Advances in Clinical Medicine
Vol.
13
No.
02
(
2023
), Article ID:
61172
,
8
pages
10.12677/ACM.2023.132211
HO-1在重症急性胰腺炎引起的肠黏膜损伤中的作用及其机制
张井银1,李医铭2,姜英建1,李洪波1,王江1,张佃良1*
1青岛大学附属青岛市立医院普外一科,山东 青岛
2青岛大学附属青岛市海慈医院神经内三科,山东 青岛
收稿日期:2023年1月8日;录用日期:2023年1月28日;发布日期:2023年2月9日

摘要
目的:本研究旨在探究HO-1对SAP大鼠肠黏膜的保护作用及机制。方法:选取SPF级健康雄性SD大鼠32只,随机分为假手术组(SO组)、重症急性胰腺炎组(SAP组)、HO-1激活组(SAP + Hemin组)、HO-1抑制组(SAP + Znpp组),每组8只。采用5%牛磺胆酸钠逆行注入胆胰管的方法建立大鼠SAP模型,SO组胆胰管注入等量生理盐水。干预组大鼠分别在建模前24 h腹腔注射Hemin和Znpp,其余组腹腔注射等量生理盐水。各组大鼠在手术后24 h检测大鼠血清淀粉酶、TNF-α、IL-6、IL-10、DAO活性以及D-LA、FABP2和内毒素水平;检测回肠组织MDA含量以及SOD和GPX的活性水平;苏木精–伊红(HE)染色观察胰腺、回肠病理变化;免疫荧光染色检测HO-1及Occludin、ZO-1的表达情况;TUNEL检测肠上皮细胞凋亡水平。结果:SAP组大鼠血清AMY、TNF-α、IL-6、DAO活性以及D-LA、FABP2和内毒素水平明显高于SO组,IL-10显著低于SO组(均P < 0.05)。与SAP组相比,SAP + Hemin组显著增加HO-1的表达,减轻了SAP全身炎症反应、MDA含量以及SOD和GPX的活性明显降低,肠上皮细胞凋亡减少(均P < 0.05)。同时,上调HO-1的表达减轻了对肠紧密连接蛋白表达的抑制作用,血清DAO活性及D-LA、FABP2和内毒素水平显著降低(均P < 0.05)。相反,SAP + Znpp组则表现出相反的实验结果。结论:H0-1通过减轻炎症、氧化应激及抗凋亡的作用来减轻重症急性胰腺炎肠粘膜损伤。
关键词
血红素加氧酶-1,重症急性胰腺炎,肠黏膜屏障,凋亡,氧化应激

Effects and Mechanism of HO-1 in Intestinal Mucosal Injury Caused by Severe Acute Pancreatitis
Jingyin Zhang1, Yiming Li2, Yingjian Jiang1, Hongbo Li1, Jiang Wang1, Dianliang Zhang1*
1Department of the First General Surgery, Qingdao Municipal Hospital, Qingdao University, Qingdao Shandong
2Department of the Third Neurology, Qingdao Haici Hospital, Qingdao University, Qingdao Shandong
Received: Jan. 8th, 2023; accepted: Jan. 28th, 2023; published: Feb. 9th, 2023

ABSTRACT
Objective: To investigate the protective effect and mechanism of HO-1 on intestinal mucosa in SAP rats. Methods: Thirty-two SPF-grade healthy male SD rats were selected and randomly divided into sham-operated group (SO group), severe acute pancreatitis group (SAP group), HO-1 activation group (SAP + Hemin group), and HO-1 inhibition group (SAP + Znpp group), eight rats in each group. The rat SAP model was established through retrograde injection of 5% sodium taurocholate into the biliopancreatic duct, and the SO group was injected with equal amounts of saline into the biliopancreatic duct. Hemin and Znpp were injected intraperitoneally 24 h before modeling in the intervention group, while equal amounts of saline were injected intraperitoneally in the other group. At 24 hours after surgery, serum amylase, TNF-α, IL-6, IL-10, DAO, D-LA, FABP2 and endotoxin levels were measured in rats; the MDA content and the activity levels of SOD and GPX in ileal tissue were measured; Hematoxylin and eosin (HE) staining for pathological changes in the pancreas and ileum; Immunofluorescence staining detection of HO-1 and Occludin, ZO-1 expression; TUNEL detection of apoptosis level in intestinal epithelial cells. Results: The serum AMY, TNF-α, IL-6, DAO, D-LA, FABP2 and endotoxin levels of rats in SAP group were significantly higher than those in SO group, and IL-10 was significantly lower than that in SO group (all P < 0.05). Compared with the SAP group, the SAP + Hemin group significantly increased HO-1 expression, attenuated systemic inflammatory response, MDA content, and significantly reduced SOD and GPX activities, and decreased intestinal epithelial cell apoptosis (all P < 0.05). Meanwhile, upregulation of HO-1 expression attenuated the inhibitory effect on intestinal tight junction protein expression, and serum DAO, D-LA, FABP2 and endotoxin levels were significantly reduced (all P < 0.05). However, the SAP + Znpp group showed the opposite experimental results. Conclusion: HO-1 attenuates intestinal mucosal injury in severe acute pancreatitis by reducing inflammation, oxidative stress and anti-apoptotic effects.
