Advances in Psychology
Vol.08 No.02(2018), Article ID:23819,9
pages
10.12677/AP.2018.82025
The Impact of Targeted Memory Reactivation on Memory Consolidation: A Review
Xiang Deng, Xiaoling Wang
School of Psychology, Southwest University, Chongqing
Received: Feb. 4th, 2018; accepted: Feb. 19th, 2018; published: Feb. 26th, 2018

ABSTRACT
Targeted memory reactivation is a fairly important technique that has the potential to influence the course of memory formation through application of cues during imperceptible states. In general, cueing memory at the slow-wave-sleep stage can promote the consolidation of procedural and declarative memory. However, the effect of cueing memory during wake and the rapid eye move sleep has no unified. Studies have shown that TMR is associated with sleep parameters and accompanied by activation of memory related brain areas, such as Hippocampus, thalamus and the prefrontal cortex. Olfactory stimulus and auditory stimulus are adapted to TMR, which is influenced by the way the stimulus represent and the relationship of cuing materials and memory. The goal of the future study is to clear the neural mechanism of TMR and to explore safer and more effective ways of adjusting memory representations to better suit individual needs, as well as its potential applications in improving the abilities of study, creativity and problem solving.
Keywords:Targeted Memory Reactivation, Memory Consolidation, Sleep, Slow Wave Sleep
目标记忆重激活对记忆巩固影响的研究综述
邓翔,王小玲
西南大学心理学部,重庆
收稿日期:2018年2月4日;录用日期:2018年2月19日;发布日期:2018年2月26日

摘 要
目标记忆重激活(Targeted memory reactivation, TMR)是一种研究记忆巩固的重要方法,通过进行记忆线索的提示诱导促进记忆的巩固。通常,发生在慢波睡眠阶段的提示可促进程序性和陈述性记忆巩固。而在清醒和快速眼动睡眠阶段进行提示对记忆巩固的效果尚无统一认识。在神经机制方面,TMR和睡眠参数有关并伴随着记忆相关脑区的激活,如海马、丘脑、前额叶皮层。TMR使用的线索材料通常为嗅觉刺激或听觉刺激,这些线索刺激呈现的时机、时长以及与目标材料之间的关系会对TMR产生影响。未来研究可以从澄清睡眠促进记忆巩固的神经机制,开发更具可操作性的TMR新技术着手,帮助大众提高学习能力、创造力和问题解决能力。
关键词 :目标记忆重激活,记忆巩固,睡眠,慢波睡眠

Copyright © 2018 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
睡眠是记忆巩固的重要阶段,新获得的短时记忆在此期间转变为更稳定持久的长时记忆(Born, Rasch, & Gais, 2006)。在过去很长的一段时间里,人们普遍认为睡眠对于学习信息的主要作用在于被动的保护这些记忆不受干扰(Rasch & Born, 2013)。随着研究的深入,人们发现其实在睡眠期间,存储的记忆信息并非静止不动,而是受到有规律、动态的管理(Saletin & Walker, 2012; Stickgold, 2005; McGaugh, 2000),是记忆巩固的理想时间窗(Stickgold, 2013)。早期研究发现在睡眠期间会出现记忆表征神经元的快速自发激活,巩固所存储的相关记忆。通过记录老鼠觉醒和睡眠期海马位置细胞的活动,研究者发现在睡眠过程中会重现觉醒期的海马激活模式(McNaughton et al., 1994; Skaggs & McNaughton, 1996);在人类的虚拟空间任务和图片位置记忆任务研究中,也发现了海马类似的激活且伴随着学习表现能力的提升(Peigneux et al., 2004; Deuker et al., 2013)。因此,是否可以人为的控制这些记忆表征的神经元激活,从而达到促进记忆巩固的效果呢?
