Hans Journal of Food and Nutrition Science
Vol.
08
No.
03
(
2019
), Article ID:
31796
,
13
pages
10.12677/HJFNS.2019.83025
Analysis on Basic Physicochemical Properties and Antioxidant Activities of the Starch from Acorn
Xiaoxin Chen, Xiujie Li, Xiaoqing Shi, Linqiang Li*
College of Food Engineering and Nutritional Science, Shaanxi Normal University, Xi’an Shaanxi

Received: Jul. 25th, 2019; accepted: Aug. 14th, 2019; published: Aug. 21st, 2019

ABSTRACT
This paper explored physicochemical properties of acorn starch, and its antioxidant activity in vitro. The swelling capacity, the hydrolysis capacity, content of amylose and amylopectin, characteristic functional groups, thermodynamic properties, crystal structure, micromorphology and tannins compositions of acorn starch were analyzed. Its reducing power and its scavenging activity on superoxide anion ( ), hydroxy radicals (∙OH), and 1,1-diphenyl-2-picrylhydrazyl radical (DPPH∙) were evaluated. The results showed that the swelling capacity of acorn starch was (68.00 ± 13.35) g/g, and the glucose conversion rate was (0.23 ± 0.025) g/g. The mass percent of amylose and amylopectin were 19% and 81%, respectively. Characteristic functional groups of the infrared spectra of acorn starch were similar to those of potato and glutinous rice starch. Onset temperature of phase transition was at 101.19˚C, and the peak temperature was at 103.32˚C. Acorn starch crystal structure exhibited A-type. Microscopic morphology showed spherical. The content of tannins in starch was (1.60 ± 0.11) g/100g, and the main components were ellagic acid (1.54 g/100g) and a small amount of quercetin (0.064 g/100g). The acorn starch reducing power on ferricyanide, and its scavenging effects on , ∙OH, and DPPH∙ were significant. Acorn starch is one kind of the general starch, and its tannins is mainly made up of ellagic acid and its antioxidant activitives are good.
Keywords:Acorn Starch, Physicochemical Properties, Antioxidant Activity, Tannins Compositions

橡子淀粉基本理化特性及其抗氧化作用分析
陈晓欣,李秀婕,石晓晴,李林强*
陕西师范大学食品工程与营养科学学院,陕西 西安

收稿日期:2019年7月25日;录用日期:2019年8月14日;发布日期:2019年8月21日

摘 要