Keywords:Heme Oxygenase-1, Severe Acute Pancreatitis, Intestinal Mucosal Barrier, Apoptosis, Oxidative Stress

Copyright © 2023 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 前言
重症急性胰腺炎(SAP)是急性胰腺炎的一种常见亚型,SAP的死亡率很高,通常死于胰腺重症感染和全身多器官的衰竭 [1] 。肠黏膜屏障被称为“多器官衰竭的引擎”。在SAP诱导的肠粘膜损伤过程中,肠道的微生物和内毒素通过肠粘膜屏障进入血液或淋巴系统而侵入远处器官 [2] 。SAP肠粘膜损伤的发病机制复杂,目前尚未完全阐明。研究发现全身炎症反应、氧化应激及肠上皮细胞死亡(如凋亡、自噬和铁死亡)参与损伤机制 [3] [4] [5] 。
血红素加氧酶-1 (HO-1)是炎症反应、氧化应激和细胞死亡的重要调节因子 [6] 。HO-1血红素分解的关键酶,血红素被分解为一氧化碳(CO)、胆绿素和游离铁 [7] 。HO-1可在胃肠道粘膜层表达,在应激源(如缺血缺氧、重金属、细胞因子等)刺激时表达增强 [8] 。HO-1通过消除肠紧密连接蛋白(tight junction proteins, TJs)失调和减弱上皮细胞损伤来维持肠粘膜屏障的完整性 [9] 。然而,HO-1在SAP肠黏膜屏障中的作用机制尚未见报道,故我们推测HO-1表达异常可能是SAP肠黏膜损伤的重要机制。本研究通过建立大鼠SAP肠黏膜损伤模型,探究HO-1在重症急性胰腺炎引起的肠黏膜损伤的作用及其机制。
2. 材料与方法
2.1. 实验动物分组及处理
本实验中动物操作均参照《实验动物管理条例》进行,符合动物伦理学要求,由青岛大学动物护理与实验委员会批准。选用32只健康成年雄性SD大鼠,体重250~300 g,由青岛大学动物中心提供。按照随机数字表法将分为四组:假手术组(SO组)、重症急性胰腺炎组(SAP组)、HO-1激动组(SAP + Hemin组)和HO-1抑制组(SAP + Znpp组),每组8只。
大鼠术前禁食12 h。通过腹腔注射3%戊巴比妥钠(20 mg/kg)麻醉。建立SAP大鼠模型:打开腹腔,在大鼠肝门附近暂时夹闭胆胰管,随后用导管穿过十二指肠,向胆胰管缓慢注射5%牛磺胆酸钠(1 ml/kg)。SO组胰胆管注射等量无菌生理盐水。SAP + Hemin组在SAP大鼠模型建立24小时前,缓慢腹腔注射HO-1激活剂Hemin (30 mg/kg)。SAP + Znpp组在SAP大鼠模型建立24小时前,缓慢腹腔注射HO-1抑制剂Znpp (20 mg/kg)。假手术组和SAP组大鼠术前24小时腹腔注射等量生理盐水。
2.2. 样本采集
各组大鼠术后24小时重新腹腔注射3%戊巴比妥钠(20 mg/kg)麻醉。使用静脉穿刺针采集大鼠下腔静脉血液样本,离心获得血清,并保存在−20℃冰箱中,供后续血清分析。分离胰腺和回肠组织,一部分立即在4%多聚甲醛中固定,用于制备组织切片,其余部分−80℃干燥保存,供后续分析。
2.3. 血清检测
使用自动生化分析仪测量血清淀粉酶(AMY)水平(检测试剂盒购于南京建成生物工程研究所)。严格按照试剂盒说明书检测大鼠血清肿瘤坏死因子-α (TNF-α)、白细胞介素-6 (IL-6)和白细胞介素-10 (IL-10)、血清二胺氧化酶(DAO)、D-乳酸(D-LA)和肠道脂肪酸结合蛋白(FABP2)、血清内毒素水平(试剂盒购于江苏酶免实业有限公司)。
2.4. 肠道氧化应激测定
将部分肠粘膜在预冷的砂浆中研磨,然后在低温均质机中加入冰冷的缓冲液进行组织均质。我们使用试剂盒检测肠粘膜中的丙二醛(MDA)、超氧化物歧化酶(SOD)含量和谷胱甘肽过氧化物酶(GPX)活性(检测试剂盒购于南京建成生物工程研究所,批次分别为:A003-1-1、A001-3-1、H545-1-1)。
2.5. 苏木精–伊红染色观察胰腺、回肠病理变化
胰腺、回肠组织用4%多聚甲醛固定。石蜡包埋,切片,然后用苏木精和伊红染料对组织切片进行染色,在光学显微镜下观察组织病理学变化。
2.6. 免疫荧光染色检测HO-1和TJs在大鼠肠道中的表达
切片在37℃的烤箱中放置15分钟。在免疫染色过程中,将载玻片与兔抗HO-1、兔抗ZO-1和兔抗occludin一抗在4℃孵育过夜。切片用PBS冲洗,并用Alexa Fluor山羊抗兔IgG在室温下孵育30分钟。随后用DAPI染色5分钟。使用荧光显微镜观察并拍摄。