基于此想法,睡眠与记忆研究领域发展了一种新型的研究技术:目标记忆重激活(targeted memory reactivation, TMR) (Oudiette & Paller, 2013)。典型的TMR实施过程包含联结编码和线索提示诱导两个阶段。首先,在编码阶段学习与线索刺激联结的目标记忆材料。然后在被试无察觉的状态下再次呈现这些线索,诱导目标记忆的神经表征再次激活,达到促进记忆巩固的目的,其中线索提示诱导是核心,常运用于睡眠阶段(见图1)。线索材料通常为特异的气味(Rasch et al., 2007)、声音(Rudoy et al., 2009),其呈现的方式方法与TMR的效果密切相关。TMR被认为可以有选择性的对记忆进行操作,这有助于揭示记忆巩固的一般规律,对于研究记忆形成的具体机制有着重要的意义。
本文旨在通过综合TMR的研究,探讨TMR对陈述性、程序性和情绪性记忆的巩固以及创造性和社会认知的影响,并结合现有理论框架来讨论该技术的神经机制,为未来的研究方向、潜在应用提供建议。
2. 提示诱导时机对记忆巩固的影响
研究发现TMR可以促进陈述性记忆巩固,能够使新获得的记忆整合到已有的知识网络中,提升知识概化、图示化和特征提取,从而产生更多创造性的和充满洞察力的问题解决策略(Sterpenich et al., 2014; Rudoy et al., 2009);此外,TMR还可以通过促进重复性自动化的动作技能以及显性知识的习得来提高程序性记忆的巩固和重组(Antony et al., 2012; Schoenauer, Geisler, & Gais, 2014; Cousins et al., 2014);不过,
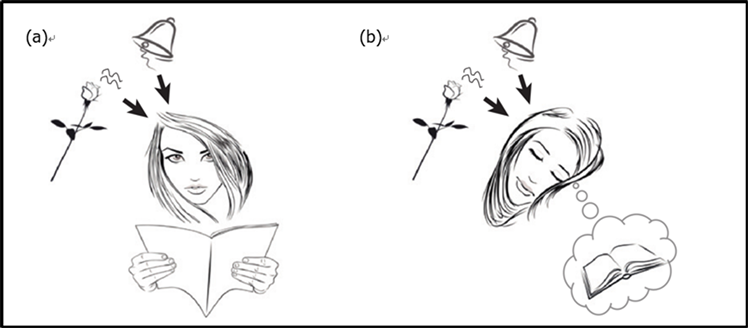 (资料来源:Oudiette& Paller, 2013)
(资料来源:Oudiette& Paller, 2013)
Figure 1. A typical protocol for targeted memory reactivation during sleep, the same stimuli are presented (b) in association with learning and (a) during sleep (Oudiette & Paller, 2013)
图1. 目标记忆重激活实施流程示意图,首先进行联结编码学习(a),再在睡眠期间使用线索提示诱导记忆重激活(b)
TMR应用于情绪性记忆的研究结果仍存在冲突,恐惧消退(Barnes & Wilson, 2014; Rolls et al., 2013)和恐惧增强(Hars & Hennevin, 1987; Hauner et al., 2013; He et al., 2015; Cairney et al., 2014)的现象都有报道。
2.1. 慢波睡眠期
目前尚不得知TMR提示诱导何时实施最佳。但研究表明,在慢波睡眠(Slow Wave Sleep, SWS)阶段进行诱导最有可能促进记忆的巩固(Schouten et al., 2017)。
2.1.1. 陈述性记忆巩固
在SWS阶段实施TMR提示诱导后,被试会有更好的记忆表现(Rasch et al., 2007; Rudoy et al., 2009; Diekelmann et al., 2012; Rihm et al., 2014; Creery et al., 2015; Schreiner, Goeldi, & Rasch, 2015; Diekelmann et al., 2011; Schreiner & Rasch, 2015),记忆被强化并转化为更稳定的形式,从而抵抗其他输入信息的干扰,不容易被遗忘(Diekelmann et al., 2011)。Rasch等人在图片位置记忆任务中,使用嗅觉材料作为线索刺激,在SWS阶段进行提示诱导,发现被试对线索联结的图片位置记忆效果更好,陈述性记忆得到了巩固(Rasch et al., 2007)。除此之外,在SWS阶段进行提示诱导也可以促进以陈述性记忆为基础的高级认知功能,如外语的学习(Gomez, Bootzin, & Nadel, 2006)。另外,TMR进行提示诱导之后需要一定的时间来实现记忆的稳定和强化,而在这个关键时间窗内若出现第二个听觉刺激很可能会破坏记忆的巩固和增强(Schreiner, Lehmann, & Rasch, 2015)。Schreiner等人在词汇学习任务中,使用听觉材料作为背景线索,在SWS期进行词汇声音提示诱导之后立即给予听觉反馈。发现不论反馈的是正确词汇、错误词汇或纯音,记忆的增强效应都会被破坏(Schreiner & Rasch, 2015; Schreiner, Lehmann, & Rasch, 2015)。Oudiette等人还发现,TMR的效果还与陈述性记忆内容关联的价值高低有关。当记忆内容与价值高低联结时,觉醒期进行提示诱导会促进与线索对应的低价值联结的记忆巩固,但SWS期进行提示诱导时,所有低价值联结的记忆都得到了巩固(Oudiette et al., 2013)。
2.1.2. 程序性记忆
迄今为止的绝大多数TMR研究都主要集中于海马依赖的记忆模式,而海马同样是程序性学习的主要控制脑区(Albouy et al., 2013),那么至少在理论上我们可以认为TMR同样可以促进程序性记忆的巩固。Antony等人在SWS阶段进行听觉诱导促进了程序性记忆的巩固(Antony et al., 2012)。在该研究中,听觉线索是程序性记忆任务本身的内在组成部分,是运动行为的结果,线索刺激和目标记忆材料联结紧密。类似的研究也发现,当声音与程序性任务紧密联结时,在SWS阶段再次呈现学习时的旋律会使得被试后测时有更好的记忆表现(Schoenauer, Geisler, & Gais, 2014)。但Rasch等人使用嗅觉材料作为线索刺激对程序性记忆进行TMR时,却发现对程序性记忆没有效果。他们推测,有可能是因为气味线索不能与目标程序性记忆建立紧密的联结,从而不适合作为程序性记忆的线索刺激材料。此外,Cousins等人在SWS阶段进行提示诱导后,发现被试获得的外显知识量增加并且反应速度更快(Cousins et al., 2014),这说明TMR还可以促进记忆发生质变。
2.1.3. 情绪性记忆
