Advances in Clinical Medicine
Vol.
13
No.
10
(
2023
), Article ID:
73463
,
8
pages
10.12677/ACM.2023.13102190
长期补充低剂量褪黑素对大鼠心跳骤停后 线粒体功能的保护作用
胡亚楠,刘毅,徐龙河*
中国人民解放军总医院第三医学中心麻醉科,北京
收稿日期:2023年9月6日;录用日期:2023年10月1日;发布日期:2023年10月10日

摘要
目的:通过长期服用保健药物来预防心跳骤停诱发的死亡是错综复杂且较少探索的复苏策略。褪黑激素是一种改善睡眠的保健品,有研究报道其在冬眠动物的唤醒期不断升高,在血液回流后可以提高存活率,并减少再灌注损伤。本研究旨在探索每日预防性补充保健药物褪黑激素对减少心跳骤停危害的影响。方法:研究对象为SD雄性大鼠,240~340克。共分为3组:对照组(Sham组);心跳骤停组(CA组);和药物预处理组(Mel组)。Mel组,大鼠每天接受腹腔注射10 mg/kg的褪黑素,持续14天。8分钟窒息诱导心跳骤停后进行手动心肺复苏。复苏后24小时进行神经学评分,取海马组织观测线粒体的完整性、动力学改变和功能损伤。结果:Mel组的存活率高于CA组(38.89% vs. 31.81%, P = 0.641)。ΔΨm在CA组、Mel组低于Sham组(P < 0.05)。ROS、线粒体肿胀和线粒体呼吸速率在CA组低于Sham组(P < 0.05)。ΔΨm、线粒体肿胀、线粒体呼吸速率在Mel组高于CA组(P > 0.05)。ROS在Mel组低于CA组(P = 0.535)。结论:心跳骤停复苏后加重了线粒体损伤。长期补充低剂量褪黑素改善了大鼠心跳骤停后线粒体功能。
关键词
心肺复苏,心跳骤停,褪黑素,缺血–再灌注,线粒体

Protective Effect of Long-Term Low-Dose Melatonin Supplementation on Mitochondrial Function after Cardiac Arrest in Rats
Yanan Hu, Yi Liu, Longhe Xu*
Department of Anesthesiology, The Third Medical Center of Chinese PLA General Hospital, Beijing
Received: Sep. 6th, 2023; accepted: Oct. 1st, 2023; published: Oct. 10th, 2023

ABSTRACT
Objective: Prophylactic pharmacotherapy for health care in reducing death after cardiac arrest (CA) is an elusive and less explored strategy. Melatonin, a natural health product contributing to sleep, was elevated upon arousal from torpor in hibernating mammals, which increased survival after blood return and reduced reperfusion injury. In this study, we sought to find the effects of long-term daily prophylactic supplement with melatonin as a health care medicine on the victim of CA. Methods: SD rats, 240~340 g, were used in this study. Sham, CA, and melatonin + CA (Mel) groups were included. The rats in the Mel group received daily IP injection of melatonin 10 mg/kg for 14 days. CA was induced by 8 mins asphyxia and followed by manual CPR. The endpoint was 24 h after resuscitation. Neurological outcome was assessed. Hippocampal mitochondrial function was observed. Results: Survival in the Mel group was higher than in the CA group (38.89% vs. 31.81%, P = 0.641). ΔΨm was lower in the CA group and Mel group than in the Sham group (P < 0.05). ΔΨm, mitochondrial swelling and mitochondria respiratory rate were higher in the Mel group than in the CA group (P > 0.05). ROS production was lower in the Mel group (P = 0.535). Conclusions: CA induced mitochondrial injury. Long-term daily prophylactic supplement with low dosage of melatonin improved neuronal mitochondrial function after CA.
