伯酰胺以中等到优秀的收率得到相应的α-氨基腈,Cbz、Fmoc、Ac和Bz等保护基团、醇羟基、酚羟基、羧基以及酰胺α、β位手性中心均不受影响(图5)。
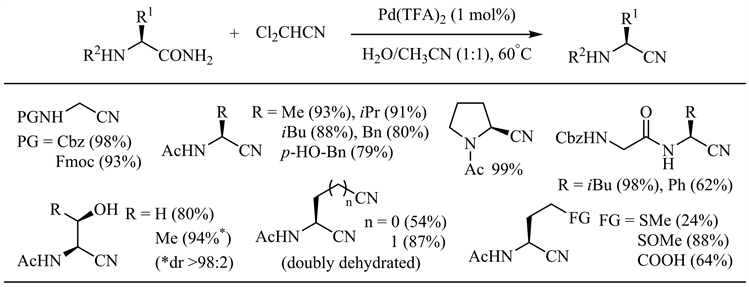
Figure 5. Palladium catalyzed amide dehydration using dichloroacetonitrile as dehydrating agent
图5. 二氯乙腈作脱水剂的钯催化酰胺脱水
2.3. 硅试剂参与的金属催化脱水
近年来,硅试剂参与的金属催化酰胺脱水反应不断见诸报道 [27] - [47]。Nagashima小组于2008年报道了羰基三钌簇合物催化下硅试剂参与的酰胺脱水反应 [27]。反应最初生成的N,N-双硅基酰胺通过硅基迁移得到N,O-双硅基亚胺酸酯,后者顺式消除得到相应的腈和硅氧化物。在上述反应条件下,芳香族和脂肪族伯酰胺均取得了良好到优秀的收率,芳香酰胺邻位位阻和对位强供电子基对收率影响不大,氯、溴和碳碳双键等官能团亦表现出较好的兼容性(图6)。Beller小组随后报道了羰基铁配合物催化的酰胺脱水 [28]。相比于上述钌催化体系,底物范围扩展到杂芳基-2-甲酰胺(中等收率),而烟酰胺在此条件下无反应(图7)。值得注意的是,肉桂酰胺的双键大部分被还原(33%收率)。
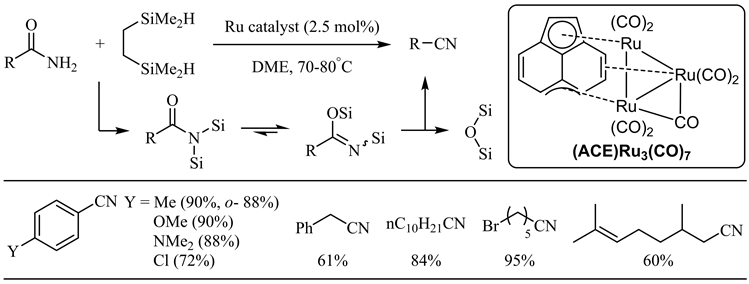
Figure 6. Ruthenium catalyzed silylative dehydration of amides
图6. 硅试剂参与的钌催化酰胺脱水
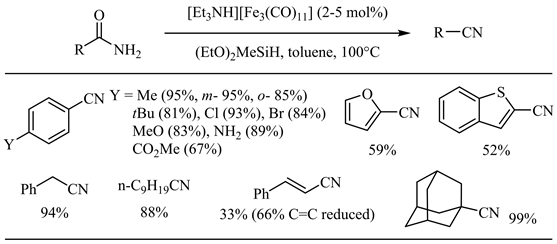
Figure 7. Iron catalyzed silylative dehydration of amides
图7. 硅试剂参与的铁催化酰胺脱水
2018年,Buchwald等人通过密度泛函理论(DFT)计算发现上述反应历程中硅氧化物顺式热消除一步能垒较高(ΔG > 35 kcal/mol),针对这一问题,他们发展出更加温和且高效的铜催化体系 [38],通过低能垒的β-消除极大地促进了脱水过程,使反应在室温下顺利进行并大大拓展了官能团兼容性和底物适用范围(图8)。此外,黄正及其同事们于2019年报道了三乙基硼烷/醋酸钾催化下苯基硅烷或聚甲基氢硅氧烷(PMHS)参与的酰胺脱水 [43],这一简单实用的催化体系同样可在温和条件下(室温或50℃)以良好到优秀的收率将一系列脂肪族、芳香族伯酰胺转化为相应的腈。

Figure 8. Iron catalyzed silylative dehydration of amides
图8. 硅试剂参与的铜催化酰胺脱水
3. 非金属及有机催化的酰胺脱水
3.1. 四丁基氟化铵(TBAF)和氮杂环卡宾(NHC)催化的酰胺脱水
Beller小组和Mandal小组分别报道了四丁基氟化铵(TBAF) [48] 和氮杂环卡宾(NHC) [49] 催化的酰胺脱水反应(图9),后者同样通过低能垒的β-消除得到相应的腈,因而具有广泛的底物适用范围,且对羟基、氨基、杂芳基如(吡啶、噻吩)等配位性基团有较好的兼容性。
3.2. 三苯氧磷、DMSO和环丙烯酮催化的酰胺脱水
2018年,Malkov等人报道了三苯氧磷催化的Appel型酰胺脱水 [50],脂肪族、芳香族酰胺(包括吡啶、噻吩等杂芳基酰胺)以及α-羟基(氨基)酰胺衍生物等均可以优秀的收率转化为相应的氰基化合物,但N-Boc保护基在此条件下不兼容。DMSO催化的Swern型酰胺脱水 [51] 和环丙烯酮催化脱水 [52] 也已见诸报道(图10)。
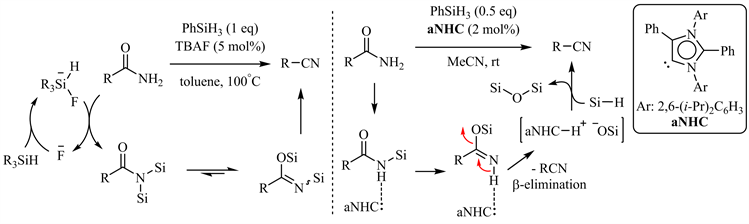
Figure 9. TBAF and a NHC catalyzed amide dehydration
图9. 四丁基氟化铵和氮杂环卡宾催化的酰胺脱水
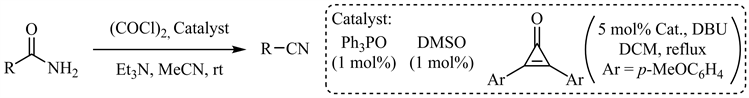
Figure 10. Ph3PO, DMSO and cyclopropenone catalyzed amide dehydration
图10. 三苯氧磷、DMSO和环丙烯酮催化的酰胺脱水
4. 总结
本文系统介绍了近年来发展的金属、非金属和有机催化的酰胺脱水制备腈的方法,并初步探讨了各种方法的反应机理、底物适用范围、官能团兼容性等问题。相比于金属催化的酰胺脱水体系,非金属和有机小分子催化的相关报道仍然较少。对比Buchwald等人发展的铜催化体系和Mandal小组的氮杂环卡宾体系,二者在产率、底物范围和官能团兼容性等方面不相上下。因此,有机催化在这一领域有望取得更多的应用。此外,更加温和、高效、高选择性和原子经济性的催化脱水策略和相关机理也有待进一步探索。
文章引用
王明慧. 酰胺催化脱水制备腈的研究进展
Research Progress of Catalytic Dehydration of Amides to Nitriles[J]. 有机化学研究, 2021, 09(04): 59-67. https://doi.org/10.12677/JOCR.2021.94008
参考文献
- 1. Pollak, P., Romeder, G., Hagedorn, F. and Gelbke, H.-P. (2000) Nitriles. In Bohnet, M., Brinker, C.G. and Cornils, B., Eds., Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 251-265. https://doi.org/10.1002/14356007.a17_363