SWS阶段进行TMR提示诱导对于情绪性记忆影响的研究存在争议(Diekelmann & Born, 2015)。Barnes和Wilson以气味做线索刺激,在呈现气味刺激时给予老鼠电击,形成恐惧记忆联结,结果老鼠在SWS期进行气味诱导之后恐惧记忆增强(Barnes & Wilson, 2014);Cairney等人将情绪图片(正性/负性图片)运用到图片位置任务中,并使用语义相关的声音作为线索刺激,在SWS阶段进行提示诱导后,被试的负性情绪记忆被巩固(Cairney et al., 2014);而何佳等人在听觉线索呈现时给予被试轻微的电刺激,形成恐惧记忆联结,在SWS期进行提示诱导后被试的恐惧反应消退(He et al., 2015)。另外,Groch等人将模棱两可的场景图片与正性或负性词对联结,并以这些词的读音作为线索材料,在SWS阶段再次播放这些词的读音。结果发现,被试对进行了提示诱导的情景图片的记忆更好,而且相比于没有进行提示诱导的情景图片,被试对与提示诱导后的情景图片相似的新模糊场景的解释会与线索刺激的情绪性一致,且情绪性更高。该研究为青少年临床心理学的研究打开了一扇新的窗户(Groch et al., 2016)。
2.2. 觉醒与快速眼动睡眠期
与大多数研究一致发现SWS期进行TMR提示诱导可以促进记忆巩固不同,觉醒期和快速眼动(Rapid Eye Movement, REM)睡眠阶段的研究争议较大。
在觉醒阶段进行TMR诱导的各项研究结果存在矛盾。有研究报道在此阶段进行提示诱导能促进记忆巩固(Fuentemilla et al., 2013),也有报道发现能破坏记忆巩固(Diekelmann et al., 2011; Barnes & Wilson, 2014; He et al., 2015),但更多的研究表明觉醒期间进行TMR对记忆巩固没有影响(Rasch et al. 2007; Rudoy et al., 2009; Antony et al., 2012; Schoenauer, Geisler, & Gais, 2014; Cousins et al., 2014; Hauner et al., 2013; Bendor & Wilson, 2012)。这可能是因为觉醒期间进行线索刺激会使得学习过的记忆返回到活跃和不稳定的状态,从而更容易受到干扰,进而产生遗忘(Diekelmann et al., 2011)。此外,Oudiette等人还发现,当记忆目标为陈述性记忆时,在觉醒期进行提示诱导的效果还与记忆任务所关联的价值高低有关。当记忆内容与价值高低联结时,觉醒期进行提示诱导会促进与线索刺激对应的低价值联结记忆的巩固(Oudiette et al., 2013)。对于程序性记忆任务而言,无论研究者采用嗅觉线索(Rasch et al., 2007)还是听觉线索(Cousins et al., 2014; Schoenauer, Geisler, & Gais, 2014; Antony et al., 2012)诱导时,在清醒期间进行TMR提示诱导都对程序性记忆巩固没有影响。对于情绪性记忆任务的研究结果与SWS阶段类似,有些研究没有发现TMR对巩固记忆的效应(Hauner et al., 2013; Hars, Hennevin, & Pasques, 1985),而另一些研究则报告恐惧情绪记忆消失(Barnes & Wilson, 2014; He et al., 2015)。
在REM睡眠期间,Sterpenich等人使用听觉材料作为线索刺激研究陈述性记忆,发现提示诱导会使得陈述性记忆的回忆速度和错误再认率都提升,表明在REM睡眠期间进行听觉TMR可以促使陈述性记忆泛化和图示化(Sterpenich et al., 2014)。而Cordi等人在REM睡眠期间使用嗅觉材料作为线索刺激时,TMR没能促进陈述性记忆的巩固(Cordi et al., 2014)。此外,Rasch及其同事的研究则表明,当使用传统的手指敲击任务来探查运动技能时,在REM睡眠期间进行嗅觉的TMR提示诱导对程序性记忆没有效果。研究者认为,诸如手指敲击这类程序性记忆任务不能有效地与气味线索建立紧密的联结,因此提示诱导不能引起记忆表征的激活(Rasch et al., 2007)。至于情绪性记忆,Hars等人发现在REM睡眠阶段进行TMR提示诱导会增强恐惧记忆(Hars, Hennevin, and Pasques, 1985),而在另一项研究则使得记忆的情绪性降低(Rihm & Rasch, 2015)。
3. 神经机制及理论解释
3.1. TMR的神经机制
睡眠期进行TMR诱导的效果与睡眠电生理活动相关。研究发现TMR效果与δ波能量(Rihm et al., 2014)、θ波能量(Schreiner, Lehmann, & Rasch, 2015)、纺锤波能量(Schreiner, Lehmann, & Rasch, 2015)、纺锤波密度(Fuentemilla et al., 2013)都成正相关。在睡眠阶段呈现线索刺激之后会诱导相应的纺锤波活动(Rihm et al., 2014; Schreiner, Lehmann, & Rasch, 2015; Creery et al., 2015; Cox et al., 2014),并且在应用TMR的词汇学习实验中,词汇学习的提升会伴随着慢波振荡数量的增加以及增强的慢纺锤波能量(Schreiner & Rasch, 2015)。而在Cox等人的研究中,尽管没能发现TMR之后记忆表现和纺锤波之间的直接关联,但其结果表明纺锤波振幅和密度对背景线索刺激确实有响应变化,这说明TMR效果确实与纺锤波之间存在联系(Cox et al., 2014)。
运用功能磁共振成像技术(functional Magnetic Resonance Imaging, fMRI),研究人员发现在SWS阶段进行TMR提示诱导可以成功诱发与记忆相关的脑区的激活。如左侧海马(left hippocampus) (Rasch et al., 2007; Diekelmann et al., 2011)、右旁侧海马皮层(right parahippocampal cortex) (van Dongen et al., 2012)、扣带皮层(retrosplenial cortex)、颞叶皮层(temporal cortex)以及额内侧皮层区域(medial frontal areas) (Diekelmann et al., 2011),其中海马在TMR中的作用尤为重要。Bendor和Wilson通过研究小鼠发现,在学习阶段建立声音线索和空间记忆任务之间的联结之后,在睡眠期间再次使用该声音线索进行诱导可以促进相应的海马神经元的重激活(Bendor & Wilson, 2012)。Fuentemilla等人通过对比海马病变的慢性颞叶癫痫患者和健康被试进行TMR之后的记忆效果,进一步证明了海马对于TMR的重要作用(Fuentemilla et al., 2013)。还有研究表明采用嗅觉线索对陈述性记忆进行TMR时,对比觉醒期,在SWS阶段进行TMR可以诱发更大的海马激活(Rasch et al., 2007)。此外,研究发现在SWS期间提示诱导能引起这些脑区更强的激活(Rasch et al., 2007; Diekelmann et al., 2011),使旁海马与内侧前额叶保持更强的连接(Rasch et al., 2007; Diekelmann et al., 2011; van Dongen et al., 2012)。这些结果与新皮层网络会随着记忆的不断巩固,将越来越多的涉及记忆加工过程的观点相符(Rasch & Born, 2013)。