本文探讨橡子淀粉基本理化特性及其体外抗氧化效果。分析橡子淀粉润胀能力、水解能力、直链淀粉和支链淀粉含量、特征官能团、热力学特性、晶型结构、微观形貌、单宁组成、还原能力及清除超氧阴离子( )、羟自由基(∙OH)、1,1-二苯基-2-三硝基自由基(DPPH∙)的能力。结果表明:橡子淀粉润胀能力为(68.00 ± 13.35) g/g,葡萄糖转化率为(0.23 ± 0.025) g/g,直链淀粉和支链淀粉质量分数分别为19%和81%;橡子淀粉红外光谱峰型特征与马铃薯淀粉、糯米淀粉相似,相变起始温度在101.19℃,高峰温度在103.32℃,晶型结构为A型,微观形貌呈球形,其淀粉中单宁含量为(1.60 ± 0.11) g/100g,主要为鞣花酸(1.54 g/100g)和少量槲皮素(0.064 g/100g),对铁氰化钾的还原能力、清除 、∙OH和DPPH∙的效果良好。橡子淀粉具有一般淀粉的基本特性,其单宁主要组分为鞣花酸,具有较好的抗氧化作用。
关键词 :橡子淀粉,理化特性,抗氧化,单宁组成

Copyright © 2019 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
碳水化合物是人们获取能量的主要来源,淀粉是由葡萄糖分子聚合成的复合碳水化合物。不少研究已证实摄入过量碳水化合物(主要为果糖,葡萄糖,蔗糖等单糖或双糖)可能会导致肥胖症 [1] [2] [3]、心血管疾病 [1] [4]、糖尿病 [1] [5] [6] [7] 等。相对于单糖和双糖,淀粉餐后血糖反应较低,或能控制病情 [5],但是过量摄入淀粉也可能导致这些疾病的发生 [2]。因此,开发各种功能性淀粉备受关注,特别是天然功能性淀粉,该淀粉中因具有特定功能因子,能预防某类疾病。
橡树是一种重要的野生植物资源,它的果实橡子富含淀粉(约58%) [8]、单宁、单不饱和脂肪酸、α-和γ-生育酚等物质,在过去常用作动物饲料 [9]。橡子粉具有食疗作用,在一些国家和地区都有食用橡子的传统。目前,我国有些山区除直接用橡子做成橡子面外,还做成橡子豆腐、橡子粉丝、橡子酱、橡子羹等食品。而在国外古希腊人和日本Jōmon时期人曾以橡子为食,橡子也曾是北美印第安人获取淀粉、脂肪和蛋白质的重要来源,尤其是在受地形限制粮食作物生产困难的东西部地区;韩国人用橡子制作一种名为“dotorimuk”的果冻,更用橡子淀粉制成叫作“dotori”的面条 [10]。
但由于橡子粉味苦涩,甚至可能有一定的毒性 [11] [12],影响了其食用价值。因此将橡子粉水洗成橡子淀粉会使其毒性降低至安全剂量内,令人可食用。近年来已有对橡子淀粉特性的研究,主要集中在橡子中淀粉的含量 [8] [13]、淀粉晶体结构的类型 [10]、热力学特性等 [8] [10] [14],相关研究结果尚存在一定争议。而对其中的活性物质如单宁类化合物的性质和作用研究较少。本文通过研究橡子淀粉的基本理化性质及其中单宁抗氧化效果,以丰富橡子淀粉研究理论并为其开发利用提供理论基础。
2. 材料与方法
2.1. 材料与试剂
橡子,采自秦岭山脉西麓,自然风干,超微粉碎,置棕色瓶阴凉处存放备用。
1,1-二苯基-2-苦基肼自由基(DPPH, C18H12N5O6),美国Sigma公司。单宁类物质标准品:没食子酸、原儿茶素、表没食子儿茶素、儿茶素、原花青素B2、绿原酸、4-羟基苯甲酸、表儿茶素、咖啡酸、表儿茶素没食子酸酯、芦丁、金丝桃苷、鞣花酸、槲皮苷、根皮苷、槲皮素,纯度 ≥ 98%,购自中国国家标准物质中心,其它试剂均为国产分析纯。
2.2. 仪器与设备
722型可见分光光度计,上海光谱仪器有限公司;傅里叶变换红外光谱仪(Fourier Transform Infrared Spectrometer, FTIR),德国布鲁克公司;热分析系统,美国TA公司;粉末X射线衍射仪,德国布鲁克公司;Motic BA 200双目生物显微镜,北京翔天智远科技有限公司;高效液相色谱仪,美国Waters公司。
2.3. 方法
2.3.1. 橡子淀粉的制备及其基本理化特性分析
橡子淀粉的制备:参考文献 [15] 的方法。
1) 橡子淀粉溶胀能力、转化葡萄糖能力及其直/支链淀粉含量的测定
分别参考文献 [16]、文献 [16] [17]、文献 [18] 的方法。
2) 橡子淀粉特征官能团傅里叶变换红外光谱分析
称取约0.1 g橡子淀粉,加入10~20 g干燥KBr,将两者混匀,用研钵研磨,然后用药勺小心将磨好混合粉末移入模具中,将压好片的溴化钾薄片取出模具进行傅里叶变换红外光谱分析,以马铃薯淀粉和糯米淀粉为对照。
3) 橡子淀粉差示扫描量热仪(DSC)分析
配制质量浓度为5、10、15 g/100g的橡子淀粉乳液,DSC分析条件为:配制橡子淀粉乳液室温条件下置约1 h,温度范围30℃~120℃,加热速率为10℃/min,氮气流量为40 mL/min。
4) 橡子淀粉颗粒晶体结构X-射线衍射仪(XRD)测定
分析条件:Cu靶,陶瓷X光管(2.2 kW),θ/θ测角仪(采用光学编码器技术与步进马达双重定位),LynxEye阵列探测器。
2.3.2. 橡子淀粉形貌显微观察及其单宁含量测定
1) 橡子淀粉形貌显微观察
配制质量百分数为1%的橡子淀粉溶液,向载玻片中央滴入少许样液,滴2滴碘液,进行光学显微镜观察。
2) 橡子淀粉单宁含量测定
采用酒石酸亚铁分光光度比色法。无水乙醇配制浓度分别为0.0、0.02、0.04、0.06、0.08、0.10、0.12、0.14 mg/mL的单宁酸标准溶液,即分别取1 mg/mL的单宁酸标准溶液0、0.2、0.4、0.6、0.8、1.0、1.2、1.4 mL,依次加入到装有5、4.8、4.6、4.4、4.2、4.0、3.8、3.6 mL pH为7.5磷酸氢二钠-氢氧化钠缓冲液的10 mL容量瓶中,再加入酒石酸亚铁溶液5 mL,在680 nm处测定吸收值,作线性回归分析,求得回归方程y = 4.803x − 0.0061,R2 = 0.9987。