- 2. Wöuhrle, D. and Knothe, G. (1988) Polymers from Nitriles.VII. Polymerization of Fumaronitrile with Triethylamine Asinitiator. Journal of Polymer Science Part A: Polymer Chemistry, 26, 2435-2447. https://doi.org/10.1002/pola.1988.080260915
- 3. Fleming, F.F., Yao, L, Ravikumar, P.C., Funk, L. and Shook, B.C. (2010) Nitrile-Containing Pharmaceuticals: Efficacious Roles of the Nitrile Pharmacophore. Journal of Medicinal Chemistry, 53, 7902-7917. https://doi.org/10.1021/jm100762r
- 4. Ahren, B., Landin-Olsson, M., Jansson, P.A., Svensson, M., Holmes, D. and Schweizer, A. (2004) Inhibition of Dipeptidyl Peptidase-4 Reduces Glycemia, Sustains Insulin Levels, and Reduces Glucagon Levels in Type 2 Diabetes. The Journal of Clinical Endocrinology & Metabolism, 89, 2078-2084. https://doi.org/10.1210/jc.2003-031907
- 5. Bhatnagar, A.S. (2007) The Discovery and Mechanism of Action of Letrozole. Breast Cancer Research and Treatment, 105, 7-17. https://doi.org/10.1007/s10549-007-9696-3
- 6. Jakesz, R., Jonat, W., Gnant, M., Mittlboeck, M., Greil, R., Tausch, C., Hilfrich, J., Kwasny, W., Menzel, C., Samonigg, H., Seifert, M., Gademann, G., Kaufmann, M. and Wolfgang, J. (2005) Switching of Postmenopausal Women with Endocrine-Responsive Early Breast Cancer to Anastrozole after 2 Years’ Adjuvant Tamoxifen: Combined Results of ABCSG Trial 8 and ARNO 95 Trial. Lancet, 366, 455-462. https://doi.org/10.1016/S0140-6736(05)67059-6
- 7. Larock, R.C. (2018) Comprehensive Organic Transformations: A Guide to Functional Group Preparations, 3rd Edition, John Wiley & Sons, Inc., Hoboken. https://doi.org/10.1002/9781118662083
- 8. Zhong, Y.-H., Lee, J., Reamer, R.A. and Askin, D. (2004) New Method for the Synthesis of Diversely Functionalized Imidazolesfrom N-Acylated α-Aminonitriles. Organic Letters, 6, 929-931. https://doi.org/10.1021/ol036423y
- 9. Storz, T., Heid, R., Zeldis, J., Hoagland, S.M., Rapisardi, V., Hollywood, S. and Morton, G. (2011) Convenient and Practical One-Pot Synthesis of 4-Chloropyrimidinesvia a Novel Chloroimidate Annulation. Organic Process Research & Development, 15, 918-924. https://doi.org/10.1021/op1002352
- 10. Yeung, K.S., Farkus, M.E., Kadow, J.F. and Meanwell, N.A. (2005) ABase-Catalyzed, Direct Synthesis of 3, 5-Disubstituted 1,2,4-Triazoles from Nitriles and Hydrazides. Tetrahedron Letters, 46, 3429-3432. https://doi.org/10.1016/j.tetlet.2005.02.167
- 11. Horneff, T., Chuprakov, S., Chernyak, N., Gevorgyan, V. and Fokin, V.V. (2008) Rhodium-Catalyzed Transannulation of 1,2,3-Triazoles with Nitriles. Journal of the American Chemical Society, 130, 14972-14974. https://doi.org/10.1021/ja805079v
