Material Sciences
Vol.
09
No.
04
(
2019
), Article ID:
29761
,
9
pages
10.12677/MS.2019.94045
Catalytic Performance and Room Temperature Ferromagnetism of TiO2 Doped with Non-Metallic Elements
Yarong Lu*, Maihemuti Maimaiti*, Mamatrishat Mamat#
School of Physics and Technology, Xinjiang University, Urumqi Xinjiang
Received: Mar. 25th, 2019; accepted: Apr. 11th, 2019; published: Apr. 18th, 2019

ABSTRACT
Titanium dioxide (TiO2) is regarded as one of the important photocatalytic and diluted magnetic materials for a long time, and it has wide applications in the field of the solar water splitting, sewage disposal and spin electronics and so on. Therefore, doping with proper elements to improve and expand its scope of application has become the focus of recent research. Comparatively speaking, the nonmetal elements can improve the performance of TiO2 in reducing the band gap, improving the light absorbing capacity, reducing the electron and hole recombination and other aspects. In this paper, we mainly summarize the effect of nonmetal elements doping on room temperature ferromagnetism and catalytic performance of TiO2. The review may contribute to the further studying and wider application of TiO2.
Keywords:Titanium Dioxide, Photocatalytic Properties, Room Temperature Ferromagnetism
非金属元素掺杂TiO2的光催化性能和室温铁磁性综述
鲁雅荣*,麦合木提·麦麦提*,买买提热夏提·买买提#
新疆大学物理科学与技术学院,新疆 乌鲁木齐
收稿日期:2019年3月25日;录用日期:2019年4月11日;发布日期:2019年4月18日

摘 要
二氧化钛(TiO2)被认为是一种重要的光催化和稀释铁磁性材料,在太阳能水分解、污水处理和自旋电子学领域具有广泛的应用。因此,通过离子掺杂来改善其光催化和室温铁磁性已成为近年来的研究热点。相比而言,非金属元素可以减小TiO2禁带宽度,提高光吸收能力和催化性能、减少电子和空穴的复合速率。本文主要综述了室温下TiO2掺杂非金属元素对其催化性能和铁磁性能的影响及其研究进展,将对TiO2的进一步的研究和推广具有重要意义。
关键词 :二氧化钛,光催化性能,室温铁磁性

Copyright © 2019 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
二氧化钛(TiO2)是一种重要的金属氧化物,具有优异的光催化性能,价格低廉且化学性能稳定,广泛应用于光催化剂、染料敏化太阳能电池、锂离子电池等领域。1972,Fujishima和Hond首次报道了TiO2作为催化剂可以提高水在半导体电极上的电化学光解能力 [1] 。从此,纳米TiO2的制备工艺、性能改善及实际应用的研究工作成为半导体研究领域的热门话题 [2] [3] 。但是,纯相TiO2的禁带宽度为3.28 eV,只能吸收紫外光(<380 nm)的能量,无法充分利用可见光(400~750 nm),极大地阻碍了其在实际生活中的应用。因此,提高TiO2的太阳能利用率和光催化活性成为科学家关注的热点。改善TiO2性能的方法一般有:贵金属沉积 [4] 、表面光敏化 [5] 、半导体复合 [6] 和离子掺杂 [7] 。TiO2还是典型的稀释磁性氧化物半导体(Diluted magnetic oxide semiconductors, DMOSs),2001年Matsumot等 [8] 首次在Co-TiO2中发现了室温铁磁性(Room temperature ferromagnetism, RTFM)的存在。从此引起科学界对TiO2的RTFM的高度重视,进一步扩大了TiO2在自旋电子器件领域的应用。本文主要综述了室温下TiO2掺杂非金属元素对其催化性能和铁磁性能的影响及其研究进展。
