Material Sciences
Vol.07 No.08(2017), Article ID:22822,10
pages
10.12677/MS.2017.78096
Synthesis and Properties of Novel Rattle-Type Fe@Fe3O4@TiO2 Nanochains
Xuebin Wei1, Shichuan Li1, Runze Tang1, Kai Yao2, Zunning Zhou1*
1School of Mechatronical Engineering, Beijing Institute of Technology, Beijing
2Shangdong Special Industry Group CO., LTD, Zibo Shandong
*通讯作者。

Received: Nov. 10th, 2017; accepted: Nov. 22nd, 2017; published: Nov. 28th, 2017

ABSTRACT
Rattle-type Fe@Fe3O4@TiO2 nanochains were synthesized by a facile magnetic-field-induced (MFI) assembly method. The morphology and composition of the as-prepared products were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). The diameter of the aligned nanoparticles (NPs) in the magnetic rattle-type chains is about 200 nm, and the length of the nanochain is about 2 - 4 μm. Vibrating sample magnetometer (VSM) measurement reveals that the Fe@Fe3O4@TiO2 nanochains are ferromagnetic with a saturation magnetization of 30.9 emu/g. The electromagnetic characteristics of Fe@Fe3O4@TiO2 nanochains were investigated at 1 - 18 GHz by vector network analyzer. The nanochains show super microwave absorption performance due to the presence of multi-shell structure and chainlike structure.
Keywords:Rattle-Type Structure, MFI Assembly, Nanochains, Kirkendall Effect, Absorption Performance
新型核壳Fe@Fe3O4@TiO2纳米链的 合成与性能
魏学宾1,李石川1,汤润泽1,姚凯2,周遵宁1*
1北京理工大学机电学院,北京
2山东特种工业集团有限公司,山东 淄博

收稿日期:2017年11月10日;录用日期:2017年11月22日;发布日期:2017年11月28日

摘 要
本文采用简单的磁场诱导自组装法制备了核壳Fe@Fe3O4@TiO2纳米链,并通过透射电镜、扫描电镜、X射线衍射、X射线光电子能谱表征其形貌和组成。纳米粒子的粒径在200 nm左右,链长约为2~4 μm。利用振动磁强计研究其磁性能,发现纳米链为铁磁性,饱和磁化强度为30.9 emu/g。采用矢量网络分析仪调查了Fe@Fe3O4@TiO2在1~18 GHz频段的电磁特性结果显示由于多重核壳结构和链状结构的存在,粒子链具有优异的微波吸收性能。
关键词 :核壳结构,磁性诱导自组装,纳米链,柯肯达尔效应,吸波性能

Copyright © 2017 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