2.7. 原位末端标记(TUNEL)法检测细胞凋亡
使用用末端脱氧核苷酸转移酶介导的缺口末端标记(TUNEL)试剂盒(购于武汉博士德生物工程有限公司)检测肠上皮细胞凋亡情况,具体参照试剂盒说明。DAPI染色细胞核为蓝色,Tunel阳性细胞为红色。使用共聚焦激光扫描显微镜观察并拍摄。
2.8. 统计学方法
采用SPSS 20.0软件进行数据分析处理。正态分布的计量数据用均数 ± 标准差(x ± s)表示,组间比较采用单因素方差分析,两两比较用LSD-t检验。P < 0.05认为差异具有统计学意义。
3. 结果
3.1. 血清淀粉酶、TNF-α、IL-6、IL-10水平比较
SAP组AMY、TNF-α、IL-6、IL-10水平明显高于SO组,差异有统计学意义(P < 0.05)。然而SAP + Hemin组AMY、TNF-α、IL-6显著低于SAP组,IL-10显著高于SAP组,差异均具有统计学意义(P < 0.05);SAP + Znpp组AMY、TNF-α、IL-6水平明显高于SAP组,IL-10明显低于SAP组,见表1。
Table 1. Comparison of serum levels of AMY, TNF-α, IL-6 and IL-10 in each group (n = 8, )
表1. 各组大鼠血清AMY、TNF-α、IL-6、IL-10水平比较(n = 8, )
注:AMY示淀粉酶;TNF-α示肿瘤坏死因子-α;IL-6示白细胞介素-6;IL-10示白细胞介素-10;SO组示假手术组;SAP组示重症急性胰腺炎组;SAP + Hemin组示HO-1激动剂干预组;SAP + Znpp组示HO-1抑制剂干预组。
3.2. 各组大鼠回肠组织中MDA含量以及SOD和GPX的活性比较
SAP组回肠组织中MDA含量明显高于SO组,SOD和GPX的活性明显低于SO组,差异具有统计学意义(P < 0.05);与SAP组相比,SAP + Hemin组MDA含量显著降低,SOD和GPX的活性显著增高,而SAP + Znpp组MDA含量显著升高,SOD和GPX的活性显著降低,差异均具有统计学意义(P < 0.05),见表2。
Table 2. Comparison of MDA content and SOD and GPX activity in ileum of rats in each group (n = 8, )
表2. 各组大鼠回肠组织中MDA含量以及SOD和GPX的活性比较(n = 8, )
注:MDA示丙二醛;SOD示超氧化物歧化酶;GPX示谷胱甘肽过氧化物酶;SO组示假手术组;SAP组示重症急性胰腺炎组;SAP + Hemin组示HO-1激动剂干预组;SAP + Znpp组示HO-1抑制剂干预组。
3.3. 各组血清DAO活性以及D-LA、FABP2和内毒素水平比较
与SO组相比,SAP组DAO活性及D-LA、FABP2和内毒素的浓度水平明显升高,差异具有统计学意义(P < 0.05)。然而,SAP + Hemin组DAO活性及D-LA、FABP2和内毒素显著低于SAP组,差异具有统计学意义(P < 0.05);SAP + Znpp组DAO活性及D-LA、FABP2和内毒素水平明显高于SAP组,差异具有统计学意义(P < 0.05),见表3。
Table 3. Comparison of serum DAO activity and concentrations of D-LA, FABP2 and endotoxin (n = 8, )
表3. 血清DAO活性以及D-LA、FABP2和内毒素的浓度比较(n = 8, )
注:DAO示二胺氧化酶;D-LA示D-乳酸;FABP2示肠道脂肪酸结合蛋白;SO组示假手术组;SAP组示重症急性胰腺炎组;SAP + Hemin组示HO-1激动剂干预组;SAP + Znpp组示HO-1抑制剂干预组。
3.4. 大鼠胰腺、回肠组织病理变化
SO组胰腺(A)、回肠组织(E)病理学未见明显变化。SAP大鼠胰腺组织(B)出现小叶结构模糊,间质水肿、炎性浸润、胰腺腺泡细胞空泡化或坏死等病理学改变;回肠组织(F)出现组织水肿、出血、绒毛脱落、黏膜细胞坏死、炎性浸润等病理学改变。与SAP组相比,SAP + Hemin组胰腺(C)小叶结构尚清楚,间质水肿减轻,胰腺腺泡坏死减少,回肠组织(G)绒毛较完整,水肿减轻,肠粘膜细胞坏死减少等表现;然而,SAP+Znpp组胰腺(D)则表现出小叶结构模糊加重,间质水肿加重、胰腺腺泡细胞坏死增加等病理变化,回肠组织(H)绒毛脱落,水肿加重,肠粘膜细胞坏死增加,炎性浸润增加。具有代表性的胰腺、回肠组织病理损伤见图1。