3.2. 理论解释
睡眠对记忆影响的研究由来已久,对于睡眠与记忆关系的理论解释有很多,其中,突触稳态假说和系统巩固假说是被广泛接受的两种假说。而目前TMR神经机制的研究也主要参考这两种假说。突触稳态假说主要强调学习记忆的神经基础是中枢神经系统的可塑性。清醒时机体接受外界刺激引起突触联结增强,睡眠则是通过电生理慢震荡活动尤其是慢波活动,使突触联结成比例地减弱(Tononi & Cirelli, 2006),从而达到记忆巩固的效果;系统巩固假说主要强调从外界获得的信息暂存于海马,并逐渐转移至新皮层以进行长期存储。在学习过程中,传入信息快速地从新皮层传递至海马,然后在海马进行巩固。而在SWS阶段,乙酰胆碱和糖皮质激素都处于较低的状态,这种激素状态和电生理活动协同促进海马记忆痕迹的自发重激活并促使其向新皮质的巩固;而在REM睡眠阶段,乙酰胆碱和糖皮质激素释放增加,从而提升情绪性记忆和程序性记忆的巩固(Diekelmann & Born, 2010);两个假说都强调了慢波活动的重要作用,都可以在一定程度上解释TMR的神经活动过程。但是由于TMR的研究普遍关注于海马的作用,所以大部分研究都是用系统巩固假说来进行讨论解释。
然而最近的研究发现,在SWS阶段使用乙酰胆碱酯酶抑制剂毒扁豆碱提高被试的乙酰胆碱水平,结果并没有对听觉提示诱导的TMR的效果造成影响(Klinzing et al., 2017)。这表明在睡眠阶段进行TMR促进记忆巩固与睡眠促进记忆巩固可能除了上面所述的相似之处之外还具有不同的神经机制。因此,未来还需进行更多的对比研究来加以说明。
4. TMR成功实施的影响因素
TMR是一种促进记忆巩固的有效方法,但不少研究都表明TMR的效果受到实施方法的影响。如线索刺激材料的类型,线索刺激和学习材料之间的联结度(Rasch et al., 2007),线索刺激和目标记忆是否有特异性关联(Donohue & Spencer, 2011);线索刺激呈现的时长和时机都会在一定程度上影响TMR效果(Schreiner, Lehmann, & Rasch, 2015; He et al., 2015)。
4.1. 线索刺激类型
Rihm等人的研究表明,在学习和睡眠过程中呈现相同的气味是TMR促进陈述性记忆巩固所必需的条件。值得注意的是,使用嗅觉诱导的TMR研究表明,嗅觉线索刺激不需要与目标刺激材料在语义上相关,嗅觉刺激线索的情绪效价(即被试感到愉快或不愉快的程度)也不会影响后续陈述性记忆巩固的效果,这说明嗅觉线索对于陈述性记忆的诱导没有特异性(Rihm et al., 2014)。同样在以嗅觉刺激为提示诱导的TMR对情绪性记忆的研究中也有类似的结果(Hauner et al., 2013)。然而,在Rasch等人的研究中发现当使用嗅觉材料作为线索刺激时,不管提示诱导发生在SWS阶段、REM或清醒阶段都不会促进程序性记忆的巩固(Rasch et al., 2007)。因此,对于陈述性和情绪性记忆而言,嗅觉线索刺激不需要和目标记忆材料进行有意义联结,只需在觉醒学习和睡眠阶段都呈现一致的嗅觉线索材料;而对于程序性记忆的TMR,嗅觉材料可能并不适合作为线索刺激。
与嗅觉刺激不同,研究表明各种类型的听觉刺激如单一声音(如猫的喵喵声) (Rudoy et al., 2009),文字读音(Schreiner & Rasch, 2015; Schreiner, Lehmann, & Rasch, 2015; Groch et al., 2016)和旋律(Antony et al., 2012; Schoenauer, Geisler, & Gais, 2014; Cousins et al., 2014)作为声音背景线索进行TMR时都能促进记忆的巩固。一方面,这可能是因为嗅觉和听觉刺激在大脑中的处理方式不同(Potargowicz, 2008)。人体接受到的气味信息会绕过丘脑直接从嗅球传递到海马和杏仁核,而且嗅觉处理过程可能不直接涉及到技能学习/储存的大脑区域,如小脑、纹状体和运动皮层(Rasch et al., 2007)。因此,气味线索特别适合陈述性记忆和情绪性记忆的TMR研究(Zelano& Sobel, 2005)。另一方面,听觉刺激相比于嗅觉刺激可以很好的与记忆材料建立特异联结,甚至作为记忆内容的一部分。因此,听觉诱导的TMR能够广泛的应用到各类型记忆巩固(Rudoy et al., 2009; He et al., 2015; Antony et al., 2012)以及一些高级认知功能的研究中(Hu et al., 2015; Groch et al., 2016)。此外,研究表明当听觉线索与记忆材料没有特异联结时,听觉诱导的TMR也可以使得词汇回忆率提高(Fuentemilla et al., 2013),降低内隐偏差分数(Hu et al., 2015)。
4.2. 线索刺激呈现方法
TMR的效果还受到线索刺激呈现方式的影响。在觉醒期间进行TMR提示诱导,除了个别结果外(Fuentemilla et al., 2013),大多数的研究结果都表明不能促进记忆的巩固(Rasch et al., 2007; Rudoy et al., 2009; Antony et al., 2012; Schoenauer, Geisler, & Gais, 2014; Cousins et al., 2014; Hauner et al., 2013; Bendor & Wilson, 2012; Hars, Hennevin, & Pasques, 1985),甚至消退记忆(更多的遗忘) (Diekelmann et al., 2011; Barnes & Wilson, 2014; He et al., 2015);而在SWS期间实施提示诱导绝大多数的研究结果都显示能够促进记忆的巩固(Rasch et al., 2007; Rudoy et al., 2009; Diekelmann et al., 2011; Diekelmann et al., 2012; Rihm et al., 2014; Creery et al., 2015; Schreiner, Goeldi, & Rasch, 2015; Antony et al., 2012; Schoenauer, Geisler, & Gais, 2014; Cousins et al., 2014; Barnes & Wilson, 2014; Cairney et al., 2014) (除了少数研究(Oudiette et al., 2013; Hars & Hennevin, 1987; Hauner et al., 2013; He et al., 2015));至于在REM阶段进行TMR对于记忆的影响,目前的研究结果存在很大的争议,有研究报道TMR对陈述性记忆和程序性记忆没有效果(Rasch et al., 2007; Cordi et al., 2014),而有的研究则表明TMR能够促进陈述性记忆的泛化和整合(Sterpenich et al., 2014),也有研究报道认为TMR能使得情绪性记忆的消退或者增强。