近年来,一维磁性纳米结构由于其特殊的光、电、磁性能备受关注 [1] [2] 。迄今,各种各样的方法以用来制备一维磁性纳米结构,如模板法 [3] ,偶极力自组装法 [4] ,磁场诱导自组装法 [5] [6] [7] 等。磁场诱导自组装法由于其操作相对简单和具有通用性,是制备一维磁性纳米结构的最常见的方法之一。例如,王辉等人通过简单的磁场诱导自组装方法制备了具有优异稳定性的一维弱铁磁纳米结构 [8] 。殷亚东课题组通过磁性自组装工艺获得的纳米链,可以在磁场中排列并衍射可见光 [1] 。
核壳纳米结构是提高纳米粒子化学和胶体稳定性的一种特殊结构 [9] 。最近,报道了许多有趣的核壳纳米结构,如多层中空结构 [10] 、蛋黄结构 [11] [12] 和其它各向异性 [13] [14] 结构。尤其是蛋黄型纳米粒子,由于其在核心和外壳之间存在空隙,具有较高的比表面积、较大的孔体积和化学稳定性,在吸附 [15] ,水处理 [16] 和药物递送 [17] 等领域有广泛应用 [18] 。例如,何千军课题组开发了一种新的超顺磁性核壳型纳米粒子,其具有优异的靶向给药能力和独特的超声波触发NO释放的特征 [19] 。
在本文中,通过磁场诱导自组装法制备了蛋黄型的Fe@Fe3O4@TiO2纳米链。基于柯肯达尔效应提出了空心纳米结构的形成机理 [20] 。而Fe@Fe3O4@TiO2纳米链的电磁波吸收性能优于报道的纯Fe3O4 [21] ,有望成为一种新型吸收材料。
2. 实验部分
2.1. 试剂与仪器
无水乙醇,正丁醇,钛酸四丁酯(TBOT),硼氢化钠(NaBH4),十六烷基三甲基溴化铵(CTAB),七水硫酸亚铁(FeSO4∙7H2O),以上试剂均购自北京化工厂,纯度均为分析纯。JSM7001F扫描电子显微镜,JEM2010F透射电子显微镜,Lakeshore 7410振动磁强计,Mastersizer 2000激光粒度仪,Perkin Elmer PHI 5600光谱仪,矢量网络分析仪(Agilent HP8722ES),傅里叶红外光谱仪(EQUINOX 55,BRUKER)。
2.2. Fe@Fe3O4@TiO2纳米链合成实验
1) 在氩气保护下,将CTAB (5 mmol)和FeSO4∙7H2O (1.5 mmol)溶解于250 mL去离子水中。在机械搅拌下缓慢加入10 ml新鲜制备的NaBH4 (0.8 M)溶液。约3 min后,透明溶液突然变黑,表明生成铁纳米粒子。15 min后,将溶液暴露于由氩气和空气(9:1,v/v)组成的气氛中。1 h后,通过磁性分离收集黑色沉淀,分别用去离子水和无水乙醇先后洗涤三次,除去表面活性剂和其他杂质。
2) 将沉淀物重新分散在55 mL丁醇和乙醇(10:1,v/v)溶液中,移入烧瓶并磁力搅拌。10 min后,将TBOT (0.45 mL)和无水乙醇(10 ml)的混合溶液缓慢滴入烧瓶中并保持25℃。然后将溶液搅拌1 h,使TBOT在颗粒表面上的充分吸附。最后,将15 mL乙醇水溶液(3:7,v/v)滴加到烧瓶中。8 h后,通过磁性分离收集沉淀。分别用去离子水和无水乙醇反复洗涤磁性纳米链,除去杂质,并在50℃的真空烘箱中干燥。
3. 结果与讨论
3.1. 形貌和结构分析
图1为样品的SEM图像。从图1(a)中可以看出大多数颗粒组装成链,长度在2~4 μm。进一步放大图像,可以看到,纳米粒子的形状平滑,尺寸相对均匀,在50~300 nm左右(图1(b))。在相应的能量色散光谱中观察到O,Ti和Fe元素的峰值(图1(c))。并且O,Ti和Fe元素的质量分数分别约为61.47%,6.46%,32.07%,这与添加的试剂的量一致。碳元素的存在来自于在测试过程之前应用的导电粘合剂。
图2是样品的TEM图像,从图中可看出粒子的直径几乎在50至300 nm之间,这与上述结果相同。此外,颗粒是均匀球形的,具有中空的双层壳结构,壳厚度约为25 nm,中空结构的厚度约为15 nm (图2(a)的右插图)。由于纳米晶体具有较大的比表面积,处于不稳定状态 [22] [23] ,这导致纳米晶体的聚集,形成较大的颗粒(图2(b))。当然,外界磁场的存在也有助于纳米晶体的聚集。图2(c)是采用激光粒度仪得到的粒径分布数据,大多数颗粒的直径在50~250 nm之间,这与SEM和TEM的结果一致。
3.2. 成分分析
Fe@Fe3O4@TiO2纳米链的形成过程如图3所示。根据反应式 [24] :
(1)
(2)
(3)
(4)
