Journal of Organic Chemistry Research
Vol.
07
No.
02
(
2019
), Article ID:
31030
,
6
pages
10.12677/JOCR.2019.72012
Recent Advances in Cycloaddition Reactions of Azaoxyallyl Cations
Li Sun, Wenhai Li*
School of Science, China Pharmaceutical University, Nanjing Jiangsu
Received: June 5th, 2019; accepted: June 20th, 2019; published: June 27th, 2019

ABSTRACT
The cycloaddition reactions of azaoxyallyl cations as 1,2- or 1,3-dipole formed in situ from α-halo hydroxamates under alkaline action, which has recently become a hot topic in the field of chemical synthesis due to their mildness, low cost and simple operation. This paper reviews the recent advances of the cycloaddition reaction of azaoxyallyl cations.
Keywords:Azaoxyallyl Cations, Cycloaddition, Synthesis
氮氧烯丙基阳离子环加成反应的最新进展
孙丽,黎文海*
中国药科大学理学院,江苏 南京
收稿日期:2019年6月5日;录用日期:2019年6月20日;发布日期:2019年6月27日

摘 要
碱性作用下由α-卤代羟肟酸酯原位形成的氮氧烯丙基阳离子作为1,2-或1,3-偶极子参与的环加成反应,条件温和,成本低廉,操作简单,已成为近年来化学合成研究领域的一大热点。本文综述了其环加成反应领域的最新进展。
关键词 :氮氧烯丙基阳离子,环加成,合成

Copyright © 2019 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
杂环化合物在医药、农药、材料和精细化工品等许多领域发挥着重要作用 [1] [2] [3] 。近年来,α-卤代羟肟酸酯,一类容易获得且稳定的氮氧烯丙基阳离子的前体,已被广泛开发作为合成杂环的关键底物。2011年,Jeffrey课题组率先发现环状双烯与α-卤代羟肟酸酯的[3 + 4]-环加成反应以良好的收率和非对映选择性生成了各种双环内酰胺衍生物,并实现了氮氧烯丙基阳离子中间体的实验捕获 [4] 。由于这一开创性的工作,随后,一系列涉及氮氧烯丙基阳离子的[2 + 4]-,[3 + 1]-,[3 + 2]-和[3 + 3]-的环加成反应被开发出来。本文在宣俊教授 [5] 对原位形成的氮氧烯丙基阳离子部分总结的基础上,重点介绍了近两年新的涉及氮氧烯丙基阳离子的[2 + m]-和[3 + m]-的环加成反应。
2. 氮氧烯丙基阳离子的[2 + m]-环加成反应
[2 + 4]-环加成反应
2018年,张健课题组实现了原位形成的氮氧烯丙基阳离子在Cs2CO3介导下与由N-(2-氯甲基)芳基酰胺原位消除产生的氮杂邻亚甲基苯醌(aza-o-QMs)的[2 + 4]-环加成反应(图1) [6] 。机理显示:1和2分别原位形成氮氧烯丙基阳离子和氮杂邻亚甲基苯醌中间体,然后,位于氮氧烯丙基阳离子氧原子γ位置的氢通过质子转移生成中间体3,随后与氮杂邻亚甲基苯醌中间体环合产生最终产物4。