样品制备:以1:10 (g/mL)的料液比,用无水乙醇浸提橡子淀粉12 h,400× g离心15 min,倾出上层清液即为橡子淀粉单宁样品液。依照上文标准曲线制作方法测定样品吸光值,代入下式即得橡子淀粉单宁质量百分数。
(1)
式中:Y,橡子淀粉单宁质量百分数,%;C,样液质量浓度,mg/mL。
2.3.3. 橡子淀粉抗氧化活性的测定
1) 橡子淀粉对铁氰化钾还原能力的测定
采用普鲁士蓝法,参考Amarowicz等 [19] 和Kaur等 [20] 的方法。以1:10 (g/Ml)的料液比,用70% (v/v)分别制备质量浓度为1.00、0.75、0.50、0.25、0.10 mg/mL的样液,各取1 mL,分别加入2.5 mL磷酸缓冲液(pH 6.6, 0.2 M)、1 mL 1% (质量百分数) K3Fe(CN)6溶液,于50℃水浴处理20 min,冰浴冷却,加入2.5 mL 10% (质量百分数)三氯乙酸溶液,在400× g下离心10 min,取上清液2.5 mL,加入2.5 mL蒸馏水和0.5 mL 质量分数为0.1%氯化铁溶液,摇匀,在可见分光光度计710 nm处测定吸光值A。样品还原能力越强,反应后溶液的吸光度越大。按下式计算其还原能力。
(2)
式中:Y,还原能力,%;A1,样液吸光值;A0,对照吸光值;A2,空白组吸光值。
2) 橡子淀粉对 清除能力的测定
采用PMS-NADH体系测定橡子淀粉对超氧阴离子的消除能力,参考Guorong等 [21] 的方法,作部分修改。50 μL不同质量浓度的样液加入到1 mL 0.1 mol/L磷酸盐缓冲液(Ph = 7.4)中,并依次加入150 μmol/L的NBT、60 μmol/L的PMS和468 μmol/L的NADH。置于25℃条件下8 min,测量其在波长560 nm处的吸光度。
(3)
式中:Y,样液对超氧阴离子清除能力,%;A1,样液吸光值;A0,对照吸光值。
3) 橡子淀粉对∙OH清除能力的测定
采用水杨酸法 [22],测定橡子淀粉对∙OH清除能力。在10 mL试管中加入2 mL不同浓度的样品,2 mL 6 mmol/L FeSO4溶液,2 mL 6 mmol/L水杨酸-乙醇溶液,2 mL 6 mmol/L H2O2溶液,充分混匀后于37℃水浴30 min,于562 nm处测定吸光值A,以加蒸馏水为空白对照,按下式计算对∙OH的清除能力。
(4)
式中:Y,羟自由基清除率,%;A1,样品吸光值;A0,蒸馏水代替样品的对照吸光值;A2,以蒸馏水代替样品和H2O2的空白吸光值。
4) 橡子淀粉对DPPH∙清除能力的测定
参考Dudonné等 [23] 和Negi等 [24] 的方法。取1 mg DPPH溶于约20 mL无水乙醇中,取DPPH溶液2 mL,向其中逐渐加样品液,当溶液颜色基本褪去时,此加样量即为样品的最大用量,以等差递减设置5个加样量;取DPPH溶液2 mL加入到小试管(或玻璃瓶)中,加无水乙醇1 mL,充分混合,测定其A0值(519 nm);取DPPH溶液2 mL加入到小试管中,加样品液x μL,再加(1000 − x) μL无水乙醇至总体积为3 mL,静置30 min后测定其A值(519 nm),依次测定样品不同添加量A值,按下式计算其对DPPH∙的清除能力。
(5)
式中:Y,DPPH∙清除率,%;A0,对照吸光值;A,样品吸光值。
2.3.4. 橡子淀粉单宁组成HPLC分析
分析条件:Waters 2478型紫外检测器和示差折光检测器,波长280 nm,色谱柱Agilent5 TC-C18 (250 mm × 4.6 mm × 5 μm),柱温30℃,进样体积20 μL,流动相B 为0.1% (v/v)甲酸水溶液,流动相D为甲醇。
梯度洗脱:流速为0.5 mL/min,0~7 min (95% B液,5% D液);7~9 min (70% B液,30% D液);9~14 min (50% B液,50% D液);14~22 min (10% B液,90% D液);22~25 min (95% B液,5% D液)。所有样品进样前用0.45 μm的滤膜过滤。HPLC分析所得组分以其标准品进行比对确认。
标准曲线制作:16种多酚标准品分别配成质量浓度为1.2 mg/mL的甲醇溶液,按1:1、1:2、1:4稀释后分别进样20 μL,以峰面积对浓度分别制作各种标准品的标准曲线,求回归方程。橡子多酚纯化物样品用甲醇溶解后,进样检测,用回归方程计算各种成分的含量。
2.4. 数据处理
所有试验均平行重复5次,试验数据采用SPSS16.0软件进行方差分析(One-Way ANOVA),Turkey’s检验5%水平进行多重比较,结果以平均值 ± 标准差 表示。
3. 结果与分析
3.1. 橡子淀粉溶胀能力和转化葡萄糖能力的测定结果