在NaBH4的辅助下,Fe2+首先还原成Fe纳米粒子。同时,由于较高的表面能,小的Fe纳米粒子聚集形成较大的纳米团簇。阶段2是TiO2的包覆和纳米链的形成阶段。在磁力搅拌下,由于纳米簇之间的磁偶极–偶极相互作用比布朗运动和静电排斥强 [25] [26] ,纳米簇将沿着磁感线方向以头尾相连的方式聚集在一起并重新排列。本实验中外部磁场强度为200 Gs。在自组装过程中,包覆过程也在同时进行。首先,TBOT水解,然后脱水生成TiO2。正丁醇降低了TBOT的水解速率,使得TiO2均匀地生长在颗粒表面。TiO2的存在削弱了样品的饱和磁化强度,提高了纳米链的抗氧化性能和稳定性。此外,由于纳米级的柯肯达尔效应 [20] ,Fe3O4和Fe之间形成了一层中空结构(图4)。反应溶液中的少量氧气导致反应(2)发生。随着时间的推移,由于氧的向内扩散速率远远小于Fe的向外扩散速率 [24] ,Fe颗粒越来越小,进而形成了中空结构。图5证实了上述过程。从图中可以看出纳米链的元素分布。Fe分布在纳米链的中间部分,而O和Ti分布在整个纳米链上。O和Ti的分布不均匀,纳米链中部O和Ti的含量明显低于边缘部分,表明颗粒是蛋黄式结构。
采用X射线衍射(XRD)进一步证实了样品的结构,结果如图6(a)所示。谱线在30.1˚,35.4˚,37.0˚,43.0˚,53.9˚,56.9˚,62.6˚的特征峰分别对应(220),(311),(222),(400),(422),(511),和(440)晶面,

Figure 1. (a), (b) are the SEM images of Fe@Fe3O4@TiO2; (c) is the EDS image of Fe@Fe3O4@TiO2
图1. (a), (b)是Fe@Fe3O4@TiO2的SEM图;(c)是Fe@Fe3O4@TiO2的EDS能谱图

Figure 2. (a), (b) are the TEM images of Fe@Fe3O4@TiO2; (c)is the particle size distribution of the Fe@Fe3O4@TiO2
图2. (a) (b)是Fe@Fe3O4@TiO2的TEM图;(c)是Fe@Fe3O4@TiO2的粒径分布图

Figure 3. Schematic of nanochains preparation
图3. 纳米链制备的示意图
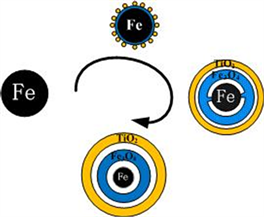
Figure 4. Synthesis of Fe@Fe3O4@TiO2 NPs
图4. Fe@Fe3O4@TiO2纳米颗粒形成示意图

Figure 5. TEM mapping images of Fe@Fe3O4@TiO2; (a) TEM image of nanochain; (b) (c) (d) are distribution images of Fe, O, Ti
图5. (a)是Fe@Fe3O4@TiO2的TEM图;(b),(c),(d)分别是Fe,O,Ti的元素分布图

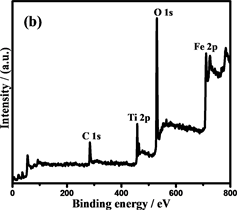
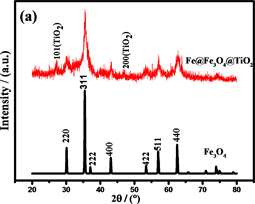
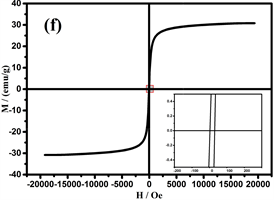
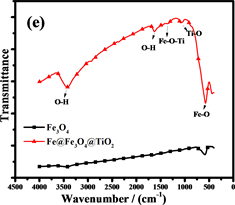
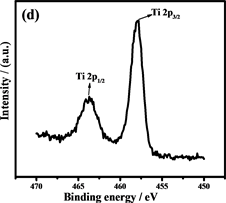
Figure 6. (a) is the XRD patterns of Fe3O4 andFe@Fe3O4@TiO2; (b),(c), (d) are the XPS spectrum of Fe@Fe3O4@TiO2, (e) is the FTIR analysis of Fe3O4 and Fe@Fe3O4@TiO2;(f) are the Hysteresis loop of Fe@Fe3O4@TiO2
图6. (a)是Fe3O4和Fe@Fe3O4@TiO2的XRD图,(b),(c),(d)是Fe@Fe3O4@TiO2的XPS图,(e)是Fe3O4和Fe@Fe3O4@TiO2的FTIR图,(f)是Fe@Fe3O4@TiO2的磁滞回线