- 12. Ganesan, M. and Nagaraaj, P. (2020) Recent Developments in Dehydration of Primary Amides to Nitriles. Organic Chemistry Frontiers, 7, 3792-3814. https://doi.org/10.1039/D0QO00843E
- 13. Blum, J. and Fisher, A. (1970) A Novel Synthesis of Nitriles from Secondary Amides. Tetrahedron Letters, 11, 1963-1966. https://doi.org/10.1016/S0040-4039(01)98128-6
- 14. Blum, J., Fisher, A. and Greener, E. (1973) The Catalytic Decomposition of Secondary Carboxamides by Transition-Metal Complexes. Tetrahedron, 29, 1073-1081. https://doi.org/10.1016/0040-4020(73)80064-X
- 15. Campbell, J.A., McDouglad, G., McNab, H., Rees, L.V.C. and Tyas, R.G. (2007) Laboratory Scale Synthesis of Nitriles by Catalysed Dehydration of Amides and Oximes under Flash Vacuum Pyrolysis (FVP) Conditions. Synthesis, 20, 3179-3184. https://doi.org/10.1055/s-2007-990782
- 16. Itagaki, S., Kamata, K., Yamaguchi, K. and Mizuno, N. (2013) Amonovacant Lacunary Silicotungstate as an Efficient Heterogeneous Catalyst for Dehydration of Primary Amides Tonitriles. ChemCatChem, 5, 1725-1728. https://doi.org/10.1002/cctc.201300063
- 17. Watanabe, Y., Okuda, F. and Tsuji, Y.J. (1990) Ruthenium Complex Catalyzed Dehydration of Carboxamides to Nitriles in the Presence of Urea Derivatives. Journal of Molecular Catalysis, 58, 87-94. https://doi.org/10.1016/0304-5102(90)85181-G
- 18. Furuya, Y., Ishihara, K. and Yamamoto, H. (2007) Perrhenic Acid-Catalyzed Dehydration from Primary Amides, Aldoximes, N-Monoacylureas, and α-Substituted Ketoximes to Nitrile Compounds. Bulletin of the Chemical Society of Japan, 80, 400-406. https://doi.org/10.1246/bcsj.80.400
- 19. Sueoka, S., Mitsudome, T., Mizugaki, T., Jitsukawa, K. and Kaneda, K. (2010) Supported Monomeric Vanadium Catalyst for Dehydration of Amides to Form Nitriles, Chemical Communications, 46, 8243-8245. https://doi.org/10.1039/c0cc02412k
- 20. Ruck, R.T. and Bergman, R.G. (2004) Zirconium-Mediated Conversion of Amides to Nitriles: A Surprising Additive Effect. Angewandte Chemie International Edition, 43, 5375-5377. https://doi.org/10.1002/anie.200461064
- 21. Maffioli, S.I., Marzorati, E. and Marazzi, A. (2005) Mild and Reversible Dehydration of Primary Amides with PdCl2 in Aqueous Acetonitrile. Organic Letters, 7, 5237-5239. https://doi.org/10.1021/ol052100l
- 22. Manjula, K. and Pasha, M.A. (2007) Rapid Method of Converting Primary Amides to Nitriles and Nitriles to Primary Amides by ZnCl2 Using Microwaves under Different Reaction Conditions. Synthetic Communications, 37, 1545-1550. https://doi.org/10.1080/00397910701230147