2. TiO2的光催化性能
2.1. TiO2的光催化机理
二氧化钛的光催化原理基于能带理论上,见图1 [9] 。半导体的能带结构由充满电子的低能价带(Valence band, VB),空的高能导带(Conduction band, CB)和禁带(Forbidden band, FB)三部分组成。当入射光的能量大于等于TiO2的禁带宽度时,价带中的电子受到激发从价带跃迁到导带,然后价带顶部就会产生空穴,空带上接收电子后成为导带,由此产生电子–空穴对,这些电子和空穴与物质发生氧化反应达到催化效果。
早期,离子掺杂的研究多以掺杂过渡金属元素收缩能带,主要利用Ti原子d轨道的导带和掺杂原子的d轨道重叠得到 [10] [11] [12] ,见图2(b) [13] 。但是之后的研究表明,金属元素可能引起热不稳定与电子陷阱,会降低TiO2的光催化效率。因此,科学家开始考虑引入非金属元素,如最活跃的H元素,第二主族和第三主族中与O原子半径和电负性接近的B,C,N,F,S,P等元素,这些元素的引入替代了原来的O-Ti-O结构,减少了TiO2的能带宽度,光催化性能也显著提高 [10] [11] [14] - [21] 。一般来说,非金属元素的掺杂会导致低能价带和高能导带之间会形成一个“附加能级”,由此减小带隙宽度,见图2。另一方面,这种“附加能级”也将延长光生电子和空穴的停留时间降低复合速率,增强材料的可见光响应效率。在掺杂方式方面,将非金属原子加入到晶格中主要有三种方式:第一种是非金属原子取代晶格中的O,因为原子半径较小的光学元素不会影响TiO2晶体结构的稳定性;第二种是晶格中的Ti原子被非金属原子所取代;第三种是非金属原子掺入晶格的间隙位置。
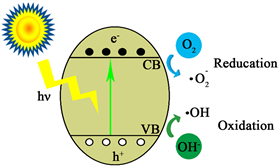
Figure 1. Photocatalytic process of TiO2 [9]
图1. 二氧化钛光催化过程示意图 [9]
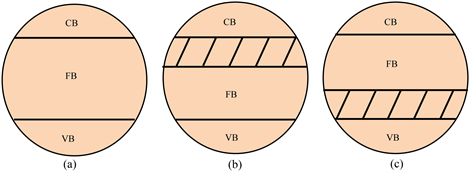
Figure 2. Diagram of energy band of TiO2, (a) Un-doped case; (b) Doped with metallic elements; (c) Doped with nonmetallic elements [13]
图2. TiO2的能带图,(a) 未掺杂;(b) 金属元素掺杂;(c) 非金属元素掺杂 [13]
2.2. TiO2的光催化研究进展
迄今为止,已有大量文献报道了TiO2在光催化剂方面的应用,并证明掺杂非金属元素可以减小禁带宽度,扩大TiO2在可见光的吸收范围。图3显示了几种常见的非金属元素掺杂对TiO2带隙的影响。很显然,无论是哪种元素的引入,X-TiO2的能带宽度都会减小。
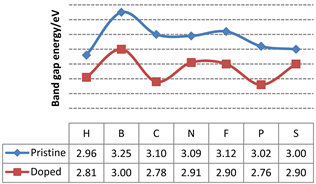
Figure 3. Band gap energy of pristine TiO2 and doped TiO2 with H [22] , B [23] , C [24] , N [21] , F [25] , P [26] , S [27]
图3. 掺杂H [22] ,B [23] ,C [24] ,N [21] ,F [25] ,P [26] 和S [27] TiO2的能带宽度
N的取代是研究最广泛并被认为最有效的掺杂元素。2001年,Asahi等 [28] 在Science上报道了TiO2晶格中少量N元素取代O后观察到O的2p轨道与N的2p轨道杂化,缩短了TiO2的带隙,实验证明亚甲基蓝和气态乙醛的太阳光光降解速率与未掺杂TiO2相比明显加快。Nakamura等 [29] 提出N的引入将TiO2的光响应范围从紫外光区扩展到可见光区,并且认为N在价带顶部形成附加能级。随着研究的进一步深入,Diwaid等 [30] 研究认为间隙N比取代N的可见光响应效果好,间隙N将TiO2的禁带宽度从3.0 eV降低到2.4 eV。这些研究已经证明,TiO2−xNx的薄膜和粉末在低于本征带隙3.0 eV的光吸收和可见光的光催化活性方面相比纯TiO2显著提高,但是相比之下,取代N对于降低金红石TiO2带隙的效果不高。Chen等 [31] 采用高温氧化法制备了C、N、S掺杂的TiO2,表明N掺杂可以改变TiO2的晶体结构,N-TiO2的带边吸收约为415 nm (3.0 eV),接近金红石相,与原始TiO2不同(390 nm)。近几年N元素与其他金属/非金属元素的共掺杂也被证明能有效提高TiO2的催化能力 [32] 。非金属/金属掺杂带来的能带缩小主要利用非金属掺杂可以减小禁带宽度,扩大光响应效应和金属元素掺杂阻碍电子–空穴重新配位,双重作用达到提高光催化效率的效果 [19] [22] [33] [34] [35] [36] 。非金共掺杂是寻求最合适的掺杂比例能最大限度的减小TiO2的禁带宽度,扩大其可见光的响应效率。