图1结果表明,橡子淀粉的溶胀能力为(68.00 ± 13.35) g/100g,接近马铃薯淀粉的溶胀能力(64.04 ± 7.40) g/100g,显著低于糯米淀粉的溶胀能力(121.68 ± 5.81) g/100g (P < 0.05),这与Correia等 [25] 和Irinislimane等 [26] 对橡子淀粉溶胀力的研究结果基本一致。图2结果表明,橡子淀粉葡萄糖转化率为(0.23 ± 0.025) g/g,显著高于马铃薯淀粉(0.011 ± 0.00084) g/g和糯米淀粉(0.049 ± 0.0060) g/g (P < 0.05),结果提示橡子淀粉可能更易于α-淀粉酶水解。直链淀粉和支链淀粉含量的测定结果表明,橡子淀粉中直链淀粉和支链淀粉质量分数分别为19%和81%,与文献 [16] 报道马铃薯淀粉直链淀粉和支链淀粉含量的结果相近。
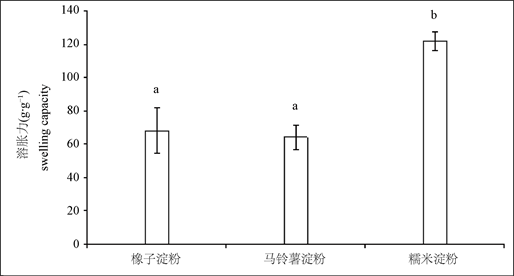 注:图中不同小写字母表示差异显著(P < 0.05),下图同。
注:图中不同小写字母表示差异显著(P < 0.05),下图同。
Figure 1. Comparison on swelling capacity of starch from acorn, potato and glutinous rice
图1. 橡子淀粉与马铃薯淀粉、糯米淀粉溶胀能力比较

Figure 2. Comparison on glucose conversion rate of starch from acorn, potato and glutinous rice
图2. 橡子淀粉与马铃薯淀粉、糯米淀粉葡萄糖转化率比较
3.2. 橡子淀粉特征官能团傅里叶变换红外光谱分析结果
橡子淀粉傅里叶变换红外光谱分析结果表明,其具有3420~3320 cm−1 -OH伸缩振动吸收带,2958~2912 cm−1 C-H伸缩振动吸收带,2100~2000 cm−1 C-H面外变形振动吸收带,1656~1593 cm−1 -OH弯曲振动吸收带,1421~1311 cm−1 C-H弯曲振动吸收带为吸收,1149 cm−1环上C-O伸缩振动吸收带,1049~1004 cm−1 -OH变角振动吸收带,904~846 cm−1糖苷键振动吸收带,570~500 cm−1 CCC和CCO变形振动吸收带,其与马铃薯淀粉和糯米淀粉傅里叶变换红外光谱峰型特征基本相似(图3)。
 注:A是糯米淀粉,B是马铃薯淀粉,C是橡子淀粉。
注:A是糯米淀粉,B是马铃薯淀粉,C是橡子淀粉。
Figure 3. FTIR analysis of acorn starch main groups
图3. 橡子淀粉主要基团傅里叶变换红外光谱分析
3.3. 橡子淀粉热力学和晶体结构分析
橡子淀粉热处理相变起始温度约在101.19℃左右,相变的高峰温度约在103.32℃左右(图4),结果提示橡子淀粉在未达到相变温度前,其应无明显的黏度效应。淀粉颗粒X射线衍射图主要呈现3种不同的晶体结构类型,即A、B和C型。经过和这3种标准晶型X射线衍射图特征峰逐一比对,橡子淀粉晶型结构为A型(图5),这与Stevenson等 [10] 和Yoo等 [27] 的研究结果一致。

Figure 4. Heating DSC curve of acorn starch
图4. 橡子淀粉热力学分析DSC曲线

Figure 5. X-ray diffraction pattern of cystal structure of starch granules
图5. 橡子淀粉颗粒晶体结构XRD分析
3.4. 橡子淀粉单宁含量测定结果和其颗粒形貌显微观察
橡子淀粉单宁质量百分数为(1.30 ± 0.20)%,显著低于橡子粉(7.30 ± 0.18)% (P < 0.05),结果表明水洗淀粉可能是脱除其中橡子单宁、降低其毒性的一种有效方法(图6)。橡子淀粉颗粒形貌光学显微镜观察呈球形。综上研究结果表明橡子淀粉具有普通淀粉的一般特性。

Figure 6. Comparison on tannins mass percentage of acron powder and acorn starch
图6. 橡子粉和橡子淀粉单宁质量百分数的比较
3.5. 橡子淀粉抗氧化活性
还原力是衡量抗氧化物质提供电子能力的重要指标,抗氧化剂通过自身的还原作用给出电子而使自由基变为稳定的分子,从而失去活性。还原力越强,则其抗氧化能力越强。由图7(a)可见,橡子淀粉的还原能力呈现良好的剂量效应线性相关(R2 = 0.9966),其对 的清除效果随着总酚质量浓度的提高而增强,在总酚浓度0~1.00 mg/mL 的范围内呈现良好的线性关系(R2 = 0.9543),IC50为0.50 mg/mL,橡子淀粉的浓度为1.00 mg/mL时,清除率达79.07% (图7(b));同样其对∙OH的清除效果也随着总酚质量浓度的提高而增强,在总酚浓度为0~1.00 mg/mL内其剂量效应关系表现良好的线相关性(R2 = 0.9261),IC50为0.37 mg/mL,在测试的浓度最大浓度1.00 mg/mL时,清除率达97.05% (图7(c)),这与Sánchez-Burgos等 [28] 的研究结果一致;橡子淀粉单宁在总酚浓度为0~1.00 mg/mL的范围内对DPPH∙的清除效果也呈现一定程度的剂量效应关系(R2 = 0.7228) (图7(d)),Uddin等 [29] 也有相似的报道。
 (a)
(a)
 (b)
(b)
 (c)
(c)
 (d)注:(a) 为橡子淀粉单宁的还原能力,(b) 为橡子淀粉单宁对
的清除效果,(c) 为橡子淀粉单宁对∙OH的清除效果,(d) 为橡子淀粉单宁对DPPH∙的清除效果。