Keywords:CPR, Cardiac Arrest, Melatonin, Ischemia-Reperfusion, Mitochondria

Copyright © 2023 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
心跳骤停后缺血再灌注(IR)损伤是致命的,且具有严格的时间依赖性 [1] 。长期预防性药物治疗以扩大内源性脑修复机制,在CA期间诱导及时的神经保护从而减少死亡是一个难以捉摸和较少探索的策略。
基于各种实验模型证明的褪黑素的有益作用,目前已被用于治疗各种疾病,包含从神经退行性疾病到中风、IR和恶性肿瘤等 [2] [3] 。大量基础研究表明褪黑素还是基于冬眠疗法的重要组成部分,可用于深度失血和失血性休克 [4] 。在一种基于冬眠哺乳动物的全容量治疗方法中,大鼠失血60%构建休克模型,在失血1小时后在小体积复苏液中加入神经保护酮β-羟丁酸和褪黑素的混合物,与对照组相比显著增加了大鼠的远期存活时间长达10天 [5] 。另一项同类研究中,β-羟丁酸和褪黑素复苏液不仅增加了失血性休克大鼠的存活率,与对照组相比,动物碱基赤字较低,耗氧量较高,血清代谢物谱显示,β-羟基丁酸(BHB)、琥珀酸、2-氧戊酸和己二酸增加,这种代谢改变更利于复苏后生存 [6] 。研究表明,在缺血或再灌注之前,褪黑素可显著降低可逆或不可逆的室颤发生率,并使其恢复到正常水平 [7] 。此外,作为治疗性体温过低的辅助药物,褪黑素通过减少低氧缺血后脑灰质和白质物质中的细胞死亡来增强低温保护 [8] 。因此,我们推测,长期服用褪黑素可能会加强其诱导的抗氧化能力和耐受缺血缺氧的能力,从而在CA发生时像冬眠唤醒期自发的神经功能保护作用,预防缺血再灌注损伤,降低CA死亡率。
2. 材料与方法
2.1. 实验动物
47只大鼠总共分为3组:对照组(Sham, n = 7);心跳骤停组(CA, n = 18);褪黑素预处理大鼠心跳骤停组(Mel, n = 22)。褪黑素溶解于终浓度为2%的DMSO中。Mel组大鼠每天上午10点左右接受腹腔注射褪黑素10 mg/kg,持续14天。CA组大鼠在同一时间接受腹腔注射同样浓度同样体积的DMSO。
2.2. 实验方案
实验流程图如图1所示。在第15天,所有实验大鼠在半密封的容器内进行预充氧2 min,用6%的七氟烷进行麻醉诱导5 min。大鼠气管内插管成功后顺势连接呼吸机进行机械通气,呼吸频率设为40次/min,I:E = 1:1,FiO2 = 0.5,PEEP = 5 cmH2O。潮气量大小设定为8~12 mL/kg,并在通气期间适当调节以维持呼吸末二氧化碳分压在35~45 mmHg之间。以氧气和1.5%~2.0%浓度的七氟烷混合气体进行麻醉维持。通过加热毯加热使各组间大鼠的鼓膜温度及直肠温度在37 ± 0.5℃范围之间。用肝素化后的穿刺针进行动静脉穿刺并固定,并开始记录平均动脉压(Mean arterial pressure, MAP)和心率(Heart rate, HR)。窒息前5 min静脉给予2 mg/kg的顺式阿曲库铵,然后停用七氟烷,关闭呼吸机,夹闭气管导管,记录窒息开始时间。MAP低于20 mmHg定义为大鼠CA。大鼠窒息期间体温持续维持在37 ± 0.5℃之间。窒息发生8 min后静脉给予0.01 mg/kg的肾上腺素,同时开始进行手动CPR,按压频率保持在200~300次/min,并同时连接呼吸机吸入纯氧。如果胸外按压3 min后未恢复自主循环,则再给予一次0.01 mg/kg肾上腺素,超过5 min仍未恢复自主循环,则视为复苏失败。自主循环恢复定义为MAP > 50 mmHg,所有实验动物在复苏成功后均进行机械通气1 h,并维持MAP在50 mmHg以上,如果MAP < 50 mmHg,静注1 µg的肾上腺素。通气1 h后撤掉呼吸机,在ICU中观察直至24 h。
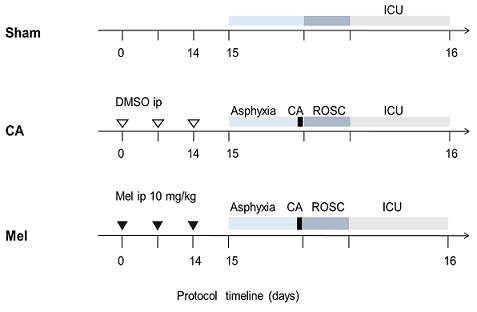
Figure 1. The experimental protocol
图1. 实验流程图
2.3. 神经功能评估
复苏后存活24 h的大鼠进行改良的神经功能损伤评分(NDS;0 = 正常,500 = 脑死亡)和整体功能评分(OPC;1 = 正常,2 = 轻度功能不良,3 = 保持清醒但严重功能不良,4 = 昏迷,5 = 死亡)。
2.4. 组织制备
每组6只大鼠在复苏后24 h断头取脑,按照组织线粒体分离试剂盒说明书进行线粒体分离提取以测定相关功能。用不含钙的PBS溶液经将海马组织洗涤一次,放置在预冷的1.5 mL离心管中,打碎,加入预冷的线粒体分离试剂,充分匀浆。将匀浆液移入一个新的离心管内,4℃、1000 g、离心5 min。第一次离心后把上清液析出,4℃、3500 g、再次离心10 min。第二次离心后,丢弃上清液,留下的沉淀即为分离得到的高纯度线粒体。
2.5. 线粒体ΔΨm测定
使用JC-1线粒体膜电位检测试剂盒对ΔΨm进行监测。JC-1聚合体(红色)与JC-1单体(绿色)的比率确定ΔΨm值。线粒体去极化表现为红/绿荧光强度比的降低。