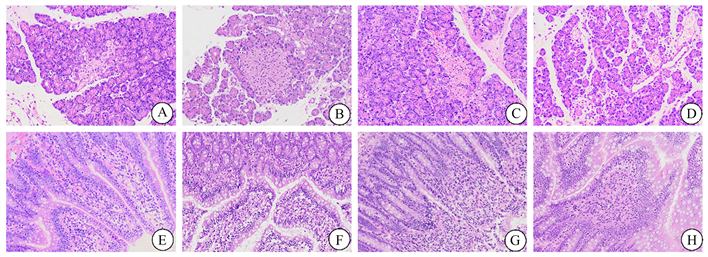
Figure 1. Representative images of rat pancreas and intestinal tissues in different groups after hematoxylin and eosin staining (original magnification, 200×). (A)~(D) Pancreatic tissues of SO, SAP, SAP + Hemin, SAP + Znpp groups; (E)~(H) Ileum tissue of SO, SAP, SAP + Hemin, SAP + Znpp group. SO group showed sham operation group; SAP group showed severe acute pancreatitis group. SAP + Hemin group showed HO-1 excitatory group. SAP + Znpp group showed HO-1 inhibition group
图1. 各组大鼠胰腺和肠道组织苏木精–伊红染色后的代表性图像(原始图像,200×)。(A)~(D)示SO、SAP、SAP + Hemin、SAP + Znpp组胰腺组织;(E)~(H)示SO、SAP、SAP + Hemin、SAP + Znpp组回肠组织。SO组示假手术组;SAP组示重症急性胰腺炎组;SAP + Hemin组示HO-1激动组;SAP + Znpp组示HO-1抑制组
3.5. 各组大鼠HO-1、Occludin、ZO-1表达水平比较
免疫荧光显微镜观察HO-1 (绿色)、ZO-1 (绿色)、Occludin (绿色)主要表达于肠黏膜上皮细胞(图2)。与SO组(A)相比,SAP组HO-1的表达明显增多(B),SAP + Hemin组表达显著增加(C),SAP + Znpp组显著减少(D),这说明我们成功有效地干预了HO-1的表达。SAP组Occludin、ZO-1 ((E)、(J))的表达明显低于SO组,SAP + Hemin组Occludin、ZO-1 ((G)、(K))的表达显著高于SAP组,SAP + Znpp组Occludin、ZO-1 ((H)、(L))的表达显著低于SAP组。具有代表性的图像见图2。
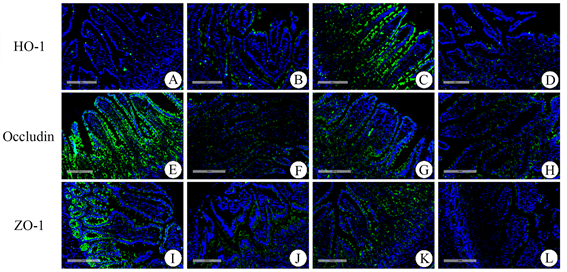
Figure 2. The expression of HO-1 (green), ZO-1 (green) and Occludin (green) proteins were detected by immunofluorescence (Original magnification, 200×). (A)~(D) The expression of HO-1 in SO, SAP, SAP + Hemin and SAP + Znpp groups; (E)~(H) The Expression of Occludin in SO, SAP, SAP + Hemin and SAP + Znpp groups; (I)~(L) The Expression of ZO-1 in SO, SAP, SAP + Hemin and SAP + Znpp groups. SO group showed sham operation group; SAP group showed severe acute pancreatitis group. SAP + Hemin group showed HO-1 excitatory group. SAP + Znpp group showed HO-1 inhibition group