C元素掺杂对纳米TiO2光催化性能的改善效果也很明显 [37] 。C掺杂通过在带隙中形成新态或氧空位提高TiO2的可见光的吸附能力和光催化活性。Shim等 [38] 通过电化学阳极氧化法制备了C掺TiO2纳米管,改性后的TiO2表现出较高的可见光光催化能力。通过控制非金属元素掺杂到TiO2晶格中的浓度,可以改变TiO2的相位、结晶度和表面结构,非金属掺杂改性后的催化剂具有更好的光催化效果。Valentin等 [39] 利用密度泛函理论计算讨论了TiO2的碳掺杂。他们认为氧环境影响掺杂效果,并得出结论,在氧气条件差和低碳浓度下,有利于用C和氧空位取代O。然而,在富氧条件下,间质和取代(对Ti) C原子是优选的。此外,碳杂质在带隙中可以诱导出若干局域占据态,可以解释可见光辐照下吸收边红移的原因。Chan等 [37] 采用溶胶–凝胶法制备C掺杂介孔TiO2,发现C掺杂的多孔TiO2薄膜的吸收光谱呈现明显的红移现象,此外,介孔尺寸对TiO2薄膜的光催化活性也有一定影响。
TiO2的光催化改性一般主要针对锐钛矿TiO2和金红石TiO2。然而,近年来,板钛矿TiO2被证明比锐钛矿相和金红石相更具有光化学活性 [40] [41] 。对于F掺杂的TiO2,锐钛矿相和板钛矿相TiO2都显示出明显的红移,Choi等研究证明H掺杂板钛矿TiO2表现出优异的可见光水解能力 [42] 。Hui等 [22] 通过第一性原理计算系统地研究了掺杂H、N及二者共掺对TiO2电化学性能的影响。研究发现三种相结构TiO2催化性能的改善依赖于相位的影响。间隙H会导致所有晶型的带隙减小,优于取代H (只优化了板钛矿型和金红石型),而N更倾向于以取代N。然而,对于金红石TiO2,间隙N原子使禁带宽度降至1.76 eV。此外,共掺杂方法缩小了锐钛矿和板钛矿TiO2的带隙,增强了可见光吸收范围,而金红石TiO2的带隙没有受到影响。重要的是,共掺杂降低了间隙区内的局部化状态,从而提高了迁移率。随着光催化性能的提高,(N,H)共掺杂的二氧化钛带隙较窄、迁移率较低等突出优点使其在光催化性能上有了很大的提高,有望在水分解领域得广泛应用。
前面已经提到H掺杂TiO2对其催化性能有较明显的提升 [22] ,然而,作为一个非常活跃的原子,H存在于所有有机材料和许多无机材料中,并表现出依赖于宿主而表现出不同的行为。特别是金属氧化物,氢通过替换氧与周围所有的金属原子平等地结合,是n型半导体的重要来源之一 [42] 。Frites等 [19] 通过在室温下阴极极化下电化学法生成氢气,将氢气引入N-TiO2。随着光电流密度的增大,对水劈裂反应的光响应逐渐增大。Feng等 [21] 通过溶胶–凝胶合成方法引入间隙B,在TiO2表层生成稳定有效的Ti3+。研究发现,间隙B导致了丰富稳定的Ti3+的形成,并形成了两组新的中间能级,加速了光生电子的迁移率,提高TiO2的可见光催化活性 [23] 。Xue等 [43] 研究了B和N共掺杂锐钛矿型TiO2,得到晶格中的Ti-O-B-N和O-Ti-B-N有助于优化孔结构,提高光响应能力。
二氧化钛的氟化会导致紫外线吸收系数的增加,优异的光反应性扩大其在建筑和汽车玻璃窗方面的应用。F的引入显著提高纳米TiO2颗粒的结晶度和吸附性,提高甲醛的降解速率 [10] [39] 。在催化降解领域,硫改性TiO2可以延长锐钛矿相的稳定性 [44] 。动力学分析表明,S掺杂TiO2的降解速率比未掺杂样品高10倍。改性后的二氧化钛在波长大于440 nm的辐照条件下,对亚甲基蓝在水溶液中的降解活性高 [27] [45] 。与纯TiO2相比,掺杂了S的TiO2在低能区存在光电流,S的3p态与价带的混合导致TiO2带隙的缩小 [27] 。P掺杂的TiO2表明,在可见光照射下,P-TiO2具有优异的光催化性能 [26] [46] 。
3. TiO2的室温铁磁性
3.1. TiO2的室温铁磁性起源
TiO2的RTFM的研究主要集中在金属掺杂,特别是含有不成对电子结构的过渡金属元素,如Mo [47] 、Co [48] 、Fe [49] 、Mn [50] 等,以及O的近邻非磁性元素掺杂,如C [51] 、N [52] [53] [54] 和S [55] 、金属/非金属元素共掺 [48] 和引入氧空位 [56] [57] 等方法。虽然TiO2的RTFM研究有很多,但是科学界对其RTFM的来源和改性机制依旧有很多争议,有些人认为RTFM的来源是过渡金属元素的引入导致的结果 [58] ,有人则认为是氧化物中的氧空位缺陷带起的磁性 [56] [59] [60] 。现虽已有Stoner-type模型 [61] 和Band coupling模型 [62] 可以解释RTFM的起源。但是,RTFM的起源问题仍有很多争议。因此,通过非磁性元素掺杂和引入氧空位研究TiO2的室温铁磁性成为两个主要的研究方向,首先探究TiO2的室温铁磁性的起源,是否与金属元素引起的磁性团簇,另外探究非金属元素掺杂改性TiO2的室温铁磁性。