与Fe3O4 (JCPDS No.19-629)的特征峰相一致。然而,在XRD图中没有明显的Fe衍射峰,表明Fe颗粒是无定形的。在包覆TiO2之后,在2θ为25.1˚和38.0˚处出现了特征峰。这些衍射峰与JCPDSNo.21-1272 (包覆TiO2)中的数据一致。然而,在30.1˚和43.0˚的特征峰与Fe3O4的特相同 [27] [28] 。由谢乐公式 [29] 计算的Fe@Fe3O4TiO2的平均晶粒尺寸为约15 nm。
通过X射线光电子能谱(XPS)表征复合材料的表面组成和Fe和Ti的化合价态。光谱(图6(b))显示纳米链由Fe,O和Ti元素组成(C为校准元素)。图6(c)是Fe 2p的XPS光谱,在710.6和723.9 eV的两个主要峰分别为Fe 2p3/2和Fe 2p1/2,这与Fe3O4的报导值相近,表明Fe2O3不存在于复合粒子中 [30] 。此外,在707.0 eV处的非常弱的峰对应为Fe0,进一步证实了复合粒子中少量Fe的存在 [31] 。同时在457.9和463.7 eV的两个峰,证明了TiO2的存在 [32] 。Fe@Fe3O4@TiO2的FTIR光谱(图6(e))也证明了纳米链中Fe3O4和TiO2的存在 [33] [34] 。
3.3. 磁性分析
可调谐的磁性作为Fe3O4的重要特性,在许多领域得到了广泛的应用 [35] [36] [37] 。图7是样品在室温(298 K)下,20kOe的磁场中测得的磁滞回线。纳米链中由于存在非磁性TiO2壳,饱和磁化强度(Ms)比Fe3O4颗粒较低为30.9 emu/g [38] 。此外,剩余磁化强度(Mr)和矫顽力(Hc)为1.8 emu/g和16.8 Oe (图7的右下插图),表明纳米链是弱铁磁性的。当颗粒尺寸小于临界尺寸时,Fe3O4将表现出超顺磁性,临界尺寸约为20 nm [39] 。综上所述,组装成链的纳米团簇由许多小的纳米晶体组成,晶粒尺寸(约15 nm)表明Fe3O4是超顺磁性的。然而,少量的Fe的存在使纳米链为弱铁磁性。
3.4. 电磁吸收性能
电磁吸收性能由材料的电磁参数决定,包括复磁导率 和复介电常数 。Fe@Fe3O4@TiO2纳米链和Fe3O4纳米粒子的电磁参数如图7所示。试样厚度为2 mm,温度为0℃,湿度为0.0%,粉末蜡复合材料的质量比为60%。
根据传输线理论 [40] [41] ,Fe@Fe3O4@TiO2纳米链和Fe3O4纳米粒子的反射损耗(RL)通过以下方程计算:
(5)
(6)
其中Zin是材料的输入阻抗; 和 分别是复磁导率和复介电常数;c是自由空间中电磁波的速度;f是微波的频率,d是吸收体的厚度。从图8(a)可以看出,磁性纳米线链RL的最大值在13.5 GHz时约为−15 dB。此外,RL在12~18 GHz的范围内低于−10 dB,这表明在高频区域具有良好的电磁吸收性能。相比之下,Fe3O4纳米粒子的RL相对较小,在14.5 GHz时,RL的最大值仅为−7 dB。
阻抗匹配和能量损耗是电磁吸收性能的主要决定性因素。材料的电磁吸收性能与材料的阻抗匹配和能量损耗性能成正比 [42] 。从方程(5)和(6)可以推断:当电磁参数满足 (损耗正切),阻抗匹配最好,结果如图7(c)和图7(f)所示。显然,纳米链的阻抗匹配性能优于Fe3O4颗粒,特别是在高频区域(12~18 GHz),阻抗匹配在13.5 GHz时达到最佳。图8(a)中材料的RL曲线证明了这一点。换句话说,由Fe,Fe3O4和TiO2形成的多层壳结构大大减小了入射的电磁波的在入射界面的散射和反射,可以最大程度进入材料(阻抗匹配)。
磁滞损耗,畴壁共振和涡流效应是磁性材料能量损耗的主要原因。磁性测量表明纳米链接近超顺磁性,表明磁滞损耗可以忽略不计。畴壁共振通常发生在1~100 MHz的范围内,因此也可以忽略。如果磁损耗仅来自涡流效应,则C (C = )的值应为恒定值 [43] 。图8(b)是Fe3O4纳米粒子和纳米链的C-f曲线。Fe3O4纳米粒子的曲线比纳米链的曲线波动大,表明纳米链具有更强的涡流效应。从图中可以看出,在5~18 GHz下,纳米链的值约为0.007。因此,涡流损耗是5~18 GHz范围内的主要磁损耗。此外,二氧化钛的存在增加了材料的各向异性能和介电损耗 [44] 。