(d)注:(a) 为橡子淀粉单宁的还原能力,(b) 为橡子淀粉单宁对
的清除效果,(c) 为橡子淀粉单宁对∙OH的清除效果,(d) 为橡子淀粉单宁对DPPH∙的清除效果。
Figure 7. Antioxidant activities of tannins from acorn starch
图7. 橡子淀粉单宁抗氧化活性
3.6. 橡子淀粉单宁类物质组成分析
橡子淀粉单宁样品HPLC分析图谱主要有1个大峰和1个小峰(图8),与单宁标准品保留时间比对结果表明橡子淀粉单宁主要为鞣花酸(1.54 g/100g),以及少量的槲皮素(0.064 g/100g),橡子淀粉单宁种类较橡子粉(图8插图)大为减少。结果提示橡子是获取鞣花酸的良好资源。

Figure 8. HPLC analysis on compositions of tannins from acorn
图8. 橡子淀粉单宁组成HPLC分析
4. 讨论
植物单宁类物质具有抗氧化 [30] [31]、抗癌 [32] [33]、抑菌 [34]、抗病毒 [35]、预防糖尿病 [36]、抑制心血管疾病 [37] 等多种生物活性。本文研究结果表明橡子淀粉具有良好的的抗氧化作用,鞣花酸应该是其抗氧化活性的关键因子。也有研究报道植物单宁具有抵抗人体衰老,提高人体的免疫力、抗肿瘤等生理作用 [38] [39] [40],Bravo等 [41] 研究发现单宁在预防和治疗流行疾病,如心血管疾病、癌症、胃溃疡、十二指肠溃疡、过敏、血管脆化、病毒和细菌感染等方面具有重要作用。Custódio等 [42] 的研究也发现橡子单宁可以缓解阿尔茨海默病和其他神经退行性疾病以及糖尿病相关的症状。Sánchez-Burgos等 [28] 研究结果表明四种橡子(Quercus resinosa, Quercus laeta, Quercus grisea, and Quercus obtusata)的水提取物均对DPPH∙和∙OH的消除率较高,其中Q.laeta和Q.grisea提取物的DPPH∙消除效果更好,而Q.grisea和Q. obtusata的水提取物可以更好防止∙OH的产生,这与本文橡子淀粉抗氧化作用研究结果一致。Nourafcan [43] 等发现橡子叶提取物对两种阳性细菌(金黄色葡萄球菌和枯草芽孢杆菌)和两种阴性细菌(肺炎克雷伯氏菌和大肠杆菌)具有良好的抗菌活性,Vinha等 [11] 也认为橡子单宁可以作为天然抗菌剂,这显然是鞣花酸生理功效之一。甚至,Korus等 [44] 还报道橡子淀粉能有效地控制乳糜泻患者的病情。
当然,橡子味苦涩,甚至具有毒性。因此,一般通过脱除橡子单宁类物质来增加其食用性。Ghahfarrokhi等 [45] 通过不同的处理包括煮沸、焙烧,水洗,酸碱和NaCl溶液处理等方法去除橡子中的单宁类化合物,本文研究结果表明水洗是脱除橡子淀粉橡子单宁类物质的一种简单有效方法。
5. 结论
淀粉是食品中提供能量的主要营养素,强化淀粉的保健作用是淀粉功能改善的一种趋势。本文研究了橡子淀粉基本理化特性,并分析了其中单宁类物质的组成及其抗氧化功能,结果表明橡子淀粉具有普通淀粉的基本特性,含有鞣花酸和槲皮素,具有良好的抗氧化作用,本研究为橡子淀粉的深度开发利用提供了理论基础。
基金项目
陕西省重点研发计划项目(2016NY-212, 2017NY-060, 2018ZDXM-NY-094);西安市科技计划项目(20193038YF026NS026)。
文章引用
陈晓欣,李秀婕,石晓晴,李林强. 橡子淀粉基本理化特性及其抗氧化作用分析
Analysis on Basic Physicochemical Properties and Antioxidant Activities of the Starch from Acorn[J]. 食品与营养科学, 2019, 08(03): 195-207. https://doi.org/10.12677/HJFNS.2019.83025
参考文献
- 1. Ludwig, D.S. (2002) The Glycemic Index: Physiological Mechanisms Relating to Obesity, Diabetes, and Cardiovascular Disease. The Journal of the American Medical Association, 287, 2414-2 423. https://doi.org/10.1001/jama.287.18.2414
- 2. Brand-Miller, J.C., Holt, S.H.A., Pawlak, D.B., et al. (2002) Glycemic Index and Obesity. The American Journal of Clinical Nutrition, 76, 281S-285S. https://doi.org/10.1093/ajcn/76.1.281S
- 3. Storlien, L.H., Kraegen, E.W., Jenkins, A.B., et al. (1988) Effects of Sucrose vs. Starch Diets on in Vivo Insulin Action, Thermogenesis, and Obesity in Rats. American Journal of Clinical Nutrition, 47, 420. https://doi.org/10.1093/ajcn/47.3.420