总的来说,在SWS睡眠阶段进行TMR最有希望促进记忆的巩固(Schouten et al., 2017)。
除此之外,线索刺激呈现的时长也会影响TMR效果。在He等人的研究中发现,接受3分钟线索刺激诱导的被试和没有接受提示诱导的被试相比,其恐惧记忆没有差异,而接受了10分钟提示诱导的被试相比于没有接受提示诱导的被试,其恐惧记忆减弱(He et al., 2015),这说明背景线索刺激呈现时长会在一定程度上影响记忆巩固的效果。但是Donohue等人在睡眠期间持续性的播放背景声音刺激,结果并没有促进记忆的巩固,研究人员推测,这可能是由于持续呈现背景声音刺激造成了被试的习惯化(Donohue & Spencer, 2011)。从现有的研究来看,目前尚不能明确TMR背景线索刺激是否有最佳的呈现时机和呈现时长。
5. 结语
睡眠期间运用TMR可以增强记忆巩固,并通过整合、重组和概括的方式对记忆进行操作(Sigman et al., 2014)。目前,已有的研究表明,TMR可以增强中年人的记忆(Backhaus et al., 2007)、可以在心理干预实施过程中缓解心理疾病如创伤后应激障碍、焦虑和抑郁等(He et al., 2015)、也可以促进洞察力的提升以及创造性地解决问题的能力(Sterpenich et al., 2014);还可以被用来消除不想要的记忆,改变偏见和减少社会偏见(Groch et al., 2016; Hu et al., 2015)等。鉴于目前的研究结果,TMR可以作为一种非侵入性和相对廉价的工具用以促进学习和记忆效果。因此,我们可以大胆想象用TMR技术去帮助有学习困难的儿童、因老龄化和疾病导致的记忆障碍、减少社会偏见、促进外语学习以及创造力和解决问题能力的发展等。
但是,目前的TMR研究水平还相当有限,实验结果也并非完全一致,在TMR能够安全有效地应用于教育或临床之前,还需要广泛的研究来解决许多开放式的问题,例如我们仍无法准确预测实施TMR后是否会增强记忆;长期使用TMR是否有潜在副作用;TMR具体的神经机制;TMR效果的持续时间等。这些问题都有待进一步研究和探索。不过随着睡眠医学的快速发展,TMR的普及应用指日可待。
文章引用
邓 翔,王小玲. 目标记忆重激活对记忆巩固影响的研究综述
The Impact of Targeted Memory Reactivation on Memory Consolidation: A Review[J]. 心理学进展, 2018, 08(02): 203-211. http://dx.doi.org/10.12677/AP.2018.82025
参考文献 (References)
- 1. Albouy, G., King, B. R., Maquet, P., & Doyon, J. (2013). Hippocampus and Striatum: Dynamics and Interaction during Acquisition and Sleep-Related Motor Sequence Memory Consolidation. Hippocampus, 23, 985-1004. https://doi.org/10.1002/hipo.22183
- 2. Antony, J. W., Gobel, E. W., O’Hare, J. K., Reber, P. J., & Paller, K. A. (2012). Cued Memory Reactivation during Sleep Influences Skill Learning. Nature Neuroscience, 15, 1114-1116. https://doi.org/10.1038/nn.3152
- 3. Backhaus, J., Born, J., Hoeckesfeld, R., Fokuhl, S., Hohagen, F., & Junghanns, K. (2007). Midlife Decline in Declarative Memory Consolidation Is Correlated with a Decline in Slow Wave Sleep. Learning & Memory, 14, 336-341. https://doi.org/10.1101/lm.470507
- 4. Barnes, D. C., & Wilson, D. A. (2014). Slow-Wave Sleep-Imposed Replay Modulates Both Strength and Precision of Memory. Journal of Neuroscience, 34, 5134-5142. https://doi.org/10.1523/JNEUROSCI.5274-13.2014
- 5. Bendor, D., & Wilson, M. A. (2012). Biasing the Content of Hippocampal Replay during Sleep. Nature Neuroscience, 15, 1439-1444. https://doi.org/10.1038/nn.3203
- 6. Born, J., Rasch, B., & Gais, S. (2006). Sleep to Remember. Neuroscientist, 12, 410-424. https://doi.org/10.1177/1073858406292647
