Material Sciences
Vol.
13
No.
04
(
2023
), Article ID:
64674
,
7
pages
10.12677/MS.2023.134037
Co3(PO4)2/Ta3N5纳米花的控制合成及 光催化解水析氢性能
孟祥磊
哈尔滨师范大学化学化工学院,黑龙江 哈尔滨
收稿日期:2023年3月6日;录用日期:2023年4月20日;发布日期:2023年4月27日

摘要
本文以TaCl5为钽源,经水热、高温氮化、光沉积过程制得Co3(PO4)2/Ta3N5纳米花。考查了Co3(PO4)2修饰量对样品光生载流子分离效率的影响规律;在Co3(PO4)2修饰量为2 wt%时,样品光电流为0.561 μA∙cm−2,是Ta3N5纳米花(0.1 μA∙cm−2)的5.61倍,载流子分离效率明显提升。表面修饰Co3(PO4)2后,形成Co3(PO4)2/Ta3N5局域异质结构;增强了样品在可见光区域的光吸收性能,降低了HER和OER过电位。在模拟太阳光照射下,样品光催化解水产氢活性为417.6 μmol∙g−1∙h−1,明显高于未修饰的Ta3N5样品(165.4 μmol∙g−1∙h−1)。
关键词
Ta3N5,Co3(PO4)2,异质结,太阳光催化,水分解产氢

Controllable Synthesis and Photocatalytic Hydrogen Evolution of Co3(PO4)2/Ta3N5 Nanoflowers
Xianglei Meng
School of Chemistry and Chemical Engineering, Harbin Normal University, Harbin Heilongjiang
Received: Mar. 6th, 2023; accepted: Apr. 20th, 2023; published: Apr. 27th, 2023

ABSTRACT
In this paper, Co3(PO4)2/Ta3N5 nanoflowers were prepared through hydrothermal, high temperature nitriding, and photochemical deposition processes, using TaCl5 as tantalum source. The effect of Co3(PO4)2 modification amount on the separation efficiency of photo generated carrier in the sample was investigated. When the modification amount of Co3(PO4)2 was 2 wt%, the photocurrent density of the sample was 0.561 μA∙cm−2, which was 5.61 times that of Ta3N5 nanoflower (0.1 μA∙cm−2), and the carrier separation efficiency was improved obviously. After surface modification of Co3(PO4)2, the local heterostructure of Co3(PO4)2/Ta3N5 was constructed, which enhanced the light absorption properties of the samples in the visible region and reduced over potential of HER and OER. Under simulated sunlight irradiation, the photocatalytic water splitting into hydrogen activity of Co3(PO4)2/Ta3N5 was 417.6 μmol∙g−1∙h−1, significantly higher than that of the unmodified Ta3N5 sample (165.4 μmol∙g−1∙h−1).
Keywords:Ta3N5, Co3(PO4)2, Heterojunction, Solar Photocatalysis, Water Splitting into Hydrogen