另外,电磁吸收特性与磁性纳米材料的小尺寸效应有关。纳米材料的高比表面积将导致形成更多的偶极点。偶极极化有助于电磁吸收性能提高。第二,在制备过程(湿化学和热处理过程)中,材料中产生许多缺陷。缺陷的存在将促进在费米能级附近建立基态。随着电磁波入射到材料上,电子从基态向费米能级跃迁,这将提高材料的电磁吸收性能。第三,来自TiO2和Fe3O4的介电损耗和磁损耗之间的互补性将有助于增强的电磁吸收性能。第四,在Fe@Fe3O4@TiO2纳米链中存在着多重界面。由于多重散射,吸收和界面极化,这些界面有助于改善电磁吸收特性。如图9(a)所示,入射波被TiO2,Fe3O4和Fe吸收。
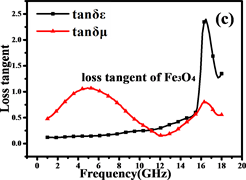
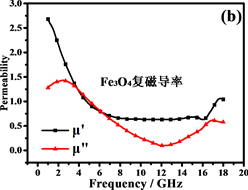

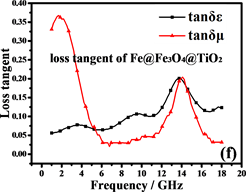

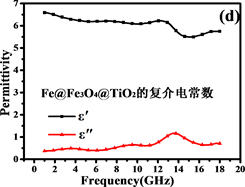
Figure 7. Electromagnetic parameters and loss tangents of Fe3O4and Fe@Fe3O4@TiO2
图7. Fe3O4and Fe@Fe3O4@TiO2的电磁参数和损耗正切
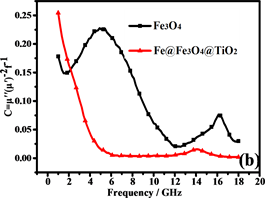
Figure 8. (a) RL of Fe3O4 and Fe@Fe3O4@TiO2; (b) C-f curves of Fe3O4 and Fe@Fe3O4@TiO2
图8. (a)是Fe3O4 and Fe@Fe3O4@TiO2的曲线;(b)是Fe3O4 and Fe@Fe3O4@TiO2的 C-f曲线

Figure 9. Schematic diagram of multiple scatterin
图9. 多重散射示意图
同时,在两个介质的边界发生散射,纳米颗粒之间的空隙可以为电磁波的散射提供更多的位置。此外,由于各种介质的存在,电磁波发生多次散射。常见的核壳材料通常只存在2~3个界面(图9(b)),入射光的散射和吸收能力受到限制。当颗粒组装成链时,形成更复杂的结构(图9(c)),这大大增加了电磁波的散射和吸收。因此,结构复杂性的增加可以提高材料的吸收性能。
4. 结论
本文通过简便的磁场诱导自组装方法制备了具有稳定结构的核壳型Fe@Fe3O4@TiO2纳米链。由于纳米级的柯肯达尔效应,内部形成蛋黄式的中空多层结构。除了水的微环境和反应时间外,氧分压对于形成中空纳米结构非常重要。TiO2的存在,提高了纳米链的抗氧化能力和能量损耗,并使得阻抗匹配性能在12~18 GHz的范围内变得更好。蛋黄式的链状结构使电磁波发生强烈的多重散射和吸收,RL小于−10 dB。因此,这种新型Fe@Fe3O4@TiO2纳米链在吸波材料中具有潜在的应用。
基金项目
国家自然科学基金(11672041)资助项目。
文章引用
魏学宾,李石川,汤润泽,姚 凯,周遵宁. 新型核壳Fe@Fe3O4@TiO2纳米链的合成与性能
Synthesis and Properties of Novel Rattle-Type Fe@Fe3O4@TiO2 Nanochains[J]. 材料科学, 2017, 07(08): 735-744. http://dx.doi.org/10.12677/MS.2017.78096
参考文献 (References)
- 1. Hu, Y., He, L. and Yin, Y. (2011) Magnetically Responsive Photonic Nanochains. Angewandte Chemie-International Edition, 50, 3747-3750.
https://doi.org/10.1002/anie.201100290 - 2. Wang, H.U.I., Yu, Y., Sun, Y., et al. (2011) Magnetic Nanochains: A Review. Nano, 6, 1-17.
https://doi.org/10.1142/S1793292011002305 - 3. Lu, H.B., Liao, L., Li, J.C., et al. (2008) Hematite Nanochain Networks: Simple Synthesis, Magnetic Properties, and Surface Wettability. Applied Physics Letters, 92, 093102.