2.6. 线粒体ROS测定
线粒体ROS使用ROS测定试剂盒进行测量。在37℃下,每个样品将反应试剂与等量的线粒体(50 μg/50μL)避光混合孵育15分钟。所有步骤都是在黑暗中完成的。使用荧光显微镜观察,接收光和发射光分别设定为490和530 nm,样品孔与对照孔的吸光度差值为ROS的产生量。
2.7. 线粒体呼吸功能测定
在25℃时,线粒体氧消耗速率通过使用Clack型氧电极测量,线粒体浓度均为1 mg/mL。添加线粒体后测得基线氧耗速率(OCR),曲线稳定后记录数据。State IV呼吸由20 μL钠琥珀酸钠(4 mM)启动。然后将20 μL丙二磷酸腺苷(ADP, 50 mM)添加到培养基中,以启动State III呼吸。
2.8. 数据分析
所有数据均以均值 ± 标准差的形式表示。使用SPSS 13.0软件进行了统计分析。生理参数指标通过One-way ANOVA和Student-Newman-Keuls检验进行分析评估。血流动力学指标通过重复测量方差分析和Bonferroni检验进行评估。卡方检验用于测试组间的OPC评分(OPC 1~2 vs. OPC 3~5)和生存率分析。Kruskal-Wallis检验用于比较各组之间的NDS。P < 0.05被认为是差异显著。
3. 结果
3.1. 基线参数和血流动力学
三组之间大鼠的体重、温度、心跳骤停前基线平均动脉压(MAP)、心率(HR)无差异。心跳骤停时间、心肺复苏时间、肾上腺素剂量在各组间无差异。
3.2. 复苏率及神经功能评分
如图2所示,复苏率在组间没有差异(CA = 50.0% VS Mel = 54.5%, P = 0.775)。Sham组24 h的存活率为100%。Mel组的24 h存活率高于CA组(38.89% VS 31.81%, P = 0.641)。CA组NDS评分和OPC评分低于Sham组(P < 0.05)。Mel组的NDS评分和OPC评分低于Sham组(P > 0.05)。Mel组的NDS评分和OPC评分高于CA组(P > 0.05)。
3.3. 线粒体功能
如图3所示,CA组和Mel组ΔΨm比Sham组显著减少(P < 0.001)。在Mel组中,ΔΨm高于CA组(P = 0.053)。
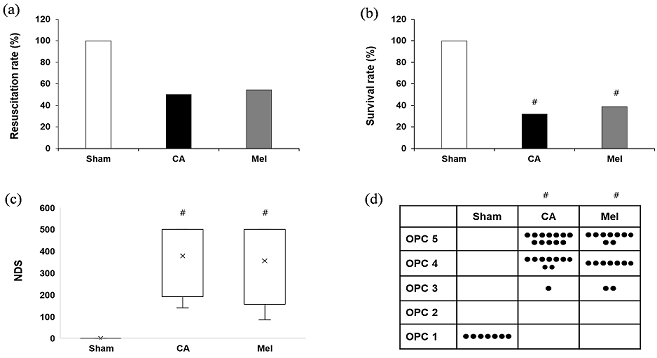
Figure 2. Resuscitation and neurological evaluation. (a) The resuscitation; (b) Survival at 24 h after resuscitation; (c) Neurological deficit score (NDS, 0~500) at 24 h after resuscitation; (d) Overall performance categories (OPC, 1~5) at 24 h after resuscitation
图2. 复苏率及神经功能评分。(a) 复苏率;(b) 24 h生存率;(c) 复苏后24 h神经功能损伤评分(NDS, 0~500);(d) 复苏后24 h的总体性能分类(OPC, 1~5)

Figure 3. Changes in mitochondrial function. (a) ΔΨm; (b) ROS; (c) Changes in mitochondria swelling; (d) The oxygen consumption rate (OCR)
图3. 线粒体功能改变。(a) ΔΨ;(b) ROS;(c) 线粒体肿胀改变;(d) 耗氧速率(OCR)
CA组线粒体ROS产生比Sham组显著增加(P = 0.016)。Mel组线粒体ROS产生比Sham组增加(P = 0.077)。线粒体ROS产生在Mel组比CA组减少,但无统计学差异(P = 0.535)。
CA组中线粒体肿胀程度明显低于Sham组(P = 0.043)。Mel组线粒体肿胀程度低于CA组(P = 0.089)。Mel组线粒体肿胀程度高于CA组(P = 0.107)。
CA组中OCR明显低于Sham组(P = 0.033)。Mel组OCR低于CA组(P = 0.101)。Mel组OCR高于CA组(P = 0.459)。
4. 讨论
本研究通过低氧诱导心跳骤停模型清楚地证明了长期预防性补充褪黑素在减轻神经损伤方面的功能作用。与以前的报告一致,这些发现可以追溯到对线粒体功能的调控。褪黑素合成的昼夜节律是由松果腺中交感神经末梢的去甲肾上腺素(NE)夜间释放所控制的 [8] 。NE的释放常常可使左室收缩功能和心输出量增加 [9] 。睡眠和呼吸的化学控制以两种方式相互作用。腹侧延髓包含中央呼吸化学感受器和交感兴奋的低氧反应性C1神经元,这些神经元的光遗传学刺激会产生剧烈的心肺刺激,进而从非快速眼动睡眠中唤醒,并伴随呼吸刺激和血压升高,褪黑素在其中发挥着不可或缺的作用。在本研究中复苏后平均动脉压在褪黑素处理组明显高于CA组,这可能是其24 h存活率较高的原因。此外,已知脑脊液中的褪黑素水平显著高于血清,进一步支持了褪黑素在大脑中的保护作用 [10] 。