Copyright © 2023 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
目前,世界人口的快速增长和社会工业的迅猛发展加剧了能源短缺与环境污染问题 [1] [2] 。氢气作为清洁绿色能源具有广阔发展前景,利用半导体光催化材料分解水产氢有望大规模推广应用。氮化钽带隙能相对较小,导带和价带的位置符合太阳能光解水产氢的需求,是一种理想的可见光响应催化剂。但是,由于其存在载流子易复合、稳定性差等缺点而限制了其实际应用。
据报道,在半导体光催化剂上负载助催化剂可以显著提高载流子分离效率,显著增强光催化活性和稳定性。Ge等 [3] 利用磷酸钴(Co-Pi)修饰的石墨化氮化碳(g-C3N4),表面沉积的磷酸钴能够捕获空穴,促进光生载流子分离和转移,进而增强了其光催化活性。
本文主要通过水热合成–高温氮化–光沉积联合技术,成功制备Co3(PO4)2/Ta3N5复合纳米光催化剂。利用仪器分析手段进行结构及性能表征,研究了Co3(PO4)2表面修饰对Ta3N5样品载流子分离效率、光吸收性能、光催化解水析氢性能的影响规律,为进一步开发高效Ta3N5基光催化剂提供了实验技术支持。
2. 实验部分
2.1. 仪器与试剂
试剂:五氯化钽(分析纯),湖南省华京粉体有限公司;乙醇(分析纯),天津市光复科技有限公司;浓盐酸(分析纯),氢氟酸(分析纯),硫酸钠(化学纯),磷酸二氢钠(分析纯),磷酸氢二钠(分析纯)天津市科密欧化学试剂有限公司;硝酸钴(分析纯),福晨化学试剂有限公司;柠檬酸钠(分析纯),天津奥普升化工有限公司。
仪器:管式炉MXG1200-40S,上海微行炉业有限公司;氙灯500 W,上海蓝晟电子有限公司;X射线衍射仪Shimadzu XRD-6000,日本岛津公司;透射电子显微镜Tecnai G2TF20,美国FEI公司;NOVA2000E型物理吸附仪,美国Quantachrome公司;紫外/可见分光光度计Shimadzu UV-2550,日本岛津公司;CHI660E电化学工作站,上海辰华有限公司;产氢测试Labsolar-IIIAG,北京泊菲莱科技有限公司。
2.2. Co3(PO4)2/Ta3N5纳米花的控制合成
2.2.1. Ta3N5纳米花的控制合成
首先,采用水热合成法,称取0.2687 g TaCl5粉末、2.2 mL HF、0.4 mL HCl、7 mL乙醇、10.4 mL水、适量柠檬酸钠,将上述药品放入烧杯中混合均匀后,磁力搅拌30 min,搅拌完成后装入25 mL聚四氟乙烯反应釜中160℃水热6 h。所得样品冷却至室温,经蒸馏水洗涤3次、过滤,再经80℃干燥2 h,制得Ta2O5纳米花。再利用高温氮化技术,取0.25 g Ta2O5粉末平铺于刚玉瓷舟中,将瓷舟放入管式炉中,在50 mL∙min−1氨气流中,经850℃氮化3 h,制得Ta3N5纳米花。
2.2.2. Co3(PO4)2/Ta3N5纳米花的控制合成
取0.2 g Ta3N5粉体,加入到50 mL磷酸盐缓冲溶液中,Co3(PO4)2修饰量分别为1.0~5.0 wt%。通入氮气后,在氮气气氛下避光暴气30 min,在500 W氙灯照射下搅拌光沉积5 h;经抽滤、洗涤、干燥,制得Co3(PO4)2/Ta3N5纳米花。
3. 结果与讨论
3.1. Co3(PO4)2修饰量对光生载流子分离效率的影响
图1为Ta3N5及不同修饰量Co3(PO4)2/Ta3N5样品的光电流密度曲线。由图1可见,与Ta3N5相比,Co3(PO4)2修饰后导致样品光电流强度增大,当Co3(PO4)2修饰量为1.0 wt%时,样品光电流强度有微弱提高;当修饰量为2.0 wt%时,光电流强度达最大值;即0.561 μA∙cm−2;修饰量继续增大至3.0~5.0 wt%时,样品光电流强度逐渐降低。表面沉积的Co3(PO4)2起到捕获空穴的作用,促进了载流子的有效分离,提高了光电流响应及其稳定性。
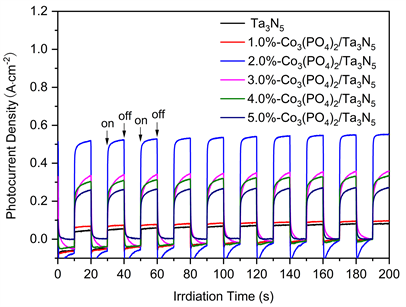
Figure 1. Photocurrent density curves of Ta3N5 and Co3(PO4)2/Ta3N5 with different modification amounts
图1. Ta3N5及不同修饰量Co3(PO4)2/Ta3N5样品的光电流密度曲线
3.2. Co3(PO4)2/Nb4N5纳米光催化剂的结构性能表征
3.2.1. XRD分析
Ta3N5和Co3(PO4)2/Ta3N5样品的XRD谱如图2所示。可见,样品位于17.32˚、24.54˚、31.50˚、35.02˚、36.14˚、39.36˚、44.23˚、46.85˚、53.69˚、57.55˚、处的特征衍射峰归属于Ta3N5的(200)、(110)、(−203)、(310)、(−113)、(−402)、(−403)、(020)、(512)、(−223)晶面(JCPDS NO.89-5200) [4] ,证实样品主晶相为Ta3N5。未观察到Co3(PO4)2特征衍射峰,这是由于Co3(PO4)2修饰量为2 wt%,远低于XRD仪器检测限。Co3(PO4)2/Ta3N5与Ta3N5相比较,特征衍射峰位置和强度基本没有变化;说明光沉积Co3(PO4)2后,对Ta3N5相结构和结晶度几乎无影响。

