Advances in Clinical Medicine
Vol.
11
No.
06
(
2021
), Article ID:
42931
,
6
pages
10.12677/ACM.2021.116364
海扶刀联合化疗后DP-CAR术式切除 胰腺癌一例
仲灏辰1,赵伟2*,曹广华1,李浩然1,李学良1,郭敬允1
1青岛大学,山东 青岛
2青岛大学附属医院,山东 青岛

收稿日期:2021年5月1日;录用日期:2021年5月13日;发布日期:2021年6月7日

摘要
胰腺癌是消化系统中常见恶性肿瘤之一,预后很差,5年生存率小于5%,多数患者在初次就诊时肿瘤已经局部转移、血管侵犯、甚至远处脏器转移,不可切除,而可切除性肿瘤仅15%~20%。本文通过对1例胰腺癌患者经术前以海扶刀和TS方案联合治疗联合DP-CAR术式扩大切除范围从而达到完全切除的目的,提高胰体尾癌患者的生活质量、生存期。
关键词
胰腺癌,海扶刀,化疗,转化治疗
A Case of Pancreatic Cancer Treated with DP Car after Combined Chemotherapy with HIFU
Haochen Zhong1, Wei Zhao2*, Guanghua Cao1, Haoran Li1, Xueliang Li1, Jingyun Guo1
1Qingdao University, Qingdao Shandong
2The Affiliated Hospital of Qingdao University, Qingdao Shandong

Received: May 1st, 2021; accepted: May 13th, 2021; published: Jun. 7th, 2021

ABSTRACT
Pancreatic cancer is one of the most common malignancies in the digestive system. The prognosis is very poor. The 5-year survival rate is less than 5%. Most of the patients had local metastasis, vascular invasion, or even distant organ metastasis at the first visit, which was unresectable, while only 15%~20% of the tumors were resectable. In order to improve the quality of life and survival time of a patient with pancreatic body and tail cancer, we expanded the scope of resection by combining HIFU and TS with DP-CAR before operation.
Keywords:Pancreatic Cancer, HIFU, Chemotherapy, Conversion Therapy

Copyright © 2021 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 病例资料
患者女性,42岁,因“腹胀1月余,腹痛2周”于2020年1月3日入院。患者1月余前无明显诱因出现腹胀,进食后加重,2周前出现腹痛,无恶心、呕吐。入院检查:视觉模拟疼痛评分法(VAS):7分;糖类抗原19-9 (CA199) 231.9 U/ml;其余肿瘤标志物血清胃泌素释放肽前体、鳞状细胞癌相关抗原、特异β人绒毛膜促性腺激素、神经元特异性烯醇化酶、糖类抗原15-3、糖类抗原72-4、糖类抗原125、甲胎蛋白、癌胚抗原均在正常范围;肝功、肾功均在正常范围;胃镜显示:慢性非萎缩性胃炎;消化系统超声(见图1):胰腺低回声结节;上腹部CT动态增强扫描显示(见图2):胰体部占位性病变并胰管扩张、胰周结节影,考虑胰腺癌伴胰周淋巴结转移可能性大;上腹部MR动态增强 + 单脏器薄层扫描(见图3):胰体部占位性病变,大小41 mm × 25 mm × 23 mm,侵犯邻近血管并胰管扩张、腹膜后肿大淋巴结,胰腺癌可能性大;超声引导下胰腺穿刺活检病理:腺癌(中分化);分期:cT4N1M0,III期;经多学科会诊、患者家属同意,决定外科手术前进行高强度聚焦超声(海扶刀,high intensity focused ultrasound,HIFU)联合化疗进行治疗。患者于2020年1月11日气管插管全身麻醉下行胰腺癌及转移淋巴结姑息性HIFU术。术前皮肤准备、脱脂脱气,待麻醉满意后,取俯卧位,治疗胰腺癌及转移淋巴结,参考术前MRI及治疗超声对胰腺病灶及转移淋巴结进行定位治疗,胰腺病灶大小约41 * 25 * 23 mm,治疗时仔细辨别肿瘤与周围组织器官关系,如皮肤、胃肠道等。