选择褪黑素作为长期补充的药物,一方面是它作为保健品具安全性,褪黑素在人体生理学中具有广泛活性,它调节情绪、睡眠、体温、运动活动、食物摄入模式、昼夜节律和免疫调节等过程 [11] ,作为保健品其常用于改善睡眠质量、缓解时差感和减少入睡潜伏期 [12] ,预防与衰老和氧化应激有关的疾病,包括2型糖尿病,心血管和神经退行性疾病或癌症等 [13] 。褪黑素被FDA归类为膳食补充剂,剂量不受限制,且未发现不良反应。另外很多研究表明褪黑素是基于冬眠疗法的重要组成部分,可用于各种缺血再灌注损伤的保护 [4] 。冬眠是一个特殊的挑战身体物理极限的状态,其特点是代谢率、体温和生理功能的大幅度下降 [14] 。通过测定循环褪黑素增加的时机表明,在冬眠和日眠状态下褪黑素的释放伴随着唤醒,它可能在这种极端的生理过渡过程中起保护作用 [15] 。CA是全身的缺血缺氧,与冬眠期相似,在短时间内导致机体严重的体温过低状态,器官灌注不足,最终组织坏死,及时的心肺复苏(CPR)逆转了缺血损害,但由于缺血诱导的代谢改变加上突然重新引入正常水平的氧气和其他基质的再灌注条件,死亡常常发生在再灌注阶段。褪黑素与大脑感知、能量代谢、自噬及昼夜节律的相关分子信号息息相关,包括细胞凋亡/坏死、线粒体融合分裂和自噬等内在的信号通路 [16] 。利用冬眠苏醒期的适应机制 [17] ,预防性应用褪黑素对预防CA后再灌注损伤具有很大的潜力。
褪黑素靶向线粒体的特异性保护能力涉及其有效的抗氧化作用。褪黑素具有抑制线粒体细胞色素C释放和随后的半胱天冬酶活化的能力以及降低缺血后体内活性氧(ROS)水平,这些细胞过程均显示其抗氧化作用从而表现出很强的神经保护特性 [18] 。一篇研究证实褪黑素在线粒体基质中产生,由细胞器释放,并与高亲和力MT1位于线粒体外膜,其配体结合结构域面向细胞质,其信号转导装置位于膜间空间 [19] 。对褪黑素亚细胞分布的测量表明,线粒体中这种吲哚的浓度大大超过了血液中的浓度 [20] 。通过使用无创荧光探针结合激光扫描显微镜直观的观察到褪黑素的抗氧化保护作用发生在线粒体中 [21] 。应用小分子MT1受体抑制剂Luzindole部分阻止了褪黑素恢复线粒体功能的能力,这表明褪黑素受体在保护线粒体中起作用 [22] 。褪黑素是通过寡肽转运蛋白PEPT1/2进入线粒体的。研究表明,当病理性线粒体呼吸链泄漏电子增加时,褪黑素可以清除ROS,减少脂质应激诱导的花生四烯酸释放,抑制线粒体DNA (mtDNA)缺失和突变 [23] 。褪黑素的这种潜能在本实验中通过检测线粒体损伤产物及呼吸功能得到了验证。
本研究存在一定限制。第一,有研究表明褪黑素具有降低体温的作用,由于本实验中引用的心跳骤停模型将温度进行了统一控制,因此未能验证褪黑素是否通过低温的保护作用提高了复苏率和存活率。第二,褪黑素在人口服剂量的范围很广,本研究只针对小剂量褪黑素的药理效果进行了研究,未来可进一步对不同剂量褪黑素的作用进行探索。
文章引用
胡亚楠,刘 毅,徐龙河. 长期补充低剂量褪黑素对大鼠心跳骤停后线粒体功能的保护作用
Protective Effect of Long-Term Low-Dose Melatonin Supplementation on Mitochondrial Function after Cardiac Arrest in Rats[J]. 临床医学进展, 2023, 13(10): 15660-15667. https://doi.org/10.12677/ACM.2023.13102190
参考文献
- 1. Kragholm, K., Wissenberg, M., Mortensen, R.N., Hansen, S.M., Malta Hansen, C., Thorsteinsson, K., Rajan, S., Lippert, F., Folke, F., Gislason, G., Kober, L., Fonager, K., Jensen, S.E., Gerds, T.A., Torp-Pedersen, C. and Rasmussen, B.S. (2017) Bystander Efforts and 1-Year Outcomes in Out-of-Hospital Cardiac Arrest. The New England Journal of Medi-cine, 376, 1737-1747. https://doi.org/10.1056/NEJMoa1601891
- 2. Renn, T.Y., Huang, Y.K., Feng, S.W., Wang, H.W., Lee, W.F., Lin, C.T., Burnouf, T., Chen, L.Y., Kao, P.F. and Chang, H.M. (2018) Prophylactic Supplement with Melatonin Successfully Suppresses the Pathogenesis of Periodontitis through Normalizing RANKL/OPG Ratio and De-pressing the TLR4/MyD88 Signaling Pathway. Journal of Pineal Research, 64, e12464. https://doi.org/10.1111/jpi.12464