- 7. Cairney, S. A., Durrant, S. J., Hulleman, J., & Lewis, P. A. (2014). Targeted Memory Reactivation during Slow Wave Sleep Facilitates Emotional Memory Consolidation. Sleep, 37, 701-707. https://doi.org/10.5665/sleep.3572
- 8. Cordi, M. J., Diekelmann, S., Born, J., & Rasch, B. (2014). No Effect of Odor-Induced Memory Reactivation during REM Sleep on Declarative Memory Stability. Frontiers in Systems Neuroscience, 8, 157. https://doi.org/10.3389/fnsys.2014.00157
- 9. Cousins, J. N., El-Deredy, W., Parkes, L. M., Hennies, N., & Lewis, P. A. (2014). Cued Memory Reactivation during Slow-Wave Sleep Promotes Explicit Knowledge of a Motor Sequence. Journal of Neuroscience, 34, 15870-15876. https://doi.org/10.1523/JNEUROSCI.1011-14.2014
- 10. Cox, R., Hofman, W. F., de Boer, M., & Talamini, L. M. (2014). Local Sleep Spindle Modulations in Relation to Specific Memory Cues. Neuroimage, 99, 103-110. https://doi.org/10.1016/j.neuroimage.2014.05.028
- 11. Creery, J. D., Oudiette, D., Antony, J. W., & Paller, K. A. (2015). Targeted Memory Reactivation during Sleep Depends on Prior Learning. Sleep, 38, 755-763. https://doi.org/10.5665/sleep.4670
- 12. Deuker, L., Olligs, J., Fell, J., Kranz, T. A., Mormann, F., Montag, C., Reuter, M., Elger, C. E., & Axmacher, N. (2013). Memory Consolidation by Replay of Stimulus-Specific Neural Activity. Journal of Neuroscience, 33, 19373-19383. https://doi.org/10.1523/JNEUROSCI.0414-13.2013
- 13. Diekelmann, S. (2015). Cueing Fear Memory during Sleep—To Extinguish or to Enhance Fear? Sleep, 38, 337-339. https://doi.org/10.5665/sleep.4484
- 14. Diekelmann, S., & Born, J. (2010). The Memory Function of Sleep. Nature Reviews Neuroscience, 11, 114-126. https://doi.org/10.1038/nrn2762
- 15. Diekelmann, S., Biggel, S., Rasch, B., & Born, J. (2012). Offline Consolidation of Memory Varies with Time in Slow Wave Sleep and Can Be Accelerated by Cuing Memory Reactivations. Neurobiology of Learning and Memory, 98, 103-111. https://doi.org/10.1016/j.nlm.2012.07.002
- 16. Diekelmann, S., Buechel, C., Born, J., & Rasch, B. (2011). Labile or Stable: Opposing Consequences for Memory When Reactivated during Waking and Sleep. Nature Neuroscience, 14, 381-386. https://doi.org/10.1038/nn.2744
- 17. Donohue, K. C., & Spencer, R. M. (2011). Continuous Re-Exposure to Environmental Sound Cues during Sleep Does Not Improve Memory for Semantically Unrelated Word Pairs. Journal of Cognitive Education and Psychology, 10, 167-177. https://doi.org/10.1891/1945-8959.10.2.167
- 18. Fuentemilla, L., Miro, J., Ripolles, P., Vila-Ballo, A., Juncadella, M., Castaner, S., Salord, N., Monasterio, C., Falip, M., & Rodriguez-Fornells, A. (2013). Hippocampus-Dependent Strengthening of Targeted Memories via Reactivation during Sleep in Humans. Current Biology, 23, 1769-1775. https://doi.org/10.1016/j.cub.2013.07.006