3.2. TiO2的室温铁磁性研究进展
Yang等 [63] 的理论计算表明C替代O之后锐钛矿TiO2的磁矩为2.0 μB,具有铁磁特性。之后他们继续研究N掺杂对金红石和锐钛矿TiO2的磁性能都有影响 [64] 。他们还计算得到不同掺杂位点得到的TiO2−xBx和Ti1−xBxO2都具有1.0 μB的磁矩 [65] 。Valentin等 [7] 也研究出取代N和间隙N都对锐钛矿TiO2的顺磁性有重要影响。Baie等 [66] 研究在锐钛矿型TiO2中的氧位掺杂N,得到N的掺杂量对磁矩的大小有一定影响。他们还计算了锐钛矿型TiO2中五种不同距离的一氧化氮,由于反向自旋取向的形成能小于平行自旋取向的形成能,他们推测N的掺杂倾向于反铁磁耦合。Ablat等 [51] 采用低温水热法制备了掺杂非磁性元素((N和C)的TiO2。实验结果表明,掺杂TiO2具有明显的M-H滞后环,具有较强的RTFM。同时,退火后饱和磁化强度(Ms)的降低表明,缺陷浓度并不是TiO2室温铁磁性行为的唯一磁源。
Drera等 [67] 结合实验和理论计算研究了N掺杂对TiO2−xNx金红石薄膜磁性的影响。样品的磁滞回线显示出,N掺杂样品的饱和磁化强度(Saturation magnetization, Ms)约为30 emu/cm3,是未掺杂样品的6倍。此外,Bao等 [70] 利用脉冲激光沉积技术成功地在N2O气氛下制备了N-TiO2薄膜。结果表明,N对样品的铁磁性有较强的影响,N取代O是铁磁性的起源。Gomez-Polo等 [71] 采用溶胶–凝胶法合成了掺杂N和C的TiO2,他们发现,真空退火后样品的Ms值和剩余磁化强度(Residual magnetization, Mr)会显著上升。结合实验和第一原理计算,Xu等 [53] 研究了未掺杂、N掺杂、Ru掺杂和Ru-N共掺杂锐钛矿型TiO2纳米管(TNTS)薄膜的室温铁磁性。实验与理论计算结果高度一致。所有样品在室温下均显示锐钛矿结构和铁磁性,其,Ms值为:Ru-TiO2 > Ru/N-TiO2 > TiO2 > N-TiO2。氧空位在TNTS的RTFM中起着重要作用。他们认为铁磁性的来源是O的2p态、Ru的4d态和N的2p态的轨道杂化形成的。
基于以上综述,表1列出了通过不同实验方法和理论计算,三种非金属元素N,C,H对掺杂改性TiO2的光催化性能和室温铁磁性的总结。从表中可以清楚地看出,掺杂后二氧化钛的室温铁磁性和光催化性能有了显著的改善。例如,RTFM的性能与Ms和Mr的增加有关;带隙和吸收波长的变化反映了非金属元素掺杂改善了TiO2的光催化性能和光降解能力;光电流密度的增加意味着可见光下水解能力的提高。此外,介孔尺寸的变化表明,非金属元素可以形成一种新的状态,该状态正好位于价带上方,减小了TiO2的带隙,提高TiO2的光催化性能。
Table 1. Performances of TiO2 doped with N, C and H
表1. N,C,H掺杂改性TiO2
4. 总结
本文主要研究了几种非金属元素掺杂TiO2对其光催化和室温铁磁性能的影响。性能的变化与掺杂位点、掺杂浓度和TiO2的结构有关。此外,自旋极化态的形成增强了TiO2的磁性能,而间隙态的形成伴随着禁带宽度的显著减小,提高了TiO2的光催化活性。综上所述,非金属或非磁性元素可以显著提高催化性能和室温铁磁性。制备具有比表面积高、复合速率低、光催化效率高和热稳定性优异等特点的改性TiO2,将是未来光催化材料主要的研究方向。
基金项目
国家自然科学基金项目(61366001)支持。
文章引用
鲁雅荣,麦合木提·麦麦提,买买提热夏提·买买提. 非金属元素掺杂TiO2的光催化性能和室温铁磁性综述
Catalytic Performance and Room Temperature Ferromagnetism of TiO2 Doped with Non-Metallic Elements[J]. 材料科学, 2019, 09(04): 338-346. https://doi.org/10.12677/MS.2019.94045
参考文献
- 1. Fujishima, A. and Honda, K. (1972) Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature, 238, 37-38. https://doi.org/10.1038/238037a0
- 2. 潘伟伟, 刘世华, 陈秋松. 制备方法对纳米TiO2形貌和光催化性质的影响[J]. 材料科学, 2018, 8(6): 643-649.