图2. 免疫荧光检测SO、SAP、SAP + Hemin、SAP + Znpp组回肠组织HO-1 (绿色)、ZO-1 (绿色)、Occludin (绿色)的表达(原始图像,200×)。(A)-(D) SO、SAP、SAP + Hemin、SAP + Znpp组HO-1的表达;(E)~(H) SO、SAP、SAP + Hemin、SAP + Znpp组Occludin的表达;(I)~(L) SO、SAP、SAP + Hemin、SAP + Znpp组ZO-1的表达。SO组示假手术组;SAP组示重症急性胰腺炎组;SAP + Hemin组示HO-1激动剂干预组;SAP + Znpp组示HO-1抑制剂干预组
3.6. 各组大鼠肠粘膜上皮细胞凋亡的比较
脱氧核糖核苷酸末端转移酶介导的缺口末端标记法(TUNEL)检测SO、SAP、SAP + Hemin、SAP + Znpp组肠上皮细胞凋亡(红色)情况。与SO组(A)相比,SAP组(B)肠上皮细胞凋亡明显增加。然而,SAP + Hemin组(C)肠上皮细胞凋亡显著低于SAP组,SAP + Znpp组(D)显著高于SAP组。具有代表性的图像见图3。

Figure 3. Representative images of terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling (TUNEL) assay (original magnification, 200×). (A)~(D) Apoptosis of intestinal epithelial cells in SO, SAP, SAP + Hemin, SAP + Znpp groups.SO group showed sham operation group; SAP group showed severe acute pancreatitis group. SAP + Hemin group showed HO-1 excitatory group. SAP + Znpp group showed HO-1 inhibition group
图3. 脱氧核糖核苷酸末端转移酶介导的缺口末端标记法(TUNEL)代表性图像(原始图像,200×)。(A)~(D) SO、SAP、SAP + Hemin、SAP + Znpp组肠上皮细胞凋亡情况。SO组示假手术组;SAP组示重症急性胰腺炎组;SAP + Hemin组示HO-1激动剂干预组;SAP + Znpp组示HO-1抑制剂干预组
4. 讨论
重症急性胰腺炎诱发肠粘膜屏障损伤会加重病情,增加死亡率。本研究以SAP肠道黏膜屏障损伤为基点,探究HO-1在重症急性胰腺炎引起的肠黏膜损伤的作用及其机制。本研究中,SAP组术后大鼠血清AMY及炎症因子水平的变化和胰腺、回肠组织HE染色病理改变表明我们成功建立了大鼠SAP肠损伤模型。
HO-1在调节生物和代谢过程中具有重要作用以及参与多种疾病的发病机制,目前成为国内外研究的热点 [8] [10] 。我们的实验结果显示,在多种应激源刺激下SAP大鼠回肠组织中HO-1表达增强,说明HO-1对肠组织的一种保护机制。我们的实验表明,全身炎症因子、肠道氧化应激和肠上皮细胞凋亡参与了SAP肠粘膜损伤的病理过程。上调HO-1表达显著减轻肠粘膜损伤,证实HO-1具有强大的抗炎抗氧化作用。
肠道是一个独特的器官,内有高浓度的有害物质,包括微生物和毒素。肠粘膜具有半透性的屏障作用,允许营养吸收和免疫感应,同时限制有害微生物通过肠上皮进入循环系统 [11] 。检测过度生长的细菌释放的代谢物可以帮助诊断肠屏障功能障碍。D-乳酸(D-LA)是肠道内多种微生物释放的产物,血清D-LA水平升高是肠道缺血的重要特征 [12] 。二胺氧化酶(DAO)仅在肠绒毛上皮细胞中合成,当肠绒毛上皮细胞受损时,DAO被释放进入血液中 [13] 。肠道脂肪酸结合蛋白(FABP2)由受损的肠上皮细胞释放 [14] 。同时,我们检测了大鼠血清内毒素水平,间接反应了肠粘膜屏障的功能。我们的实验显示血清DAO、D-LA、FABP2及内毒素水平与肠粘膜损伤程度相符。
紧密连接蛋白是肠粘膜物理屏障的重要组成部分,维持着肠上皮细胞的稳态。HO-1促进肠紧密连接蛋白的表达,从分子角度说明了HO-1在SAP肠黏膜中的潜在价值。肠上皮细胞的持续更新是有效平衡肠内消化液及肠内菌群所必需的。正常情况下,细胞凋亡在维持肠上皮细胞稳态中起着重要作用。肠上皮细胞过度凋亡导致肠黏膜屏障功能受损 [15] 。然而,HO-1作为细胞保护分子可对抗多种形式细胞死亡,包括坏死、凋亡和调节性细胞死亡(RCD)程序,如焦亡和铁死亡 [6] 。本研究发现HO-1显著降低SAP大鼠肠上皮细胞凋亡水平。SAP诱导的肠黏膜损伤涉及复杂的细胞死亡过程,其他类型的细胞死亡及内在关系值得进一步探讨。