- 19. Gomez, R. L., Bootzin, R. R., & Nadel, L. (2006). Naps Promote Abstraction in Language-Learning Infants. Psychological Science, 17, 670-674. https://doi.org/10.1111/j.1467-9280.2006.01764.x
- 20. Groch, S., McMakin, D., Guggenbuhl, P., Rasch, B., Huber, R., & Wilhelm, I. (2016). Memory Cueing during Sleep Modifies the Interpretation of Ambiguous Scenes in Adolescents and Adults. Developmental Cognitive Neuroscience, 17, 10-18. https://doi.org/10.1016/j.dcn.2015.10.006
- 21. Hars, B., & Hennevin, E. (1987). Impairment of Learning by Cueing during Postlearning Slow-Wave Sleep in Rats. Neuroscience Letters, 79, 290-294. https://doi.org/10.1016/0304-3940(87)90446-0
- 22. Hars, B., Hennevin, E., & Pasques, P. (1985). Improvement of Learning by Cueing during Postlearning Paradoxical Sleep. Behavioural Brain Research, 18, 241-250. https://doi.org/10.1016/0166-4328(85)90032-4
- 23. Hauner, K. K., Howard, J. D., Zelano, C., & Gottfried, J. A. (2013). Stimulus-Specific Enhancement of Fear Extinction during Slow-Wave Sleep. Nature Neuroscience, 16, 1553-1555. https://doi.org/10.1038/nn.3527
- 24. He, J., Sun, H. Q., Li, S. X., Zhang, W. H., Shi, J., Ai, S. Z., Li, Y., Li, X. J., Tang, X. D., & Lu, L. (2015). Effect of Conditioned Stimulus Exposure during Slow Wave Sleep on Fear Memory Extinction in Humans. Sleep, 38, 423-431. https://doi.org/10.5665/sleep.4502
- 25. Hu, X., Antony, J. W., Creery, J. D., Vargas, I. M., Bodenhausen, G. V., & Paller, K. A. (2015). Unlearning Implicit Social Biases during Sleep. Science, 348, 1013-1015. https://doi.org/10.1126/science.aaa3841
- 26. Klinzing, J. G., Kugler, S., Soekadar, S. R., Rasch, B., Born, J., & Diekelmann, S. (2017). Odor Cueing during Slow-Wave Sleep Benefits Memory Independently of Low Cholinergic Tone. Psychopharmacology, 235, 291-299.
- 27. McGaugh, J. L. (2000). Memory—A Century of Consolidation. Science, 287, 248-251. https://doi.org/10.1126/science.287.5451.248
- 28. McNaughton, M., Wilson, A., & Bruce, L. (1994). Reactivation of Hippocampal Ensemble Memories during Sleep.
- 29. Oudiette, D., & Paller, K. A. (2013). Upgrading the Sleeping Brain with Targeted Memory Reactivation. Trends in Cognitive Sciences, 17, 142-149. https://doi.org/10.1016/j.tics.2013.01.006
- 30. Oudiette, D., Antony, J. W., Creery, J. D., & Paller, K. A. (2013). The Role of Memory Reactivation during Wakefulness and Sleep in Determining Which Memories Endure. Journal of Neuroscience, 33, 6672-6758. https://doi.org/10.1523/JNEUROSCI.5497-12.2013
- 31. Peigneux, P., Laureys, S., Fuchs, S., Collette, F., Perrin, F., Reggers, J., Phillips, C., Degueldre, C., Del Fiore, G., Aerts, J., Luxen, A., & Maquet, P. (2004). Are Spatial Memories Strengthened in the Human Hippocampus during Slow Wave Sleep? Neuron, 44, 535-545. https://doi.org/10.1016/j.neuron.2004.10.007
- 32. Potargowicz, E. (2008). Smell: The Unappreciated Human Sense. Postepy Higieny I Medycyny Doswiadczalnej, 62, 87-93.