- 3. Noman, M.T., Ashraf, M.A. and Ali, A. (2019) Synthesis and Applications of Nano-TiO2: A Review. Environmental Science and Pollution Research, 26, 1-31.
- 4. Zielińska-Jurek, A. and Hupka, J. (2014) Preparation and Characterization of Pt/Pd-Modified Titanium Dioxide Nanoparticles for Visible Light Irradiation. Catalysis Today, 230, 181-187. https://doi.org/10.1016/j.cattod.2013.09.045
- 5. 郭莉, 王丹军, 付峰, 等. 叶绿素铜敏化二氧化钛光催化剂的合成及性能研究[J]. 江西农业大学学报, 2010, 32(4): 819-823.
- 6. Li, X.Z., Li, F.B., Yang, C.L., et al. (2001) Photocatalytic Activity of WOx-TiO2 under Visible Light Irradiation. Journal of Photochemistry & Photobiology A Chemistry, 141, 209-217. https://doi.org/10.1016/S1010-6030(01)00446-4
- 7. Valentin, D.C., Finazzi, E., Pacchioni, G., et al. (2007) N-Doped TiO2: Theory and Experiment. Chemical Physics, 339, 44-56. https://doi.org/10.1016/j.chemphys.2007.07.020
- 8. Matsumoto, Y., Murakami, M., Shono, T., et al. (2001) Room-Temperature Ferromagnetism in Transparent Transition Metal-Doped Titanium Dioxide. Science, 291, 854-856. https://doi.org/10.1126/science.1056186
- 9. 范鸿梅. 黑色TiO2的改性及其光催化性能研究[D]: [硕士学位论文]. 乌鲁木齐: 新疆大学, 2018.
- 10. Hattori, A. and Tada, H. (2001) High Photocatalytic Activity of F-Doped TiO2 Film on Glass. Journal of Sol-Gel Science and Technology, 22, 47-52. https://doi.org/10.1023/A:1011260219229
- 11. Zhao, J., Li, W., Xue, L., et al. (2017) Low Temperature Synthesis of Water Dispersible F-Doped TiO2 Nanorods with Enhanced Photocatalytic Activity. RSC Advances, 7, 21547-21555. https://doi.org/10.1039/C7RA00850C
- 12. Wang, Z., Cao, M., Yang, L., et al. (2017) Hemin/Au Nanorods/Self-Doped TiO2 Nanowires as a Novel Photoelectrochemical Bioanalysis Platform. Analyst, 142, 2805-2811. https://doi.org/10.1039/C7AN00783C
- 13. 王丽, 陈永, 赵辉, 等. 非金属掺杂二氧化钛光催化剂的研究进展[J]. 材料导报, 2015, 29(1): 147-151.
- 14. Yang, X., Cao, C., Erickson, L., et al. (2009) Photo-Catalytic Degradation of Rhodamine B on C-, S-, N-, and Fe-Doped TiO2 under Visible-Light Irradiation. Applied Catalysis B Environmental, 91, 657-662. https://doi.org/10.1016/j.apcatb.2009.07.006
- 15. Jia, T., Fang, F., Yu, D., et al. (2018) Facile Synthesis and Characterization of N-Doped TiO2/C Nanocomposites with Enhanced Visible-Light Photocatalytic Performance. Applied Surface Science, 430, 133-139.
- 16. Matějová, L., Kočí, K., Troppová, I., et al. (2018) TiO2 and Nitrogen Doped TiO2 Prepared by Different Methods; on the (Micro)structure and Photocatalytic Activity in CO2 Reduction and N2O Decomposition. Journal of Nanoscience & Nanotechnology, 18, 688-698. https://doi.org/10.1166/jnn.2018.13936
- 17. Cho, S., Ahn, C., Park, J., et al. (2018) 3D Nanostructured N-Doped TiO2 Photocatalysts with Enhanced Visible Absorption. Nanoscale, 10, 9747-9751. https://doi.org/10.1039/C8NR02330A
- 18. Boytsova, E.L., Leonova, L.A. and Pichugin, V.F. (2018) The Struc-ture of Biocoats Based on TiO2 Doped with Nitrogen Study. Materials Science and Engineering, 347, 1-5. https://doi.org/10.1088/1757-899X/347/1/012025
- 19. Frites, M. and Khan, S.U.M. (2009) Visible Light Active Hydrogen Modified (HM)-n-TiO Thin Films for Photoelectrochemical Splitting of Water. Electrochemistry Commu-nications, 11, 2257-2260. https://doi.org/10.1016/j.elecom.2009.10.005
- 20. Lee, J.Y., Park, J. and Cho, J.H. (2005) Electronic Properties of N- and C-Doped TiO2. Applied Physics Letters, 87, 1516-1521. https://doi.org/10.1063/1.1991982