总治疗参数如下:12号治疗头,辐照时间857秒,平均功率286 W,病灶呈团块状灰度改变。术后声诺维超声造影显示无血供区,消融效果满意,手术顺利。HIFU术后于2020-01-16、2020-02-06、2020-02-27、2020-03-25、2020-04-15给予5周期TS方案,即白蛋白紫杉醇300 mg d1 + 替吉奥50 mg bid d1-14化疗。期间多次复查磁共振以及肝肾功等。2020-03-19上腹部MR动态增强 + 单脏器薄层扫描显示(见图4):胰体部见囊状长T1长T2信号影,DWI信号混杂,范围约27 mm × 15 mm,较前范围减小,远侧胰管扩张,胰尾萎缩。腹腔内、腹膜后未见明显肿大淋巴结。2020-05-06 (见图5)上腹部MR动态增强 + 单脏器薄层扫描显示(见图5):胰体部见囊状长T1长T2信号影,DWI信号混杂,范围约27 mm × 15 mm,较前2020.3.19 MR范围变化不大,远侧胰管扩张,胰尾萎缩。肝固有动脉、脾动静脉、肠系膜上静脉显影欠佳,胃周多发迂曲血管,腹腔内、腹膜后未见明显肿大淋巴结。2020-02-05糖类抗原19-9:178.4 U/ml;2020-02-26糖类抗原19-9:69.55 U/ml;2020-03-18糖类抗原19-9:33.86 U/ml;2020-04-14糖类抗原19-9:14.4 U/ml;2020-05-06糖类抗原19-9:13.17 U/ml。视觉模拟疼痛评分法(VAS):2分,检查评价疗效PR。经肝胆外科医师讨论、家属同意,决定行手术治疗(联合腹腔干切除的胰体尾切除术,Distal pancreatectomy with en bloc celiac axis resection, DP-CAR),于2020-05-09行联合腹腔干切除的胰体尾切除术 + 脾脏切除术 + 肠系膜上静脉切除重建术(见图6),术后病理:① 胰体尾 + 脾:胰腺大小9 * 3 * 2 cm,距胰腺断端1 cm于胰腺内见一囊性区(临床已剖开),大小约2.5 * 2.5 cm,内壁粗糙,脾脏大小16 * 8 * 4 cm,多切面切开灰红质软未见特殊,网膜组织大小35 * 15 * 1 cm,未扪及质硬区。另送检淋巴结一组。② (胰腺)中分化腺癌(范围2.5 * 2.5 cm),间质广泛纤维化,周边伴胰岛增生,可见灶状钙化及囊性变,符合化疗后改变,神经侵犯(+),未累及胰腺断端,网膜组织内未见癌转移。③ 脾脏组织,未见癌转移。④ 送检(第9组淋巴结)镜下为少许纤维、脂肪及神经组织。术后第10天出院,出院后随访至今,健在。
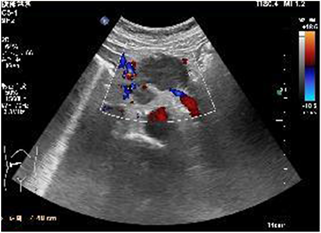
Figure 1. Digestive ultrasound
图1. 消化系统超声
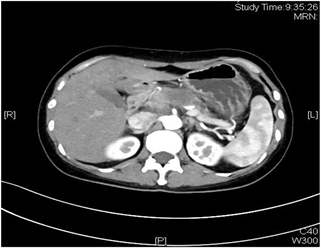
Figure 2. Dynamic contrast-enhanced CT scan of the upper abdomen
图2. 上腹部CT动态增强扫描
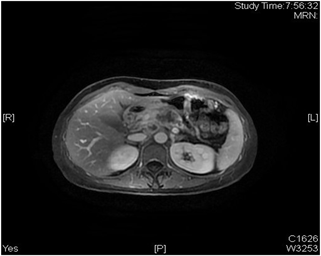
Figure 3. MR dynamic enhanced single organ thin-layer scan of upper abdomen
图3. 上腹部MR动态增强 + 单脏器薄层扫描

Figure 4. MR dynamic enhanced single organ thin-layer scan of upper abdomen
图4. 上腹部MR动态增强 + 单脏器薄层扫描
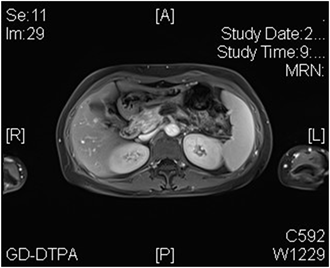
Figure 5. MR dynamic enhanced single organ thin-layer scan of upper abdomen
图5. 上腹部MR动态增强 + 单脏器薄层扫描
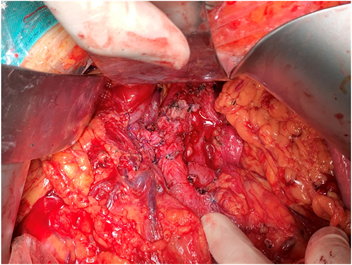
Figure 6. Pancreatectomy + splenectomy + resection and reconstruction of superior mesenteric vein
图6. 联合腹腔干切除的胰体尾切除术 + 脾脏切除术 + 肠系膜上静脉切除重建术
2. 讨论