- 4. Klokkenburg, M., Vonk, C., Claesson, E.M., et al. (2004) Direct Imaging of Zero-Field Dipolar Structures in Colloidal Dispersions of Synthetic Magnetite. Journal of the American Chemical Society, 126, 16706-16707.
https://doi.org/10.1021/ja0456252 - 5. Niu, H.L., Chen, Q.W., Ning, M., et al. (2004) Synthesis and One-Dimensional Self-Assembly of Acicular Nickel Nanocrystallites under Magnetic Fields. Journal of Physical Chemistry B, 108, 3996-3999.
https://doi.org/10.1021/jp0361172 - 6. Wang, M., He, L. and Yin, Y. (2013) Magnetic Field Guided Colloidal Assembly. Materials Today, 16, 110-116.
https://doi.org/10.1016/j.mattod.2013.04.008 - 7. Kralj, S. and Makovec, D. (2015) Magnetic Assembly of Su-perparamagnetic Iron Oxide Nanoparticle Clusters into Nano Chains and Nanobundles. ACS Nano, 9, 9700-9707.
https://doi.org/10.1021/acsnano.5b02328 - 8. Wang, H., Chen, Q.W., Sun, L.X., et al. (2009) Magnet-ic-Field-Induced Formation of One-Dimensional Magnetite Nanochains. Langmuir, 25, 7135-7139.
https://doi.org/10.1021/la900234n - 9. Guerrero Martinez, A., Perez Juste, J. and Liz Marzan, L.M. (2010) Recent Progress on Silica Coating of Nanoparticles and Related Nanomaterials. Advanced Materials, 22, 1182-1195.
https://doi.org/10.1002/adma.200901263 - 10. Yu, L., Wu, H.B. and Lou, X.W.D. (2017) Self-Templated For-mation of Hollow Structures for Electrochemical Energy Applications. Accounts of Chemical Research, 50, 293-301.
https://doi.org/10.1021/acs.accounts.6b00480 - 11. Liu, J., Xu, J., Che, R., et al. (2013) Hierarchical Fe3O4@TiO2 Yolk-Shell Microspheres with Enhanced Microwave-Absorption Properties. Chemistry-A European Journal, 19, 6746-6752.
https://doi.org/10.1002/chem.201203557 - 12. Wu, Z.C., Li, W.P., Luo, C.H., et al. (2015) Photothermal Ablation: Rattle-Type Fe3O4@CuS Developed to Conduct Magnetically Guided Photoinduced Hyperthermia at First and Second NIR Biological Windows. Advanced Functional Materials, 25, 6527-6537.
https://doi.org/10.1002/adfm.201503015 - 13. Niu, Z., Becknell, N., Yu, Y., et al. (2016) Anisotropic Phase Segregation and Migration of Pt in Nanocrystals En Route to Nanoframe Catalysts. Nature Maters, 15, 1188-1194.
https://doi.org/10.1038/nmat4724 - 14. Ge, J., Hu, Y., Zhang, T., et al. (2007) Superparamagnetic Composite Colloids with Anisotropic Structures. Journal of the American Chemical Society, 129, 8974-8975.
https://doi.org/10.1021/ja0736461 - 15. Yin, Y.Y., Zhou, S.X., Min, C., et al. (2011) Preparation of Rattle-Type Magnetic Mesoporous Carbon Spheres and Their Highly Efficient Adsorption and Separation. Journal of Colloid and Interface Science, 361, 527-533.
https://doi.org/10.1016/j.jcis.2011.05.014 - 16. Kalantari, M., Yu, M., Noonan, O., et al. (2017) Rattle-Type Magnetic Mesoporous Hollow Carbon as a High-Performance and Reusable Adsorbent For Water Treatment. Chemo-sphere, 166, 109-117.