- 21. Cristiana, D.V., Gianfranco, P., Annabella, S., et al. (2005) Characterization of Paramagnetic Species in N-Doped TiO2 Powders by EPR Spectroscopy and DFT Calculations. Journal of Physical Chemistry B, 109, 11414-11419. https://doi.org/10.1021/jp051756t
- 22. Hui, P., Zhang, Y.W., Shenoy, V.B., et al. (2011) Effects of H-, N-, and (H, N)-Doping on the Photocatalytic Activity of TiO2. Journal of Physical Chemistry C, 115, 12224-12231. https://doi.org/10.1021/jp202385q
- 23. Feng, N., Liu, F., Huang, M., et al. (2016) Unravelling the Efficient Pho-tocatalytic Activity of Boron-Induced Ti3+ Species in the Surface Layer of TiO2. Scientific Reports, 6, 1-9. https://doi.org/10.1038/srep34765
- 24. Kamani, H., Nasseri, S., Khoobi, M., et al. (2016) Sonocatalytic Degrada-tion of Humic Acid by N-Doped TiO2 Nano-Particle in Aqueous Solution. Journal of Environmental Health Science & Engineering, 14, 1-9. https://doi.org/10.1186/s40201-016-0242-2
- 25. Huang, D., Liao, S., Quan, S., et al. (2007) Preparation of Ana-tase F Doped TiO2 Sol and Its Performance for Photodegradation of Formaldehyde. Journal of Materials Science, 42, 8193-8202. https://doi.org/10.1007/s10853-007-1694-7
- 26. Shi, Q., Dong, Y., Jiang, Z., et al. (2006) Visible-Light Photo-catalytic Regeneration of NADH Using P-Doped TiO2 Nanoparticles. Journal of Molecular Catalysis B Enzymatic, 43, 44-48. https://doi.org/10.1016/j.molcatb.2006.06.005
- 27. Umebayashi, T., Yamaki, T., Yamamoto, S., et al. (2003) Sulfur-Doping of Rutile-Titanium Dioxide by Ion Implantation: Photocurrent Spectroscopy and First-Principles Band Calculation Studies. Journal of Applied Physics, 93, 5156-5160. https://doi.org/10.1063/1.1565693
- 28. Asahi, R., Morikawa, T., Ohwaki, T., et al. (2001) Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science, 293, 269-271. https://doi.org/10.1126/science.1061051
- 29. Nakamura, R., Tanaka, T. and Nakato, Y. (2004) Mechanism for Visible Light Responses in Anodic Photocurrents at N-Doped TiO2 Film Electrodes. Journal of Physical Chemistry B, 108, 10617-10620. https://doi.org/10.1021/jp048112q
- 30. Diwald, O., Thompson, T.L., Zubkov, T., et al. (2004) Photochemical Activity of Nitrogen-Doped Rutile TiO2 (110) in Visible Light. Cheminform, 35, 219-221. https://doi.org/10.1002/chin.200430022
- 31. Xiaobo, C. and Clemens, B. (2008) The Electronic Origin of the Visible-Light Absorption Properties of C-, N- and S-Doped TiO2 Nanomaterials. Journal of the American Chemical Society, 130, 5018-5027. https://doi.org/10.1021/ja711023z
- 32. 赵兴国, 张王刚, 余彬, 等. 高催化活性银氮共掺纳米TiO2薄膜的性能研究[J]. 材料科学, 2018, 8(11): 1055-1065.
- 33. Nzaba, S.K.M., Ntsendwana, B., Mamba, B.B., et al. (2018) PAMAM Templated N, Pt Co-Doped TiO2 for Visible Light Photodegradation of Brilliant Black. Environmental Science & Pollution Research, 25, 1-13. https://doi.org/10.1007/s11356-018-1717-8
- 34. Zhang, C., Zhou, Y., Bao, J., et al. (2018) Hierarchical Honey-comb Br, N Codoped TiO2 with Enhanced Visible-Light Photocatalytic H2 Production. ACS Applied Materials & In-terfaces, 10, 18796-18804. https://doi.org/10.1021/acsami.8b04947
- 35. Xiang, Q., Yu, J. and Jaroniec, M. (2011) Nitrogen and Sulfur Co-Doped TiO2 Nanosheets with Exposed {001} Facets: Synthesis, Characterization and Visible-Light Photocatalytic Activity. Physical Chemistry Chemical Physics, 13, 4853-4861. https://doi.org/10.1039/C0CP01459A
- 36. Li, M., Zhang, J., Guo, D., et al. (2012) Band Gap Engineering of Compensated (N, H) and (C, 2H) Codoped Anatase TiO2: A First-Principles Calculation. Chemical Physics Letters, 539-540, 175-179.