综上所述,本研究表明HO-1通过抗炎和抗氧化应激及抗凋亡的作用减轻SAP肠黏膜屏障损伤。我们研究对预防或减轻重症急性胰腺炎诱导的肠屏障损伤提供了新的见解。
声明
所有动物实验均经青岛大学伦理委员会批准,并按照伦理标准进行。
基金项目
国家自然科学基金面上项目(81270448, 81470890)。
利益冲突
作者宣称没有利益冲突。
文章引用
张井银,李医铭,姜英建,李洪波,王 江,张佃良. HO-1在重症急性胰腺炎引起的肠黏膜损伤中的作用及其机制
Effects and Mechanism of HO-1 in Intestinal Mucosal Injury Caused by Severe Acute Pancreatitis[J]. 临床医学进展, 2023, 13(02): 1530-1537. https://doi.org/10.12677/ACM.2023.132211
参考文献
- 1. Petrov, M.S., Shanbhag, S., Chakraborty, M., Phillips, A.R. and Windsor, J.A. (2010) Organ Failure and Infection of Pancreatic Necrosis as Determinants of Mortality in Patients with Acute Pancreatitis. Gastroenterology, 139, 813-820. https://doi.org/10.1053/j.gastro.2010.06.010
- 2. Liu, J.B., Huang, L., Luo, M. and Xia, X.M. (2019) Bacterial Translocation in Acute Pancreatitis. Critical Reviews in Microbiology, 45, 539-547. https://doi.org/10.1080/1040841X.2019.1621795
- 3. Huang, L.Q., Jiang, Y.J., Sun, Z.Q., Gao, Z.Y., Wang, J. and Zhang, D.L. (2018) Autophagy Strengthens Intestinal Mucosal Barrier by Attenuating Oxidative Stress in Severe Acute Pancreatitis. Digestive Diseases and Sciences, 63, 910-919. https://doi.org/10.1007/s10620-018-4962-2
- 4. Ma, D.L., Jiang, P.L., Jiang, Y.J., Li, H.B. and Zhang, D.L. (2021) Effects of Lipid Peroxidation-Mediated Ferroptosis on Severe Acute Pancreatitis-Induced Intestinal Barrier Injury and Bacterial Translocation. Oxidative Medicine and Cellular Longevity, 2021, Article ID: 6644576. https://doi.org/10.1155/2021/6644576
- 5. 田锐, 许飞, 王瑞兰, 谢晖, 孟潇潇, 钱永兵, 金卫, 胡家昌, 周志刚, 俞康龙. 氧化应激及凋亡与重症急性胰腺炎肠屏障功能障碍[J]. 中华急诊医学杂志, 2012, 21(10): 1088-1092.
- 6. Ryter, S.W. (2021) Heme Oxgenase-1, a Cardinal Modulator of Regulated Cell Death and Inflamma-tion. Cells, 10, Article No. 515. https://doi.org/10.3390/cells10030515