- 3. Tan, D.X., Manchester, L.C., Sainz, R.M., Mayo, J.C., Leon, J. and Reiter, R.J. (2005) Physiological Ischemia/Reperfusion Phenomena and Their Relation to Endogenous Melatonin Production: A Hypothesis. Endocrine, 27, 149-157. https://doi.org/10.1385/ENDO:27:2:149
- 4. Hsu, J.T., Kuo, C.J., Chen, T.H., Wang, F., Lin, C.J., Yeh, T.S., Hwang, T.L. and Jan, Y.Y. (2012) Melatonin Prevents Hemorrhagic Shock-Induced Liver Injury in Rats through an Akt-Dependent HO-1 Pathway. Journal of Pineal Research, 53, 410-416. https://doi.org/10.1111/j.1600-079X.2012.01011.x
- 5. Klein, A.H., Wendroth, S.M., Drewes, L.R. and Andrews, M.T. (2010) Small-Volume d-β-Hydroxybutyrate Solution Infusion Increases Survivability of Lethal Hemorrhagic Shock in Rats. Shock, 34, 565-572. https://doi.org/10.1097/SHK.0b013e3181e15063
- 6. Mulier, K.E., Lexcen, D.R., Luzcek, E., Greenberg, J.J. and Beilman, G.J. (2012) Treatment with β-Hydroxybutyrate and Melatonin Is Associated with Improved Survival in a Por-cine Model of Hemorrhagic Shock. Resuscitation, 83, 253-258. https://doi.org/10.1016/j.resuscitation.2011.08.003
- 7. Hadj-Moussa, H. and Storey, K.B. (2019) Bringing Nature Back: Using Hibernation to Reboot Organ Preservation. The FEBS Journal, 286, 1094-1100. https://doi.org/10.1111/febs.14683
- 8. Maronde, E. and Stehle, J.H. (2007) The Mammalian Pineal Gland: Known Facts, Unknown Facets. Trends in Endocrinology and Metabolism, 18, 142-149. https://doi.org/10.1016/j.tem.2007.03.001
- 9. Chonde, M., Flickinger, K.L., Sundermann, M.L., Koller, A.C., Salcido, D.D., Dezfulian, C., Menegazzi, J.J. and Elmer, J. (2019) Intra-Arrest Administration of Cyclosporine and Methylprednisolone Does Not Reduce Postarrest Myocardial Dysfunction. BioMed Research International, 2019, Article ID: 6539050. https://doi.org/10.1155/2019/6539050
- 10. Hardeland, R., Tan, D.X. and Reiter, R.J. (2009) Kynuramines, Metab-olites of Melatonin and Other Indoles: The Resurrection of an Almost Forgotten Class of Biogenic Amines. Journal of Pineal Research, 47, 109-126. https://doi.org/10.1111/j.1600-079X.2009.00701.x