- 23. Zhang, W.D., Haskins, C.W., Yang, Y. and Dai, M.J. (2014) Synthesis of Nitriles via Palladium-Catalyzed Water Shuffling from Amides to Acetonitriles. Organic & Biomolecular Chemistry, 12, 9109-9112. https://doi.org/10.1039/C4OB01825G
- 24. Dubey, P., Gupta, S. and Singh, A.K. (2017) Trinuclear Complexes of Palladium(II) with Chalcogenated N-Hetero- cyclic Carbenes: Catalysis of Selective Nitrile-Primary Amide Interconversion and Sonogashira Coupling. Dalton Transactions, 46, 13065-13076. https://doi.org/10.1039/C7DT02592K
- 25. Al-Huniti, M.H., Rivera-Chávez, J., Colón, K.L., Stanley, J.L., Burdette, J.E., Pearce, C.J., Oberlies, N.H. and Croatt, M.P. (2018) Development and Utilization of a Palladium-Catalyzed Dehydration of Primary Amides to Form Nitriles. Organic Letters, 20, 6046-6050. https://doi.org/10.1021/acs.orglett.8b02422
- 26. Okabe, H., Naraoka, A., Isogawa, T., Oishi, S. and Naka, H. (2019) Acceptor-Controlled Transfer Dehydration of Amides to Nitriles. Organic Letters, 21, 4767-4770. https://doi.org/10.1021/acs.orglett.9b01657
- 27. Hanada, S., Motoyama, Y. and Nagashima, H. (2008) Hydrosilanes Are Not Always Reducing Agents for Carbonyl Compounds but Can Also Induce Dehydration: A Ruthenium-Catalyzed Conversion of Primary Amides to Nitriles. European Journal of Organic Chemistry, 2008, 4097-4100. https://doi.org/10.1002/ejoc.200800523
- 28. Zhou, S.L., Addis, D., Das, S., Junge, K. and Beller, M. (2009) New Catalytic Properties of Iron Complexes: Dehydration of Amides to Nitriles. Chemical Communications, 45, 4883-4885. https://doi.org/10.1039/b910145d
- 29. Bezier, D., Venkanna, G.T., Sortais, J.B. and Darcel, C. (2011) Well-Defined Cyclopentadienyl NHC Iron Complex as the Catalyst for Efficient Hydrosilylation of Amides to Amines and Nitriles. ChemCatChem, 3, 1747-1750. https://doi.org/10.1002/cctc.201100202
- 30. Enthaler, S. and Weidauer, M. (2011) Copper-Catalyzed Dehydration of Primary Amides to Nitriles. Catalysis Letters, 141, Article No. 1079. https://doi.org/10.1007/s10562-011-0660-9
- 31. Enthaler, S. (2011) Straightforward Uranium-Catalyzed Dehydration of Primary Amides to Nitriles. Chemistry—A European Journal, 17, 9316-9319. https://doi.org/10.1002/chem.201101478
- 32. Enthaler, S. (2011) Straightforward Iron-Catalyzed Synthesis of Nitrilesby Dehydration of Primary Amides. European Journal of Organic Chemistry, 2011, 4760-4763. https://doi.org/10.1002/ejoc.201100754
- 33. Enthaler, S. and Inoue, S. (2012) An Efficient Zinc-Catalyzed Dehydration of Primary Amides to Nitriles. Chemistry—An Asian Journal, 7, 169-175. https://doi.org/10.1002/asia.201100493