- 37. Chan, L., Yang, S., Lixin, C., et al. (2013) Effective Photocatalysis of Functional Nanocomposites Based on Carbon and TiO2 Nanoparticles. Nanoscale, 5, 4986-4992. https://doi.org/10.1039/c3nr01033c
- 38. Shim, J., Seo, Y.S., Oh, B.T., et al. (2016) Microbial Inacti-vation Kinetics and Mechanisms of Carbon-Doped TiO2 (C-TiO2) under Visible Light. Journal of Hazardous Materials, 306, 133-139. https://doi.org/10.1016/j.jhazmat.2015.12.013
- 39. Valentin, C.D. and Pacchioni, G. (2013) Trends in Non-Metal Doping of Anatase TiO2: B, C, N and F. Catalysis Today, 206, 12-18. https://doi.org/10.1016/j.cattod.2011.11.030
- 40. Dimitrijevic, N.M., Saponjic, Z.V., Rabatic, B.M., et al. (2007) The Effect of Size and Shape of Nanocrystalline TiO2 on Photogenerated Charges, an EPR Study. Journal of Physical Chemistry C, 111, 14597-14601. https://doi.org/10.1021/jp0756395
- 41. Buonsanti, R., Grillo, V., Carlino, E., et al. (2008) Nonhydrolytic Synthesis of High-Quality Anisotropically Shaped Brookite TiO2 Nanocrystals. Journal of the American Chemical Society, 130, 11223-11233. https://doi.org/10.1021/ja803559b
- 42. Choi, M., Lee, J.H., Jang, Y.J., et al. (2016) Hydrogen-Doped Brookite TiO2 Nanobullets Array as a Novel Photoanode for Efficient Solar Water Splitting. Scientific Reports, 6, Article No. 36099. https://doi.org/10.1038/srep36099
- 43. Xue, H., Jiang, Y., Yuan, K., et al. (2016) Floating Photocatalyst of B-N-TiO2/Expanded Perlite: A Sol-Gel Synthesis with Optimized Mesoporous and High Photocatalytic Activity. Scientific Reports, 6, Article No. 29902. https://doi.org/10.1038/srep29902
- 44. Pillai, S., Mccormack, D., Periyat, P., et al. (2008) Improved High-Temperature Stability and Sun-Light-Driven Photocatalytic Activity of Sulfur-Doped Anatase TiO2. The Journal of Physical Chemistry C, 112, 7644-7652. https://doi.org/10.1021/jp0774847
- 45. Tanaka, S. (2003) Visible Light-Induced Degradation of Methylene Blue on S-Doped TiO. Chemistry Letters, 32, 330-331. https://doi.org/10.1246/cl.2003.330
- 46. Hirotaka, N., Koichi, K. and Masashi, T. (2009) Preparation and Photocatalytic Property of Phosphorus-Doped TiO2 Particles. Journal of Oleo Science, 58, 389-394. https://doi.org/10.5650/jos.58.389
- 47. Lamrani, A.F., Belaiche, M., Benyoussef, A., et al. (2012) Ferromagnetism in Mo-Doped TiO2 Rutile from Ab Initio Study. Journal of Superconductivity & Novel Magnetism, 25, 503-507. https://doi.org/10.1007/s10948-011-1317-z
- 48. Manivannan, A., Seehra, M.S., Ma-jumder, S.B., et al. (2003) Magnetism of Co-Doped Titania Thin Films Prepared by Spray Pyrolysis. Applied Physics Letters, 83, 111-113. https://doi.org/10.1063/1.1590744
- 49. Lin, C., Li, H., Zhang, K., et al. (2014) Magnetic and Optical Properties of N-Doped, Fe-Doped and (N, Fe)-Codoped Anatase TiO2. Chemical Physics Letters, 608, 186-190.
- 50. Ahmed, S.A. (2016) Annealing Effects on Structure and Magnetic Properties of Mn-Doped TiO2. Journal of Magnetism & Magnetic Materials, 402, 178-183. https://doi.org/10.1016/j.jmmm.2015.11.065
- 51. Ablat, A., Wu, R., Mamat, M., et al. (2016) Electronic Structure and Room Temperature Ferromagnetism of C Doped TiO2. Solid State Communications, 243, 7-11. https://doi.org/10.1016/j.ssc.2016.05.013
- 52. Ablat, A., Rong, W., Jian, J., et al. (2014) Room Temperature Ferromagnetism of N-Doped TiO2 Nanowires. Materials Letters, 132, 86-89. https://doi.org/10.1016/j.matlet.2014.06.040
- 53. Xu, J., Zhou, Z. and Wang, H. (2017) Origin of Ferromagnetism in Ru and N Codoped TiO2 Nanotubes: Experiments and Theory Investigations. Journal of Nanomaterials, 2017, Article ID: 2316745.
- 54. Luitel, H., Chakrabarti, M., Sarkar, A., et al. (2018) Ab-Initio Calculation and Experimental Observation of Room Temperature Ferromagnetism in 50 keV Nitrogen Implanted Rutile TiO2. Materials Research Express, 5, 026104-026112. https://doi.org/10.1088/2053-1591/aaab8c
- 55. Shi, B., Yong, L., Song, C., et al. (2008) First-Principles Investigation of the Band Structure of S-Doped TiO2. Rare Metal Materials & Engineering, 37, 623-625.