- 4. Siri-Tarino, P.W., Sun, Q., Hu, F.B., et al. (2010) Saturated Fat, Carbohydrate, and Cardiovascular Disease. The American Journal of Clinical Nutrition, 91, 502-509. https://doi.org/10.3945/ajcn.2008.26285
- 5. Hu, F.B., Van Dam, R.M. and Liu, S. (2001) Diet and Risk of Type II Diabetes: The Role of Types of Fat and Carbohydrate. Diabetologia, 44, 805-817. https://doi.org/10.1007/s001250100547
- 6. Feinman, R.D., Pogozelski, W.K., Astrup, A., et al. (2015) Dietary Carbohydrate Restriction as the First Approach in Diabetes Management: Critical Review and Evidence Base. Nutrition, 31, 1-13. https://doi.org/10.1016/j.nut.2014.06.011
- 7. Hodge, A.M., English, D.R., O’dea, K., et al. (2004) Glycemic Index and Dietary Fiber and the Risk of Type 2 Diabetes. Diabetes Care, 27, 2701-2706. https://doi.org/10.2337/diacare.27.11.2701
- 8. Molavi, H., Sma, R. and Farhoosh, R. (2018) Impact of Hydrothermal Modifications on the Physicochemical, Morphology, Crystallinity, Pasting and Thermal Properties of Acorn Starch. Food Chemistry, 245, 385-393. https://doi.org/10.1016/j.foodchem.2017.10.117
- 9. Tejerina, D., Garcíatorres, S., De Vaca, M.C., et al. (2010) Acorns (Quercus rotundifolia Lam.) and Grass as Natural Sources of Antioxidants and Fatty Acids in the “Montanera” Feeding of Iberian Pig: Intra- and Inter-Annual Variations. Food Chemistry, 124, 997-1004. https://doi.org/10.1016/j.foodchem.2010.07.058
- 10. Stevenson, D.G., Jane, J.L. and Inglett, G.E. (2010) Physicochemical Properties of Pin Oak (Quercus palustris Muenchh.) Acorn Starch. Starch/St?rke, 58, 553-560. https://doi.org/10.1002/star.200600533
- 11. Vinha, A.F., Barreira, J.C.M., Costa, A.S.G., et al. (2016) A New Age for Quercus spp. Fruits: Review on Nutritional and Phytochemical Composition and Related Biological Activities of Acorns. Comprehensive Reviews in Food Science and Food Safety, 15, 947-981. https://doi.org/10.1111/1541-4337.12220
- 12. Smith, S., Naylor, R.J., Knowles, E.J., et al. (2015) Suspected Acorn Toxicity in Nine Horses. Equine Veterinary Journal, 47, 568-572. https://doi.org/10.1111/evj.12306
- 13. 谢碧霞, 谢涛. 我国橡实资源的开发利用[J]. 中南林学院学报, 2002, 22(3): 37-41.