- 11. Paredes, S.D., Marchena, A.M., Bejarano, I., Espino, J., Barriga, C., Rial, R.V., Reiter, R.J. and Rodriguez, A.B. (2009) Melatonin and Tryptophan Affect the Activity-Rest Rhythm, Core and Peripheral Temperatures, and Interleukin Levels in the Ringdove: Changes with Age. The Journals of Gerontology: Series A, 64A, 340-350. https://doi.org/10.1093/gerona/gln054
- 12. Jan, J.E., Reiter, R.J., Wasdell, M.B. and Bax, M. (2009) The Role of the Thalamus in Sleep, Pineal Melatonin Production, and Circadian Rhythm Sleep Disorders. Journal of Pineal Research, 46, 1-7. https://doi.org/10.1111/j.1600-079X.2008.00628.x
- 13. Witt-Enderby, P.A., Radio, N.M., Doctor, J.S. and Davis, V.L. (2006) Therapeutic Treatments Potentially Mediated by Melatonin Receptors: Potential Clinical Uses in the Preven-tion of Osteoporosis, Cancer and as an Adjuvant Therapy. Journal of Pineal Research, 41, 297-305. https://doi.org/10.1111/j.1600-079X.2006.00369.x
- 14. Ren, C., Li, S., Rajah, G., Shao, G., Lu, G., Han, R., Huang, Q., Li, H., Ding, Y., Jin, K. and Ji, X. (2018) Hypoxia, Hibernation and Neuroprotection: An Experimental Study in Mice. Aging and Disease, 9, 761-768. https://doi.org/10.14336/AD.2018.0702
- 15. Frerichs, K.U., Kennedy, C., Sokoloff, L. and Hallenbeck, J.M. (1994) Local Cerebral Blood Flow during Hibernation, a Model of Natural Tolerance to “Cerebral Ischemia”. Journal of Cere-bral Blood Flow and Metabolism, 14, 193-205. https://doi.org/10.1038/jcbfm.1994.26
- 16. Pevet, P. and Challet, E. (2011) Melatonin: Both Master Clock Output and Internal Time-Giver in the Circadian Clocks Network. Journal of physiology-Paris, 105, 170-182. https://doi.org/10.1016/j.jphysparis.2011.07.001
- 17. Patil, K.D., Halperin, H.R. and Becker, L.B. (2015) Cardiac Arrest: Resuscitation and Reperfusion. Circulation Research, 116, 2041-2049. https://doi.org/10.1161/CIRCRESAHA.116.304495
- 18. Reiter, R.J., Tan, D.X., Manchester, L.C. and El-Sawi, M.R. (2002) Melatonin Reduces Oxidant Damage and Promotes Mitochondrial Respiration: Implications for Aging. An-nals of the New York Academy of Sciences, 959, 238-250. https://doi.org/10.1111/j.1749-6632.2002.tb02096.x