- 56. Nguyen, H.H., Sakai, J., Poirot, N, et al. (2006) Room Temperature Ferromagnetism Observed in Undoped Semiconducting and Insulating Oxide Thin Films. Physical Review B, 73, 2404-2407.
- 57. Santara, B., Giri, P.K., Imakita, K., et al. (2013) Evidence of Oxygen Vacancy Induced Room Temperature Ferromagnetism in Solvothermally Synthesized Undoped TiO2 Nanoribbons. Nanoscale, 5, 5476-5488. https://doi.org/10.1039/c3nr00799e
- 58. Chu, D., Zeng, Y.-P., Jiang, D., et al. (2009) Room Temperature Ferro-magnetism in Transition Metal Doped TiO2 Nanowires. Science of Advanced Materials, 1, 227-229. https://doi.org/10.1166/sam.2009.1047
- 59. Anisimov, V.I., Korotin, M.A., Nekrasov, I.A., et al. (2006) The Role of Transition Metal Impurities and Oxygen Vacancies in the Formation of Ferromagnetism in Co-Doped TiO2. Journal of Physics Condensed Matter, 18, 1695-1704. https://doi.org/10.1088/0953-8984/18/5/022
- 60. Kikoin, K. and Fleurov, V. (2007) On the Nature of Ferromagnetism in Non-Stoichiometric TiO2 Doped with Transition Metals. Journal of Magnetism & Magnetic Materials, 310, 2097-2098. https://doi.org/10.1016/j.jmmm.2006.10.792
- 61. Griessen, R. (1983) Phase Separation in Amorphous Metal Hydrides: A Stoner-Type Criterion. Physical Review B, 27, 7575-7582. https://doi.org/10.1103/PhysRevB.27.7575
- 62. Peng, H., Xiang, H.J., Wei, S.H., et al. (2009) Origin and Enhancement of Hole-Induced Ferromagnetism in First-Row d0 Semiconductors. Physical Review Letters, 102, 17201-17205. https://doi.org/10.1103/PhysRevLett.102.017201
- 63. Yang, K., Ying, D., Huang, B., et al. (2008) On the Possibility of Ferromagnetism in Carbon-Doped Anatase TiO2. Applied Physics Letters, 93, 10507-13510. https://doi.org/10.1063/1.2996024
- 64. Yang, K., Dai, Y., Huang, B., et al. (2009) Density Functional Studies of the Magnetic Properties in Nitrogen Doped TiO2. Chemical Physics Letters, 481, 99-102. https://doi.org/10.1016/j.cplett.2009.09.050
- 65. Yang, K., Dai, Y., Huang, B., et al. (2010) Density-Functional Characterization of Antiferromagnetism in Oxygen-Deficient Anatase and Rutile TiO2. Physical Review B, Condensed Matter, 81, 033202-033206. https://doi.org/10.1103/PhysRevB.81.033202
- 66. Bai, Y. and Chen, Q.N. (2008) Dopant Induced Antiferromagnetism in Anatase TiO2: First Principle Study. Solid State Communications, 147, 169-171. https://doi.org/10.1016/j.ssc.2008.05.034
- 67. Drera, G., Mozzati, M.C., Galinetto, P., et al. (2010) Enhancement of Room Temperature Ferromagnetism in N-Doped TiO2−x Rutile: Correlation with the Local Electronic Properties. Applied Physics Letter, 97, 012506-012509. https://doi.org/10.1063/1.3458699
- 68. Dong, F., Wang, H. and Wu, Z. (2009) One-Step “Green” Synthetic Approach for Mesoporous C-Doped Titanium Dioxide with Efficient Visible Light Photocatalytic Activity. The Journal of Physical Chemistry C, 113, 16717-16723. https://doi.org/10.1021/jp9049654
- 69. Li, D.I., Haneda, H., et al. (2005) Visible-Light-Driven N-F-Codoped TiO2 Photocatalysts. 2. Optical Characterization, Photocatalysis, and Potential Application to Air Purification. Chemistry of Materials, 17, 2596-2602. https://doi.org/10.1021/cm049099p
- 70. Bao, N.N., Fan, H.M., Ding, J., et al. (2011) Room Temperature Ferromagnetism in N-Doped Rutile TiO. Journal of Applied Physics, 109, 302-305. https://doi.org/10.1063/1.3535427
- 71. Gómez-Polo, C., Larumbe, S. and Pastor, J.M. (2013) Room Temperature Ferromagnetism in Non-Magnetic Doped TiO2 Nanoparticles. Journal of Applied Physics, 113, 511-514. https://doi.org/10.1063/1.4795615
NOTES
*共同第一作者。
#通讯作者。