胰腺癌是消化系统中常见恶性肿瘤之一,预后很差,5年生存率小于5% [1] [2] [3],胰腺癌中约1/4发生在胰腺体尾部,然而胰体尾癌恶性程度高,早期没有特异性的症状及体征,多数患者在初次就诊时肿瘤已经局部转移、血管侵犯(腹腔干、肝总动脉、肠系膜上动脉等)、甚至远处脏器转移,不可切除,而可切除性肿瘤仅15%~20% [4] [5] [6] [7] [8]。R0切除性手术仍然是提高胰体尾癌患者治愈率、生活质量、生存期的治疗方法 [9] [10]。回顾本病例,从以下几个方面讨论:① 该患者外科手术前,根据影像学、实验室检查、穿刺活检病理等资料,诊断为胰体尾癌,胰周淋巴结肿大、侵犯腹腔干。经海扶刀和TS方案化疗治疗后,检查评价疗效为部分缓解(PR):肿瘤最大直径及最大垂直直径的乘积缩小达50%,其他病变无增大,持续超过1个月 [11]。近年来海扶刀在胰腺癌的治疗中有着独特的优势,它是通过实时监控下对癌细胞进行灭活 [12]。在中晚期的胰腺癌患者中,很多患者存在严重的癌痛,因此癌痛的治疗非常重要,目前对胰腺癌癌痛的治疗方式由非阿片类镇痛药过度至阿片类镇痛药。对于药物难以控制的疼痛,局部治疗,将对癌痛胰腺癌患者有益。该患者经海扶刀治疗后疼痛明显减轻,视觉模拟疼痛评分法(VAS)由7分降至2分。研究报道 [13],海扶刀是一种费用低、安全、有效、精确、微创、可重复性高的方法,止疼效果明显,有88%的胰腺癌癌痛患者发生了不同程度的缓解。笔者认为,在进行海扶刀治疗的时候会发生不同类型、不同程度的并发症,如皮肤的热损伤、胰腺正常组织损伤、胰管损伤、胰瘘、胆瘘、邻近组织器官的损伤等。该患者在进行海扶刀之后常规对皮肤术区进行冰袋冷敷处理,这在一定程度减少皮肤的热损伤。准确定位能够减少胰腺正常组织、邻近组织的损伤等。② 胰腺癌相关指南 [11],晚期胰腺癌可选的化疗方案为吉西他滨单药或联合替吉奥方案,临床效果不明显。患者难以耐受推荐的FOLFIRINOX方案,因为FOLFIRINOX方案毒性较大。2013年,白蛋白结合紫杉醇被美国食品药品监督管理局(Food and Drug Administration, FDA)批准用于治疗晚期胰腺癌 [14]。替吉奥的消化道反应相对较轻 [15]。多项研究表明 [16] [17] [18],白蛋白紫杉醇联合替吉奥方案一线治疗老年晚期胰腺癌疗效较好,不良反应可耐受。此患者应用白蛋白紫杉醇联合替吉奥方案,效果明显,不良反应轻,可耐受。③ 相关文献报道 [19],海扶刀与化疗有协同效应,海扶刀联合化疗治疗后糖类抗原19-9 (CA19-9)下降明显,该患者在海扶刀联合化疗后,CA19-9血清浓度由231.9 U/ml降至13.17 U/ml,与此文献报道相符。在临床实践中,HIFU的热疗联合化疗治疗胰腺癌,可以优势互补,协同增敏,下调肿瘤标记物CA19-9,抑制肿瘤活性。④ 胰腺癌患者单纯手术治疗后(术前无新辅助治疗)局部复发率为50%~80%,腹腔转移率为30%~40%,肝转移率为40%~90% [20]。有研究证明 [21],术前放化疗能够提高胰腺癌患者的生存率。然而另一项研究表明胰腺癌术前新辅助治疗未见明显提高胰腺癌患者的生存率 [22]。笔者认为术前新辅助治疗能使肿瘤降低分期,便于手术,同时减少肿瘤扩散。此患者术前经过海扶刀和TS方案联和治疗后,多次磁共振检查发现肿瘤明显缩小,腹腔无肿大淋巴结,表明海扶刀和TS方案联和治疗效果明显。然而术前海扶刀联合TS方案治疗胰腺癌的相关研究样本量少,还需多中心大量样本的前瞻性临床研究进一步证实。⑤ 根据影像学和术中探查,该患者胰体部肿瘤,侵犯腹腔干、脾血管、肠系膜上静脉等血管,术中决定行联合腹腔干切除的胰体尾切除术 + 脾脏切除术 + 肠系膜上静脉切除重建术。联合腹腔干切除因术中切断了腹腔神经丛和神经节,因此具有长期的阵痛效果,减轻了患者的癌痛,提高患者生活治疗。有关文献报道 [23] [24],DP-CAR联合新辅助疗法安全有益的治疗方式,术前新辅助一定程度清除了隐匿性的微转移病灶 [25]。DP-CAR术式是扩大切除范围从而达到完全切除的目的,术前再以海扶刀和TS方案联合治疗,提高侵犯腹腔干的胰体尾癌患者的生活质量、生存期,使此类患者获得最大的益处。
文章引用
仲灏辰,赵 伟,曹广华,李浩然,李学良,郭敬允. 海扶刀联合化疗后DP-CAR术式切除胰腺癌一例
A Case of Pancreatic Cancer Treated with DP Car after Combined Chemotherapy with HIFU[J]. 临床医学进展, 2021, 11(06): 2533-2538. https://doi.org/10.12677/ACM.2021.116364
参考文献
- 1. Barman, S., Fatima, I., Singh, A.B., et al. (2021) Pancreatic Cancer and Therapy: Role and Regulation of Cancer Stem Cells. International Journal of Molecular Sciences, 22, 4765. https://doi.org/10.3390/ijms22094765