- 14. Aee, L.H., Hie, K.N. and Nishinari, K. (1998) DSC and Rheological Studies of the Effects of Sucrose on the Gelatinization and Retrogradation of Acorn Starch. Thermochimica Acta, 322, 39-46. https://doi.org/10.1016/S0040-6031(98)00469-9
- 15. 陈钧辉, 李俊. 生物化学实验[M]. 北京: 科学出版社, 2014: 21.
- 16. 余平, 石彦忠. 淀粉与淀粉制品工艺学[M]. 北京: 中国轻工业出版社, 2011: 27, 174.
- 17. 萧能庚, 余瑞园, 袁明秀. 生物化学实验原理和方法[M]. 第二版. 北京: 北京大学出版社, 2005: 197.
- 18. 曹龙奎, 李凤林. 淀粉制品生产工艺学[M]. 北京: 中国轻工业出版社, 2008: 246-247.
- 19. Amarowicz, R., Pegg, R.B., Rahimi-Moghaddam, P., et al. (2004) Free-Radical Scavenging Capacity and Antioxidant Activity of Selected Plant Species from the Canadian Prairies. Food Chemistry, 84, 551-562. https://doi.org/10.1016/S0308-8146(03)00278-4
- 20. Kaur, G., Jabbar, Z., Athar, M., et al. (2006) Punica granatum (Pomegranate) Flower Extract Possesses Potent Antioxidant Activity and Abrogates Fe-NTA Induced Hepatoxicity in Mice. Food & Chemical Toxicology, 44, 984-993. https://doi.org/10.1016/j.fct.2005.12.001
- 21. Du, G.R., Li, M.J., Ma, F.W., et al. (2009) Antioxidant Capacity and the Relationship with Polyphenol and Vitamin C in Actinidia Fruits. Food Chemistry, 113, 557-562. https://doi.org/10.1016/j.foodchem.2008.08.025
- 22. Liu, W., Xu, J., Jing, P., et al. (2010) Preparation of a Hydroxypropyl Ganoderma lucidum Polysaccharide and Its Physicochemical Properties. Food Chemistry, 122, 965-971. https://doi.org/10.1016/j.foodchem.2009.11.087
- 23. Dudonne, S., Vitrac, X., Coutiere, P., et al. (2009) Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. Journal of Agricultural & Food Chemistry, 57, 1768-1774. https://doi.org/10.1021/jf803011r
- 24. Negi, P.S., Jayaprakasha, G.K. and Jena, B.S. (2003) Antioxidant and Anti-Mutagenic Activities of Pomegranate Peel Extracts. Food Chemistry, 80, 393-397. https://doi.org/10.1016/S0308-8146(02)00279-0
- 25. Correia, P.R., Nunes, M.C. and Beirao-da-Costa, M.L. (2012) The Effect of Starch Isolation Method on Physical and Functional Properties of Portuguese Nut Starches. II. Q. rotundifolia Lam. and Q. suber Lam. Acorns Starches. Food Hydrocolloids, 27, 256-263. https://doi.org/10.1016/j.foodhyd.2012.06.014
- 26. Irinislimane, H. and Belhanechebensemra, N. (2017) Extraction and Characterization of Starch from Oak Acorn, Sorghum and Potato and Adsorption Application for Removal of Maxilon Red GRL from Wastewater. Chemical Engineering Communications, 204, 897-906. https://doi.org/10.1080/00986445.2017.1325739
- 27. Yoo, S.H., Lee, C.S., Kim, B.S., et al. (2012) The Properties and Molecular Structures of Gusiljatbam Starch Compared to Those of Acorn and Chestnut Starches. Starch/St?rke, 64, 339-347. https://doi.org/10.1002/star.201100104
- 28. Sanchez-Burgos, J.A., Ramirez-Mares, M.V., Larrosa, M.M., et al. (2013) Antioxidant, Antimicrobial, Antitopoisomerase and Gastroprotective Effect of Herbal Infusions from Four Quercus Species. Industrial Crops and Products, 42, 57-62. https://doi.org/10.1016/j.indcrop.2012.05.017
- 29. Uddin, G. and Rauf, A. (2012) Phytochemical Screening, Antimicrobial and Antioxidant Activities of Aerial Parts of Quercus robur L. Middle-East Journal of Medicinal Plants Research, 1, 1-4.