- 19. Suofu, Y., Li, W., Jean-Alphonse, F.G., Jia, J., Khattar, N.K., Li, J., Baranov, S.V., Leronni, D., Mihalik, A.C., He, Y., Cecon, E., Wehbi, V.L., Kim, J., Heath, B.E., Baranova, O.V., Wang, X., Gable, M.J., Kretz, E.S., Di Benedetto, G., Lezon, T.R., Ferrando, L.M., Larkin, T.M., Sullivan, M., Yablonska, S., Wang, J., Minnigh, M.B., Guillaumet, G., Suzenet, F., Richardson, R.M., Poloyac, S.M., Stolz, D.B., Jockers, R., Witt-Enderby, P.A., Carlisle, D.L., Vilardaga, J.P. and Friedlander, R.M. (2017) Dual Role of Mitochondria in Producing Melatonin and Driving GPCR Signaling to Block Cytochrome c Release. Proceedings of the National Academy of Sciences of the United States of America, 114, E7997-E8006. https://doi.org/10.1073/pnas.1705768114
- 20. Slominski, R.M., Reiter, R.J., Schlabritz-Loutsevitch, N., Ostrom, R.S. and Slominski, A.T. (2012) Melatonin Membrane Receptors in Peripheral Tissues: Distribution and Functions. Mo-lecular and Cellular Endocrinology, 351, 152-166. https://doi.org/10.1016/j.mce.2012.01.004
- 21. Perez-Gonzalez, A., Castaneda-Arriaga, R., Alvarez-Idaboy, J.R., Reiter, R.J. and Galano, A. (2019) Melatonin and Its Metabolites as Chemical Agents Capable of Directly Repairing Oxidized DNA. Journal of Pineal Research, 66, e12539. https://doi.org/10.1111/jpi.12539
- 22. Wang, X., Sirianni, A., Pei, Z., Cormier, K., Smith, K., Jiang, J., Zhou, S., Wang, H., Zhao, R., Yano, H., Kim, J.E., Li, W., Kristal, B.S., Ferrante, R.J. and Friedlander, R.M. (2011) The Melato-nin MT1 Receptor Axis Modulates Mutant Huntingtin-Mediated Toxicity. The Journal of Neuroscience, 31, 14496-14507. https://doi.org/10.1523/JNEUROSCI.3059-11.2011
- 23. Zhong, J., Tan, Y., Lu, J., Liu, J., Xiao, X., Zhu, P., Chen, S., Zheng, S., Chen, Y., Hu, Y. and Guo, Z. (2019) Therapeutic Contribution of Melatonin to the Treatment of Septic Cardiomyopathy: A Novel Mechanism Linking Ripk3-Modified Mitochondrial Performance and Endoplasmic Re-ticulum Function. Redox Biology, 26, Article ID: 101287. https://doi.org/10.1016/j.redox.2019.101287
NOTES
*通讯作者。