- 2. Luu, T. (2021) Epithelial-Mesenchymal Transition and Its Regulation Mechanisms in Pancreatic Cancer. Frontiers in Oncology, 11, Article ID: 646399. https://doi.org/10.3389/fonc.2021.646399
- 3. Siegel, R.L., Miller, K.D. and Jemal, A. (2017) Cancer Statistics, 2017. CA: A Cancer Journal for Clinicians, 67, 7-30. https://doi.org/10.3322/caac.21387
- 4. Lopez, N.E., Prendergast, C. and Lowy, A.M. (2014) Borderline Resectable Pancreatic Cancer: Definitions and Management. World Journal of Gastroenterology: WJG, 20, 10740-10751. https://doi.org/10.3748/wjg.v20.i31.10740
- 5. Ortiz, R., Quiñonero, F., Garcia-Pinel, B., et al. (2021) Nanomedicine to Overcome Multidrug Resistance Mechanisms in Colon and Pancreatic Cancer: Recent Progress. Cancers (Basel), 13, 2058. https://doi.org/10.3390/cancers13092058
- 6. Inoue, Y., Oba, A., Ono, Y., et al. (2021) Radical Resection for Locally Advanced Pancreatic Cancers in the Era of New Neoadjuvant Therapy-Arterial Resection, Arterial Divestment and Total Pancreatectomy. Cancers (Basel), 13, 1818. https://doi.org/10.3390/cancers13081818
- 7. Rai, Z.L., Feakins, R., Pallett, L.J., et al. (2021) Irreversible Electroporation (IRE) in Locally Advanced Pancreatic Cancer: A Review of Current Clinical Outcomes, Mechanism of Action and Opportunities for Synergistic Therapy. Journal of Clinical Medicine, 10, 1609. https://doi.org/10.3390/jcm10081609
- 8. Kutschat, A., Johnsen, S.A. and Hamdan, F.H. (2021) Store-Operated Calcium Entry: Shaping the Transcriptional and Epigenetic Landscape in Pancreatic Cancer. Cells, 10, 966. https://doi.org/10.3390/cells10050966
- 9. Arias-Pinilla, G.A. and Modjtahedi, H. (2021) Therapeutic Application of Monoclonal Antibodies in Pancreatic Cancer: Advances, Challenges and Future Opportunities. Cancers (Basel), 13, 1781. https://doi.org/10.3390/cancers13081781
- 10. Elsayed, M. and Abdelrahim, M. (2021) The Latest Advancement in Pancreatic Ductal Adenocarcinoma Therapy: A Review Article for the Latest Guidelines and Novel Therapies. Biomedicines, 9, 389. https://doi.org/10.3390/biomedicines9040389
- 11. 中华人民共和国国家卫生健康委员会. 胰腺癌诊疗规范(2018年版) [J]. 临床肝胆病杂志, 2019, 35(2): 281-293.