Figure 1. [2 + 4]-Cycloaddition of azaoxyallyl cations with aza-o-QMs
图1. 氮氧杂烯丙基阳离子与氮杂邻亚甲基苯醌的[2 + 4]-环加成反应
3. 氮氧烯丙基阳离子的[3 + m]-环加成反应
3.1. [3 + 2]-环加成反应
2018年,孙江涛教授开发了氮氧烯丙基阳离子与六氢-1, 3, 5-三嗪的[3 + 2]-环加成反应(图2) [7] 。在温和条件下,不需要过渡金属并以中等至优异的产率一步完成了咪唑烷-4-酮骨架的构建。同年,Ocal等人也报道了氮氧烯丙基阳离子与(E)-N-亚芳基苯胺以良好的收率和区域选择性获得咪唑烷-4-酮的类似合成方法(图2) [8] 。
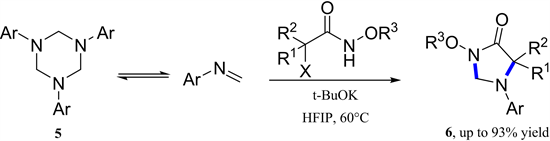
Figure 2. [3 + 2]-Cycloaddition of azaoxyallyl cations with hexahydro-1,3,5-triazi
图2. 氮氧杂烯丙基阳离子与六氢-1,3,5-三嗪的[3 + 2]-环加成反应
Singh小组报道了一种无金属和氧化剂参与的氮氧烯丙基阳离子与取代的2-(三甲基甲硅烷基)芳基三氟乙酸酯合成N-烷氧基羟吲哚的[3 + 2]-环加成反应(图3) [9] 。通过有价值的合成转化证明了这种方法的多功能性。
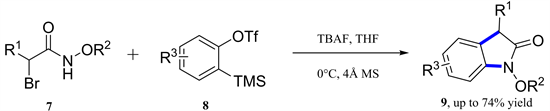
Figure 3. [3 + 2]-Cycloaddition of azaoxyallyl cations with arynes
图3. 氮氧杂烯丙基阳离子与芳烃的[3 + 2]-环加成反应
多取代吡咯烷酮是广泛存在于天然产物和生物活性分子中的结构片段 [10] [11] 。2018年,黄国生教授等报道了氮氧烯丙基阳离子和芳香族乙烯的[3 + 2]-环加成反应,以中等至良好的产率和非对映选择性生成了一系列多取代的吡咯烷酮(图4) [12] 。
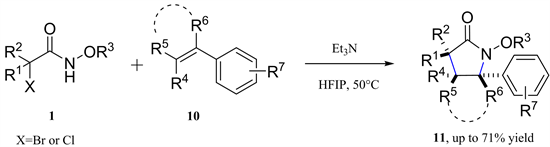
Figure 4. [3 + 2]-Cycloaddition of azaoxyallyl cations with aromatic ethylenes
图4. 氮氧杂烯丙基阳离子与芳香族乙烯的[3 + 2]-环加成反应
同年,汪舰教授等建立了α-卤代羟肟酸酯与异氰酸酯的[3 + 2]-环加成反应(图5) [13] 。捕获的中间体表明原位产生的两种离子在促进合成中起重要作用。该方案允许从简单且易获得的起始原料中快速组装乙内酰脲。
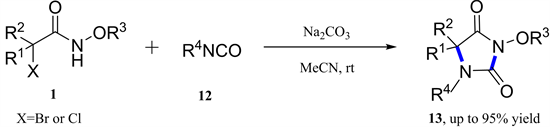
Figure 5. [3 + 2]-Cycloaddition of azaoxyallyl cations with isocyanates
图5. 氮氧杂烯丙基阳离子与异氰酸酯的[3 + 2]-环加成反应
3.2. [3 + 3]-环加成反应
由于氮氧烯丙基阳离子环加成的反应特性,2018年,我校姚和权教授等报道了其与叠氮化物的[3 + 3]-环加成反应(图6) [14] 。提供了合成1,2,3,4-四嗪的有效和实用途径。随后,孙绍发教授等通过氮氧烯丙基阳离子与氧化腈的[3 + 3]-环加成反应制备了一系列1,2,4-恶二嗪-5-酮类化合物(图7) [15] 。

Figure 6. [3 + 3]-Cycloaddition of azaoxyallyl cations with azides
图6. 氮氧杂烯丙基阳离子与叠氮化物的[3 + 3]-环加成反应
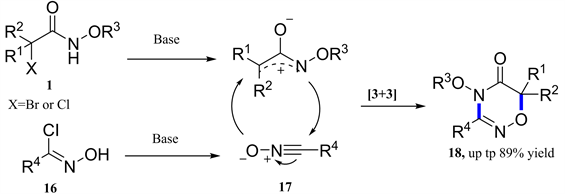
Figure 7. [3 + 3]-Cycloaddition of azaoxyallyl cations with nitrile oxides
图7. 氮氧杂烯丙基阳离子与氧化腈的[3 + 3]-环加成反应
哌嗪酮代表一类重要的氮杂环化合物 [16] [17] ,2018年,Gandon等成功报道了2-呋喃甲醇,取代苯胺和α-卤代羟肟酸酯之间的[3 + 3]-环加成反应,为构建环戊二烯[b]哌嗪酮衍生物开辟了一条新途径(图8) [18] 。该反应通过氮杂-Piancatelli环化和氮氧烯丙基阳离子的捕获完成转化,并实现了高的非对映选择性。

Figure 8. [3 + 3]-Cycloaddition of azaoxyallyl cations with 2-furylcarbinols and anilines
图8. 氮氧杂烯丙基阳离子与2-呋喃基甲醇和苯胺的[3 + 3]-环加成反应
同年,王刚等人发明了一种α-卤代羟肟酸酯与巯基乙醛或其多聚体经过环合-脱水两步反应一锅法制备2H-1,4-噻嗪-3(4H)-酮的类似[3 + 3]-环加成反应(图9) [19] ,收率良好。为1,4-噻嗪-3-酮类化合物以及含有该结构的药物合成提供了一种新方法。