https://doi.org/10.1016/j.chemosphere.2016.09.083 - 17. Zhao, W., Chen, H., Li, Y., et al. (2008) Uniform Rat-tle-Type Hollow Magnetic Mesoporous Spheres as Drug Delivery Carriers and Their Sustained-Release Property. Ad-vanced Functional Materials, 18, 2780-2788.
https://doi.org/10.1002/adfm.200701317 - 18. Liang, C., Li, Z. and Dai, S. (2008) Mesoporous Carbon Materials: Synthesis and modification. Angewandte Chemie-International Edition, 47, 3696-3717.
https://doi.org/10.1002/anie.200702046 - 19. Jin, Z., Wen, Y., Hu, Y., et al. (2017) MRI-Guided and Ultra-sound-Triggered Release of NO by Advanced Nanomedicine. Nanoscale, 9, 3637-3645.
https://doi.org/10.1039/C7NR00231A - 20. Yin, Y., Rioux, R.M., Erdonmez, C.K., et al. (2004) Formation of Hollow Nanocrystals through the Nanoscale Kirkendall Effect. Science, 304, 711-714.
https://doi.org/10.1126/science.1096566 - 21. Li, S., Zhou, Z., Zhang, T., et al. (2014) Synthesis and Characteri-zation of Ag/Fe3O4 Electromagnetic Shielding Particles. Journal of Magnetism and Magnetic Materials, 358, 27-31.
https://doi.org/10.1016/j.jmmm.2014.01.026 - 22. Thess, A., Lee, R., Nikolaev, P., et al. (1996) Crystalline Ropes of Metallic Carbon Nanotubes. Science, 273, 483-487.
https://doi.org/10.1126/science.273.5274.483 - 23. Luo, P., Nieh, T.G., Schwartz, A.J., et al. (1995) Surface Characterization of Nanostructured Metal and Ceramic Particles. Materials Science and Engineering A—Structural Materials Properties Microstructure and Processing, 204, 59-64.
https://doi.org/10.1016/0921-5093(95)09938-7 - 24. Huang, J., Chen, W., Zhao, W., et al. (2009) One-Dimensional Chainlike Arrays of Fe3O4 Hollow Nanospheres Synthesized by Aging Iron Nanoparticles in Aqueous Solution. Journal of Physical Chemistry C, 113, 12067-12071.
https://doi.org/10.1021/jp810662j - 25. Vargas, E., Melo, W.W.M., Allende, S., et al. (2015) Dipolar-Driven Formation of Cobalt Nanoparticle Chains in Polyethylene Films. Materials Chemistry and Physics, 162, 229-233.
https://doi.org/10.1016/j.matchemphys.2015.05.062 - 26. Ilg, P. (2008) Importance of Depletion Interactions for Structure and Dynamics of Ferrofluids. The European Physical Journal E, 26, 169-176.
https://doi.org/10.1140/epje/i2007-10248-6 - 27. Abbas, M., Rao, B.P., Reddy, V., et al. (2014) Fe3O4/TiO2 Core/Shell Nanocubes: Single-Batch Surfactantless Synthesis, Characterization and Efficient Catalysts for Methylene Blue Degradation. Ceramics International, 40, 11177-11186.
https://doi.org/10.1016/j.ceramint.2014.03.148 - 28. Yu, X., Liu, S. and Yu, J. (2011) Superparamagnetic γ-Fe2O3@SiO2@TiO2 Composite Microspheres with Superior Photocatalytic Properties. Applied Catalysis B: Envi-ronmental, 104, 12-20.
https://doi.org/10.1016/j.apcatb.2011.03.008 - 29. Monshi, A., Foroughi, M.R. and Monshi, M.R. (2012) Modi-fied Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World Journal of Nano Science & Engineering, 2, 154-160.
https://doi.org/10.4236/wjnse.2012.23020 - 30. Wang, T., Liu, Z., Lu, M., et al. (2013) Graphene-Fe3O4 Nano-hybrids: Synthesis and Excellent Electromagnetic Absorption Properties. Journal of Applied Physics, 113, Article ID: 024314.
https://doi.org/10.1063/1.4774243 - 31. Klaus, S., Trotochaud, L., Cheng, M.J., et al. (2016) Experimental and Computational Evidence of Highly Active Fe Impurity Sites on the Surface of Oxidized Au for the Electrocatalytic Oxidation of Water in Basic Media. ChemElectroChem, 3, 66-73.
https://doi.org/10.1002/celc.201500364 - 32. Yang, H.G. and Zeng, H.C. (2012) Preparation of Hollow Anatase TiO2 Nanospheres via Ostwald Ripening. Journal of Physical Chemistry B. Condensed Matter Materials Surfaces In-terfaces & Biophysical, 108, 3492-3495.