- 7. 张雁林, 赵金垣. 血红素加氧酶-1的细胞保护作用研究进展[J]. 环境与职业医学, 2008, 25(2): 197-202. https://doi.org/10.13213/j.cnki.jeom.2008.02.020
- 8. Puentes-Pardo, J.D., Moreno-SanJuan, S., Carazo, Á. and León, J. (2020 Heme Oxygenase-1 in Gastrointestinal Tract Health and Disease. Antioxidants, 9, Article No. 1214. https://doi.org/10.3390/antiox9121214
- 9. Zhang, Z.L., Zhang, Q.P., Li, F., Xin, Y. and Duan, Z.J. (2021) Con-tributions of HO-1-Dependent MAPK to Regulating Intestinal Barrier Disruption. Biomolecules & Therapeutics, 29, 175-183. https://doi.org/10.4062/biomolther.2020.112
- 10. 任萍萍, 段志军. 血红素氧合酶-1在胃肠道疾病中的作用研究进展[J]. 大连医科大学学报, 2022, 44(1): 69-74.
- 11. Okumura, R. and Takeda, K. (2017) Roles of Intestinal Epi-thelial Cells in the Maintenance of Gut Homeostasis. Experimental & Molecular Medicine, 49, e338. https://doi.org/10.1038/emm.2017.20
- 12. Murray, M.J., Barbose, J.J. and Cobb, C.F. (1993) Serum D(-)-Lactate Levels as a Predictor of Acute Intestinal Ischemia in a Rat Model. The Journal of Surgical Research, 54, 507-509. https://doi.org/10.1006/jsre.1993.1078
- 13. Luk, G.D., Bayless, T.M. and Baylin, S.B. (1980) Diamine Oxidase (Histaminase). A Circulating Marker for Rat Intestinal Mucosal Maturation and Integrity. The Journal of Clinical Inves-tigation, 66, 66-70. https://doi.org/10.1172/JCI109836
- 14. Funaoka, H., Kanda, T., Kajiura, S., Ohkaru, Y. and Fujii, H. (2011) De-velopment of a High-Specificity Sandwich ELISA System for the Quantification of Human Intestinal Fatty Acid-Binding Protein (I-FABP) Concentrations. Immunological Investigations, 40, 223-242. https://doi.org/10.3109/08820139.2010.534216
- 15. Sun, Z., Wang, X., Wallen, R., Deng, X., Du, X., Hallberg, E. and Andersson, R. (1998) The Influence of Apoptosis on Intestinal Barrier Integrity in Rats. Scandinavian Journal of Gastroenterology, 33, 415-422. https://doi.org/10.1080/00365529850171053
NOTES
*通讯作者Email: qdsurg@qdu.edu.cn