- 30. Ma, T., Tian, C., Luo, J., et al. (2013) Influence of Technical Processing Units on Polyphenols and Antioxidant Capacity of Carrot (Daucus carrot L.) Juice. Food Chemistry, 141, 1637-1644. https://doi.org/10.1016/j.foodchem.2013.04.121
- 31. Tian, L., Shi, X., Yu, L., et al. (2012) Chemical Composition and Hepatoprotective Effects of Polyphenol-Rich Extract from Houttuynia cordata Tea. Journal of Agricultural and Food Chemistry, 60, 4641-4648. https://doi.org/10.1021/jf3008376
- 32. Lansky, E.P. and Newman, R.A. (2007) Punica granatum (Pomegranate) and Its Potential for Prevention and Treatment of Inflammation and Cancer. Journal of Ethnopharmacology, 109, 177-206. https://doi.org/10.1016/j.jep.2006.09.006
- 33. Syed, D.N. Agaq, F. and Mukhtar, H. (2007) Pomegranate Derived Products for Cancer Chemoprevention. Seminars in Cancer Biology, 17, 377-385. https://doi.org/10.1016/j.semcancer.2007.05.004
- 34. Braga, L.C., Shupp, J.W., Cummings, C., et al. (2005) Pomegranate Extract Inhibits Staphylococcus aureus Growth and Subsequent Enterotoxin Production. Journal of Ethnopharmacology, 96, 335-339. https://doi.org/10.1016/j.jep.2004.08.034
- 35. Aviram, M., Volkova, N., Coleman, R., et al. (2008) Pomegranate Phenolics from the Peels, Arils, and Flowers Are Antiatherogenic: Studies in Vivo in Atherosclerotic Apolipoprotein e-Deficient (E0) Mice and in Vitro in Cultured Macrophages and Lipoproteins. Journal of Agricultural and Food Chemistry, 56, 1148-1157. https://doi.org/10.1021/jf071811q
- 36. Rosenblat, M., Hayek, T. and Aviram, M. (2006) Anti-Oxidative Effects of Pomegranate Juice (PJ) Consumption by Diabetic Patients on Serum and on Macrophages. Atherosclerosis, 187, 363-371. https://doi.org/10.1016/j.atherosclerosis.2005.09.006
- 37. Aviram, M. and Dornfeld, L. (2001) Pomegranate Juice Consumption Inhibits Serum Angiotensin Converting Enzyme Activity and Reduces Systolic Blood Pressure. Atherosclerosis, 158, 195-198. https://doi.org/10.1016/S0021-9150(01)00412-9
- 38. Blazquez, I., Alonso, G.L., Zalacain, A., et al. (2002) Antiradical Efficiency of Different Vegetable Tannin Extracts. Journal: American Leather Chemists Association, 97, 137-142.
- 39. Perezfons, L., Garzon, M.T. and Micol, V. (2010) Relationship between the Antioxidant Capacity and Effect of Rosemary (Rosmarinus officinalis L.) Polyphenols on Membrane Phospholipid Order. Journal of Agricultural & Food Chemistry, 58, 161-171. https://doi.org/10.1021/jf9026487
- 40. Yamada, H., Ohashi, K., Atsumi, T., et al. (2003) Effects of Tea Catechin Inhalation on Methicillin-Resistant Staphylococcus mreus in Elderly Patients in a Haspital Ward. Journal of Hospital Infection, 53, 229-231. https://doi.org/10.1053/jhin.2002.1327
- 41. Bravo, L. (2010) Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutrition Reviews, 56, 317-333. https://doi.org/10.1111/j.1753-4887.1998.tb01670.x
- 42. Custodio, L., Patarra, J., Albericio, F., et al. (2015) Phenolic Composition, Antioxidant Potential and in Vitro Inhibitory Activity of Leaves and Acorns of Quercus suber on Key Enzymes Relevant for Hyperglycemia and Alzheimer’s Disease. Industrial Crops & Products, 64, 45-51. https://doi.org/10.1016/j.indcrop.2014.11.001
- 43. Nourafcan, H., Nasrollahpour, M. and Bajalan, I. (2013) Antibacterial Activity of Leaves Extract from Oak (Quercus persica) against Some Positive and Negative Bacteria. International Journal of Farming and Allied Sciences, 2, 1153-1155.
- 44. Korus, J., Witczak, M., Ziobro, R., et al. (2015) The Influence of Acorn Flour on Rheological Properties of Gluten-Free Dough and Physical Characteristics of the Bread. European Food Research & Technology, 240, 1135-1143. https://doi.org/10.1007/s00217-015-2417-y
- 45. Ghahfaroki, M.G., Mahoonak, A.S., Alami, M., et al. (2016) Effect of Processing Treatments on Polyphenol Removal from Kernel of Two Iranian Acorns Varieties. International Food Research Journal, 24, 86-93.
NOTES
*通讯作者。