- 33. Hu, H., Wang, Z., Pan, L., et al. (2010) Ag-Coated Fe3O4@SiO2 Three-Ply Composite Microspheres: Synthesis, Characterization, and Application in Detecting Melamine with Their Surface-Enhanced Raman Scattering. Journal of Physical Chemistry C, 114, 7738-7742.
https://doi.org/10.1021/jp100141c - 34. Qian, G., Chen, F., Zhang, J., et al. (2009) The Study of Novel Fe3O4@γ-Fe2O3 Core/Shell Nanomaterials with Improved Properties. Journal of Magnetism & Magnetic Materials, 321, 1052-1057.
https://doi.org/10.1016/j.jmmm.2008.10.022 - 35. Venkatesan, M., Nawka, S., Pillai, S.C., et al. (2003) Enhanced Magnetoresistance in Nanocrystalline Magnetite. Journal of Applied Physics, 93, 8023-8025.
https://doi.org/10.1063/1.1555371 - 36. Hu, F.Q., Wei, L., Zhou, Z., et al. (2006) Preparation of Biocompatible Magnetite Nanocrystals for in Vivo Magnetic Resonance Detection of Cancer. Advanced Materials, 18, 2553-2556.
https://doi.org/10.1002/adma.200600385 - 37. Goya, G.F., Berquo, T.S., Fonseca, F.C., et al. (2003) Static and Dynamic Magnetic Properties of Spherical Magnetite Nanoparticles. Journal of Applied Physics, 94, 3520-3528.
https://doi.org/10.1063/1.1599959 - 38. Kim, E.H., Lee, H.S., Kwak, K.B., et al. (2005) Synthesis of Ferrofluid with Magnetic Nanoparticles by soNochemical Method for MRI Contrast Agent. Journal of Magnetism and Magnetic Materials, 289, 328-330.
https://doi.org/10.1016/j.jmmm.2004.11.093 - 39. Zhu, Y., Zhao, W., Chen, H., et al. (2007) A Simple One-Pot Self-Assembly Route to Nanoporous and Monodispersed Fe3O4 Particles with Oriented Attachment Structure and Magnetic Property. Journal of Physical Chemistry C, 111, 5281-5285.
https://doi.org/10.1021/jp0676843 - 40. Chen, Y.J., Zhang, F., Zhao, G.G., et al. (2010) Synthesis, Mul-ti-Nonlinear Dielectric Resonance, and Excellent Electromagnetic Absorption Characteristics of Fe3O4/ZnO Core/Shell Nanorods. Journal of Physical Chemistry C, 114, 9239-9244.
https://doi.org/10.1021/jp912178q - 41. Wen, S., Liu, Y., Zhao, X., et al. (2014) Synthesis, Multi-Nonlinear Dielectric Resonance and Electromagnetic Absorption Properties of hcp-Cobalt Particles. Journal of Magnetism and Magnetic Materials, 354, 7-11.
https://doi.org/10.1016/j.jmmm.2013.10.030 - 42. Du, Y., Liu, W., Qiang, R., et al. (2014) Shell Thick-ness-Dependent Microwave Absorption of Core-Shell Fe3O4@C Composites. ACS Applied Materials & Interfaces, 6, 12997-13006.
https://doi.org/10.1021/am502910d - 43. Wu, M.Z., Zhang, Y.D., Hui, S., et al. (2002) Microwave Magnetic Properties of Co50/(SiO2)50 Nanoparticles. Applied Physics Letters, 80, 4404-4406.
https://doi.org/10.1063/1.1484248 - 44. Zhu, C.L., Zhang, M.L., Qiao, Y.J., et al. (2010) Fe3O4/TiO2 Core/Shell Nanotubes: Synthesis and Magnetic and Electromagnetic Wave Absorption Characteristics. Journal of Physical Chem-istry C, 114, 16229-16235.
https://doi.org/10.1021/jp104445m