- 33. Rasch, B., & Born, J. (2013). About Sleep’s Role in Memory. Physiological Reviews, 93, 681-766. https://doi.org/10.1152/physrev.00032.2012
- 34. Rasch, B., Buechel, C., Gais, S., & Born, J. (2007). Odor Cues during Slow-Wave Sleep Prompt Declarative Memory Consolidation. Science, 315, 1426-1429. https://doi.org/10.1126/science.1138581
- 35. Rihm, J. S., & Rasch, B. (2015). Replay of Conditioned Stimuli during Late REM and Stage N2 Sleep Influences Affective Tone Rather than Emotional Memory Strength. Neurobiology of Learning and Memory, 122, 142-151. https://doi.org/10.1016/j.nlm.2015.04.008
- 36. Rihm, J. S., Diekelmann, S., Born, J., & Rasch, B. (2014). Reactivating Memories during Sleep by Odors: Odor Specificity and Associated Changes in Sleep Oscillations. Journal of Cognitive Neuroscience, 26, 1806-1818. https://doi.org/10.1162/jocn_a_00579
- 37. Rolls, A., Makam, M., Kroeger, D., Colas, D., de Lecea, L., & Heller, H. C. (2013). Sleep to Forget: Interference of Fear Memories during Sleep. Molecular Psychiatry, 18, 1166-1170. https://doi.org/10.1038/mp.2013.121
- 38. Rudoy, J. D., Voss, J. L., Westerberg, C. E., & Paller, K. A. (2009). Strengthening Individual Memories by Reactivating Them during Sleep. Science, 326, 1079. https://doi.org/10.1126/science.1179013
- 39. Saletin, J. M., & Walker, M. P. (2012). Nocturnal Mnemonics: Sleep and Hippocampal Memory Processing. Frontiers in Neurology, 3, 59. https://doi.org/10.3389/fneur.2012.00059
- 40. Schoenauer, M., Geisler, T., & Gais, S. (2014). Strengthening Procedural Memories by Reactivation in Sleep. Journal of Cognitive Neuroscience, 26, 143-153. https://doi.org/10.1162/jocn_a_00471
- 41. Schouten, D. I., Pereira, S. I., Tops, M., & Louzada, F. M. (2017). State of the Art on Targeted Memory Reactivation: Sleep Your Way to Enhanced Cognition. Sleep Medicine Reviews, 32, 123-131. https://doi.org/10.1016/j.smrv.2016.04.002
- 42. Schreiner, T., & Rasch, B. (2015). Boosting Vocabulary Learning by Verbal Cueing during Sleep. Cerebral Cortex, 25, 4169-4179. https://doi.org/10.1093/cercor/bhu139
- 43. Schreiner, T., Goeldi, M., & Rasch, B. (2015). Cueing Vocabulary during Sleep Increases Theta Activity during Later Recognition Testing. Psychophysiology, 52, 1538-1543. https://doi.org/10.1111/psyp.12505
- 44. Schreiner, T., Lehmann, M., & Rasch, B. (2015). Auditory Feedback Blocks Memory Benefits of Cueing during Sleep. Nature Communications, 6, 8729-8729. https://doi.org/10.1038/ncomms9729
- 45. Sigman, M., Pena, M., Goldin, A. P., & Ribeiro, S. (2014). Neuroscience and Education: Prime Time to Build the Bridge. Nature Neuroscience, 17, 497-502. https://doi.org/10.1038/nn.3672
- 46. Skaggs, W. E., & McNaughton, B. L. (1996). Replay of Neuronal Firing Sequences in Rat Hippocampus during Sleep Following Spatial Experience. Science, 271, 1870-1873. https://doi.org/10.1126/science.271.5257.1870
- 47. Sterpenich, V., Schmidt, C., Albouy, G., Matarazzo, L., Vanhaudenhuyse, A., Boveroux, P., Degueldre, C., Leclercq, Y., Balteau, E., Collette, F., Luxen, A., Phillips, C., & Maquet, P. (2014). Memory Reactivation during Rapid Eye Movement Sleep Promotes Its Generalization and Integration in Cortical Stores. Sleep, 37, 1061-1075. https://doi.org/10.5665/sleep.3762
- 48. Stickgold, R. (2005). Sleep-Dependent Memory Consolidation. Nature, 437, 1272-1278. https://doi.org/10.1038/nature04286
- 49. Stickgold, R. (2013). Parsing the Role of Sleep in Memory Processing. Current Opinion in Neurobiology, 23, 847-853. https://doi.org/10.1016/j.conb.2013.04.002
- 50. Tononi, G., & Cirelli, C. (2006). Steep Function and Synaptic Homeostasis. Sleep Medicine Reviews, 10, 49-62. https://doi.org/10.1016/j.smrv.2005.05.002
- 51. van Dongen, E. V., Takashima, A., Barth, M., Zapp, J., Schad, L. R., Paller, K. A., & Fernandez, G. (2012). Memory Stabilization with Targeted Reactivation during Human Slow-Wave Sleep. Proceedings of the National Academy of Sciences of the United States of America, 109, 10575-10580. https://doi.org/10.1073/pnas.1201072109
- 52. Zelano, C., & Sobel, N. (2005). Humans as an Animal Model for Systems-Level Organization of Olfaction. Neuron, 48, 431-454. https://doi.org/10.1016/j.neuron.2005.10.009