- 34. Mineno, T., Shinada, M., Watanabe, K., Yoshimitsu, H., Miyashita, H. and Kansui, H. (2014) Highly-Efficient Conversion of Primary Amides to Nitriles Using Indium(III) Triflate as the Catalyst. International Journal of Organic Chemistry, 4, 1-6. https://doi.org/10.4236/ijoc.2014.41001
- 35. Elangovan, S., Quintero-Duque, S., Dorcet, V., Roisnel, T., Norel, L., Darcel, C. and Sortais, J.B. (2015) Knölker-Type Iron Complexes Bearing an N-Heterocyclic Carbene Ligand: Synthesis, Characterization, and Catalytic Dehydration of Primary Amides. Organometallics, 34, 4521-4528. https://doi.org/10.1021/acs.organomet.5b00553
- 36. Xue, B.J., Sun, H.J., Wang, Y., Zheng, Y.Y., Li, X.Y., Fuhr, O. and Fenske, D. (2016) Efficient Reductive Dehydration of Primary Amides to Nitriles Catalyzed by Hydrido Thiophenolato Iron (II) Complexes under Hydrosilation Conditions. Catalysis Communications, 86, 148-150. https://doi.org/10.1016/j.catcom.2016.08.024
- 37. Ren, S., Xie, S., Zheng, T., Wang, Y., Xu, S., Xue, B., Li, X., Sun, H., Fuhr, O. and Fenske, D. (2018) Synthesis of Silyl Iron Hydride via Si–H Activation and Its Dual Catalytic Application in the Hydrosilylation of Carbonyl Compounds and Dehydration of Benzamides. Dalton Transactions, 47, 4352-4359. https://doi.org/10.1039/C8DT00289D
- 38. Liu, R.Y., Bae, M. and Buchwald, S.L. (2018) Mechanistic Insight Facilitates Discovery of a Mild and Efficient Copper-Catalyzed Dehydration of Primary Amides to Nitriles Using Hydrosilanes. Journal of the American Chemical Society, 140, 1627-1631. https://doi.org/10.1021/jacs.8b00643
- 39. Wang, Y.Y., Fu, L.Y., Qi, H.M., Chen, S.W. and Li, Y.H. (2018) Bioinspired Synthesis of Nitriles from Primary Amides via Zinc/Anhydride Catalysis. Asian Journal of Organic Chemistry, 7, 367-370. https://doi.org/10.1002/ajoc.201700664
- 40. Zheng, T., Wang, Y., Yang, Z., Sun, H. and Li, X. (2019) Catalytic Effect of Iron Hydrides on Dehydration of Primary Amides to Nitriles. Chinese Journal of Organic Chemistry, 39, 2941-2945. https://doi.org/10.6023/cjoc201903075
- 41. Ren, S., Wang, Y., Yang, D., Sun, H. and Li, X. (2019) Dehydration of Primary Amides to Nitriles Catalyzed by [CNC]-Pincer Hydrido Cobalt(III) Complexes. Catalysis Communications, 120, 72-75. https://doi.org/10.1016/j.catcom.2018.12.002
- 42. Li, Y., Zhao, Y., Wang, S. and Ma, X. (2019) Silica Supported Potassium Oxide Catalyst for Dehydration of 2-Picolinamide to form 2-Cyanopyridine. Chinese Chemical Letters, 30, 494-498. https://doi.org/10.1016/j.cclet.2018.04.012