Figure 9. Similar [3 + 3]-Cycloaddition of azaoxyallyl cations with mercaptoacetaldehyde or its multimer
图9. 氮氧杂烯丙基阳离子与巯基乙醛或其多聚体的类似[3 + 3]-环加成反应
2019年,宣俊教授报道了碱和卤素介导的氮氧烯丙基阳离子与C,N-环甲亚胺的[3 + 3]-环加成反应,该方法可以在无过渡金属催化的条件下获得各种具有C-Y键的异喹啉-稠合三嗪衍生物(图10) [20] 。

Figure 10. Similar [3 + 3]-Cycloaddition of azaoxyallyl cations with C, N-cyclic azomethine imines
图10. 氮氧杂烯丙基阳离子与C,N-环甲亚胺的[3 + 3]-环加成反应
4. 结语
在此,我们总结了利用原位形成的氮氧烯丙基阳离子参与环加成反应的最新进展。这些环加成方法不仅可以高效快捷地构建各种含氮杂环,而且为某些天然产物或活性化合物的合成提供了新的思路,显示了氮氧烯丙基阳离子中间体在有机合成和药物发现以及材料科学中的巨大潜力,将进一步引起广大科研工作者的注意。
文章引用
孙丽,黎文海. 氮氧烯丙基阳离子环加成反应的最新进展
Recent Advances in Cycloaddition Reactions of Azaoxyallyl Cations[J]. 有机化学研究, 2019, 07(02): 88-93. https://doi.org/10.12677/JOCR.2019.72012
参考文献
- 1. 陶绍木, 张建华, 彭昌亚, 等. 杂环化合物的应用和发展[J]. 中国食品添加剂, 2003(3): 31-34.
- 2. Kumari, J. (2018) Application of Heterocyclic Compounds in Everyday Life. Journal of Modern Chemistry & Chemical Technology, 9, 1-7.
- 3. Saleh, S.S., Al-Salihi, S.S. and Mohammed, I.A. (2019) Biological Activity Study for Some Heterocyclic Compounds and Their Impact on the Gram Positive and Negative Bacteria. Energy Procedia, 157, 296-306. https://doi.org/10.1016/j.egypro.2018.11.194
- 4. Jeffrey, C.S., Barnes, K.L., Eickhoff, J.A. and Carson, C.R. (2011) Generation and Reactivity of Aza-Oxyallyl Cationic Intermediates: Aza-[4 + 3] Cycloaddition Reactions for Heterocycle Synthesis. Journal of the American Chemical Society, 133, 7688-7691. https://doi.org/10.1021/ja201901d
- 5. Xuan, J., Cao, X. and Cheng, X. (2018) Advances in Heterocycle Synthesis via [3 + m]-cycloaddition Reactions Involving an Azaoxyallyl Cation as the Key Intermediate. Chemical Communications, 54, 5154-5163. https://doi.org/10.1039/C8CC00787J
- 6. Jin, Q.-M., Gao, M., Zhang, D.-J., Jiang, C.-H., Yao, N. and Zhang, J. (2018) Base-Mediated [2 + 4] Cycloadditions of in Situ Formed Azaoxyallyl Cations with N-(2-chloromethyl) Aryl Amides. Organic & Biomolecular Chemistry, 16, 7336-7339. https://doi.org/10.1039/C8OB02176G
- 7. Ji, D.-Q. and Sun, J.-T. (2018) [3 + 2]-Cycloaddition of Azaoxyallyl Cations with Hexahydro-1, 3, 5-triazines: Access to 4-Imidazolidinones. Organic Letters, 20, 2745-2748. https://doi.org/10.1021/acs.orglett.8b00951
- 8. Eyilcim, O., Issever, S., Ocal, N., Gronert, S. and Erden, I. (2018) Imidazolidin-4-Ones via (3 + 2) Cycloadditions of Aza-Oxyallyl Cations onto (E)-N-arylideneanilines. Tetrahedron Letters, 59, 3674-3677. https://doi.org/10.1016/j.tetlet.2018.08.056
- 9. Singh, R., Nagesh, K., Yugandhar, D. and Prasanthi, A.V.G. (2018) Metal- and Oxidant-Free Modular Approach to Access N-Alkoxy Oxindoles via Aryne Annulation. Organic Letters, 20, 4848-4853. https://doi.org/10.1021/acs.orglett.8b01972
- 10. Li, J., Liu, S.-C., Niu, S.-B., Zhuang, W.-Y. and Che, Y.-S. (2009) Pyrrolidinones from the Ascomycete Fungus Albonectria rigidiuscula. Journal of Natural Products, 72, 2184-2187. https://doi.org/10.1021/np900619z