- 12. Russo, S. and Saif, M.W. (2016) 2016 Gastrointestinal Cancers Symposium: Update on Pancreatic Cancer. Annals of Gastroenterology Quarterly Publication of the Hellenic Society of Gastroenterology, 29, 238-240. https://doi.org/10.20524/aog.2016.0024
- 13. 徐杰. 高强度聚焦超声治疗胰腺癌镇痛疗效体会[J]. 基层医学论坛, 2009(34): 108.
- 14. Hoy, S.M. (2014) Albumin-Bound Paclitaxel: A Review of Its Use for the First-Line Combination Treatment of Metastatic Pancreatic Cancer. Drugs, 74, 1757-1768. https://doi.org/10.1007/s40265-014-0291-8
- 15. 廖小莉, 廖思娜, 李永强, 黄钰晶, 刘志辉, 黎倩, 黄仕新, 韦军葆. 替吉奥单药治疗老年转移性胰腺癌的疗效观察[J]. 广西医科大学学报, 2018, 35(2): 234-237.
- 16. 刘艳杰, 陆翰杰. 白蛋白结合紫杉醇联合替吉奥治疗晚期胰腺癌的疗效及安全性观察[J]. 中国临床新医学, 2019, 12(9): 997-1000.
- 17. 彭小波, 颜芳, 王斌, 傅强. 白蛋白结合型紫杉醇联合替吉奥治疗吉西他滨治疗失败进展期胰腺癌的临床观察[J]. 中国癌症杂志, 2015, 25(1): 63-66.
- 18. 刘甜, 熊琦, 胡毅. 白蛋白结合型紫杉醇联合替吉奥一线治疗老年晚期胰腺癌患者的临床观察[J]. 现代肿瘤医学, 2018(5): 722-725.
- 19. 庄兴俊, 欧娟娟, 高云姝, 高百春, 顾军. 高强度聚焦超声联合放疗治疗35例胰腺癌的临床分析[J]. 重庆医学, 2010, 39(3): 283-284.
- 20. Hess, K.R., Varadhachary, G.R., Taylor, S.H., Wei, W., Raber, M.N., Lenzi, R. and Abbruzzese, J.L. (2006) Metastatic Patterns in Adenocarcinoma. Cancer, 106, 1624-1633. https://doi.org/10.1002/cncr.21778
- 21. Ammori, J.B., Colletti, L.M., Zalupski, M.M., Eckhauser, F.E., Greenson, J.K., Dimick, J., Lawrence, T.S. and McGinn, C.J. (2003) Surgical Resection Following Radiation Therapy with Concurrent Gemcitabine in Patients with Previously Unresectable Adenocarcinoma of the Pancreas. Journal of Gastrointestinal Surgery, 7, 766-772. https://doi.org/10.1016/S1091-255X(03)00113-6
- 22. Evans, D.B., Wolff, R.A. and Crane, C.H. (2001) Neoadjuvant Strategies for Pancreatic Cancer.
- 23. Yoshiya, S., Fukuzawa, K., Inokuchi, S., Kosai-Fujimoto, Y., Sanefuji, K., Iwaki, K., Motohiro, A., Itoh, S., Harada, N. and Ikegami, T. (2019) Efficacy of Neoadjuvant Chemotherapy in Distal Pancreatectomy with En Bloc Celiac Axis Resection (DP-CAR) for Locally Advanced Pancreatic Cancer. Journal of Gastrointestinal Surgery, 24, 1605-1611. https://doi.org/10.1007/s11605-019-04324-8
- 24. Powell-Brett, S., Pande, R. and Roberts, K.J. (2021) Achieving “Marginal Gains” to Optimise Outcomes in Resectable Pancreatic Cancer. Cancers (Basel), 13, 1669. https://doi.org/10.3390/cancers13071669
- 25. Ocuin, L.M., Miller-Ocuin, J.L., Novak, S.M., Bartlett, D.L., Marsh, J.W., Tsung, A., Lee, K.K., Hogg, M.E., Zeh, H.J. and Zureikat, A.H. (2016) Robotic and Open Distal Pancreatectomy with Celiac Axis Resection for Locally Advanced Pancreatic Body Tumors: A Single Institutional Assessment of Perioperative Outcomes and Survival. HPB, 18, 835-842. https://doi.org/10.1016/j.hpb.2016.05.003
NOTES
*通讯作者。