- 43. Yao, W., Fang, H., He, Q., Peng, D., Liu, G. and Huang, Z. (2019) A BEt3-Base Catalyst for Amide Reduction with Silane. The Journal of Organic Chemistry, 84, 6084-6093. https://doi.org/10.1021/acs.joc.9b00277
- 44. Das, H.S., Das, S., Dey, K., Singh, B., Haridasan, R.K., Das, A., Ahmed, J. and Mandal, S.K. (2019) Primary Amides to Amines or Nitriles: A Dual Role by a Single Catalyst. Chemical Communications, 55, 11868-11871. https://doi.org/10.1039/C9CC05856G
- 45. Li, K., Sun, H., Yang, W., Wang, Y., Xie, S., Li, X., Fuhr, O. and Fenske, D. (2020) Efficient Dehydration of Primary Amides to NitrilesCatalyzed by Phosphorus-Chalcogen Chelated Iron Hydrides. Applied Organometallic Chemistry, 34, Article No. e5337. https://doi.org/10.1002/aoc.5337
- 46. Chang, G., Li, X., Zhang, P., Yang, W., Li, K., Wang, Y., Sun, H., Fuhr, O. and Fenske, D. (2020) Lewis Acid Promoted Dehydration of Amides to Nitriles Catalyzed by [PSiP]-Pincer Iron Hydrides. Applied Organometallic Chemistry, 34, Article No. e5466. https://doi.org/10.1002/aoc.5466
- 47. Wang, Y., Zhang, H., Xie, S., Sun, H., Li, X., Fuhr, O. and Fenske, D. (2020) An Air-Stable N-Heterocyclic [PSiP] Pincer Iron Hydride and an Analogous Nitrogen Iron Hydride: Synthesis and Catalytic Dehydration of Primary Amides to Nitriles. Organometallics, 39, 824-833. https://doi.org/10.1021/acs.organomet.9b00880
- 48. Zhou, S.L., Junge, K., and Addis, D., Das, S. and Beller, M. (2009) A General and Convenient Catalytic Synthesis of Nitriles from Amides and Silanes. Organic Letters, 11, 2461-2464. https://doi.org/10.1021/ol900716q
- 49. Hota, P.K., Maji, S., Ahmed, J., Rajendran, N.M. and Mandal, S.K. (2020) NHC-Catalyzed Silylative Dehydration of Primary Amides to Nitriles at Room Temperature. Chemical Communications, 56, 575-578. https://doi.org/10.1039/C9CC08413D
- 50. Shipilovskikh, S.A., Vaganov, V.Y., Denisova, E.I., Rubtsov, A.E. and Malkov, A.V. (2018) Dehydration ofAmides to Nitriles under Conditions of a Catalytic Appel Reaction. Organic Letters, 20, 728-731. https://doi.org/10.1021/acs.orglett.7b03862
- 51. Ding, R., Liu, Y.G., Han, M.R., Jiao, W.Y., Li, J.Q., Tian, H.Y. and Sun, B.G. (2018) Synthesis of Nitriles from Primary Amides or Aldoximes under Conditions of a Catalytic Swern Oxidation. The Journal of Organic Chemistry, 83, 12939-12944. https://doi.org/10.1021/acs.joc.8b02190
- 52. Rai, A. and Yadav, L.D.S. (2013) Cyclopropenone-Catalyzed Direct Conversion of Aldoximes and Primary Amides into Nitriles. European Journal of Organic Chemistry, 2013, 1889-1893. https://doi.org/10.1002/ejoc.201300059