- 11. Ding, H., Wang, J.-N., Zhang, D.-S. and Ma, Z.-J. (2017) Derivatives of Holomycin and Cyclopropaneacetic Acid from Streptomyces sp. DT-A37. Chemistry & Biodiversity, 14, e1700140. https://doi.org/10.1002/cbdv.201700140
- 12. Zhang, Y.-X., Ma, H.-J., Liu, X.-X., Cui, X.-F., Wang, S.-H., Zhan, Z.-Z., Pu, J.-H. and Huang, G.-S. (2018) The Synthesis of Multi-Substituted Pyrrolidinones via a Direct [3 + 2] Cycloaddition of Azaoxyallyl Cations with Aromatic Ethylenes. Organic & Biomolecular Chemistry, 16, 4439-4442. https://doi.org/10.1039/C8OB00899J
- 13. Sun, S.-F., Chen, R.-X., Wang, G.-Q. and Wang, J. (2018) Sodium Carbonate Promoted [3 + 2] Annulation of Alpha-Halohydroxamates and Isocyanates. Organic & Biomolecular Chemistry, 16, 8011-8014. https://doi.org/10.1039/C8OB02321B
- 14. Xu, X.-Y., Zhang, K.-F., Li, P.-P., Yao, H.-Q. and Lin, A.-J. (2018) [3 + 3] Cycloaddition of Azides with in Situ Formed Azaoxyallyl Cations to Synthesize 1, 2, 3, 4-Tetrazines. Organic Letters, 20, 1781-1784. https://doi.org/10.1021/acs.orglett.8b00280
- 15. Wang, G.-Q., Chen, R.-X., Zhao, S., Yang, L.-F., Guo, H.-B., Sun, S.-F., Wang, J., Domena, J. and Xing, Y.-L. (2018) Efficient Synthesis of 1, 2, 4-Oxadiazine-5-Ones via [3 + 3] Cycloaddition of in Situ Generated Aza-Oxyallylic Cations with Nitrile Oxides. Tetrahedron Letters, 59, 2018-2020. https://doi.org/10.1016/j.tetlet.2018.04.025
- 16. Bell, I.M., Gallicchio, S.N., Wood, M.R., Quigley, A.G., Stump, C.A., Zartman, C.B., Fay, J.F., Li, C.C., Lynch, J.J., Moore, E.L., Mosser, S.D., Prueksaritanont, T., Regan, C.P., Roller, S., Salvatore, C.A., Kane, S.A., Vacca, J.P. and Selnick, H.G. (2018) Discovery of MK-3207: A Highly Potent, Orally Bioavailable CGRP Receptor Antagonist. ACS Medicinal Chemistry Letters, 1, 24-29. https://doi.org/10.1021/ml900016y
- 17. Kakarla, R., Liu, J., Naduthambi, D., Chang, W., Mosley, R.T., Bao, D., Steuer, H.M., Keilman, M., Bansal, S., Lam, A.M., Seibel, W., Neilson, S., Furman, P.A. and Sofia, M.J. (2014) Dis-covery of a Novel Class of Potent HCV NS4B Inhibitors: SAR Studies on Piperazinone Derivatives. Journal of Me-dicinal Chemistry, 57, 2136-2160. https://doi.org/10.1021/jm4012643
- 18. Baldé, B., Force, G., Marin, L., Schulz, E., Gandon, V. and Lebœuf, D. (2018) Synthesis of Cyclopeta[b]piperazinones via an Azaoxyallyl Cation. Organic Letters, 20, 7405-7409. https://doi.org/10.1021/acs.orglett.8b03103
- 19. 王刚, 何照林, 陈一, 等. 一种2H-1,4-噻嗪-3(4H)-酮衍生物的合成方法[P]. 中国专利, 201810616552.3. 2018-10-02.
- 20. Cheng, X., Cao, X., Zhou, S.-J., Cai, B.-G., He, X.-K. and Xuan, J. (2018) Transition-Metal Free Construction of Isoquinoline-Fused Triazines Containing Alkenyl C-X Bonds. Advanced Synthesis & Catalysis, 361, 1230-1235. https://doi.org/10.1002/adsc.201801181
NOTES
*通讯作者