Figure 2. X-ray diffraction patterns of Ta3N5 and Co3(PO4)2/Ta3N5
图2. Ta3N5和Co3(PO4)2/Ta3N5纳米花的X射线衍射图
3.2.2. SEM分析
Ta2O5、Ta3N5和Co3(PO4)2/Ta3N5样品的SEM图像如图3(a)、图3(b)、图3(c)所示。由图像可知,Ta2O5呈现由长度约为300~400 nm的纳米棒组成的纳米花;经高温氮化后,组成Ta3N5纳米花的纳米棒的长度明显缩短,少部分纳米棒伴有交联现象,是由2N替代3O引起晶格塌陷及界面耦合所致;再经光沉积后,所得Co3(PO4)2/Ta3N5纳米花与Ta3N5纳米花形貌无明显差异。

Figure 3. SEM images of (a) Ta2O5 (b) Ta3N5 and (c) Co3(PO4)2/Ta3N5
图3. (a) Ta2O5、(b) Ta3N5和(c) Co3(PO4)2/Ta3N5的SEM照片
3.2.3. HRTEM分析
图4为Co3(PO4)2/Ta3N5样品的高分辨透射电子显微镜(HRTEM)分析图像。显然,晶格间距为0.362 nm的衍射条纹对应Ta3N5的(110)晶面 [5] ,晶格间距为0.244 nm衍射条纹对应Co3(PO4)2的(031)晶面 [6] 。结果证实,Co3(PO4)与Ta3N5表面形成了紧密的Co3(PO4)2/Ta3N5异质结构,有助于光生载流子的快速分离,从而提高材料的光催化活性。
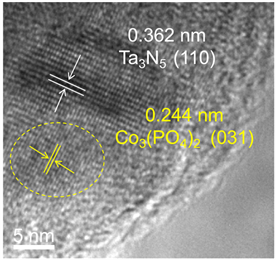
Figure 4. HRTEM image of Co3(PO4)2/Ta3N5
图4. Co3(PO4)2/Ta3N5的HRTEM照片
3.2.4. DRS分析
Ta3N5和Co3(PO4)2/Ta3N5样品的UV-vis吸收光谱如图5所示,与未修饰的Ta3N5相比,Co3(PO4)2/Ta3N5样品在380 nm以上的紫外光区及可见光区光吸收能力显著提升。Co3(PO4)2/Ta3N5样品吸收带边由Ta3N5的617 nm拓展到624 nm。Co3(PO4)2耦合后,样品可见光吸收能力增强是由于Co(II)的d-d跃迁导致在400 nm~750 nm处产生较强的光吸收 [7] [8] 。

Figure 5. UV-vis absorption spectra of Ta3N5 and Co3(PO4)2/Ta3N5 nanoflowers
图5. Ta3N5和Co3(PO4)2/Ta3N5纳米花的UV-vis吸收光谱
3.2.5. 过电位曲线分析
图6为Ta3N5和Co3(PO4)2/Ta3N5样品的线性扫描伏安法曲线。由图可知,在10 mA∙cm−2电流密度下,Co3(PO4)2/Ta3N5样品的HER过电位由Ta3N5的665 mV降低至647 mV;耦合样品的OER过电位由Ta3N5的898 mV降低至614 mV,Co3(PO4)2耦合明显降低了HER和OER过电位,提高了样品表面光催化产氢产氧反应动力学 [9] [10] 。
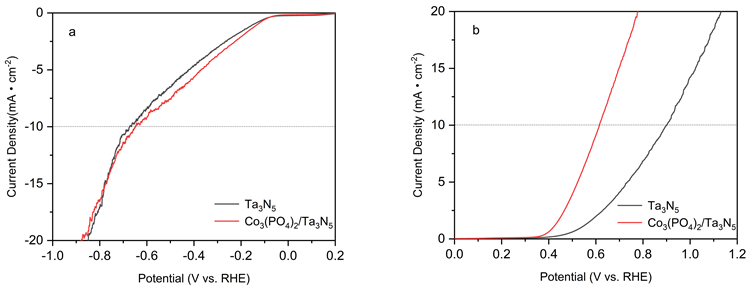
Figure 6. (a) Hydrogen evolution (b) oxygen evolution over potential diagram of Ta3N5 and Co3(PO4)2/Ta3N5 nanoflowers
图6. Ta3N5和Co3(PO4)2/Ta3N5纳米花的(a)析氢(b)析氧过电位曲线图
3.2.6. 可见光催化解水析氢活性
在500 W氙灯的模拟太阳光照射下,Co3(PO4)2/Ta3N5样品光催化解水产氢活性为417.6 μmol∙g−1∙h−1,明显高于未修饰的Ta3N5 (165.4 μmol∙g−1∙h−1)。光催化解水析氢性能的提升归因于,Co3(PO4)2耦合增强了紫外及可见光吸收能力,促进了光生载流子的分离和转移,降低了HER和OER过电位,提高了样品表面光催化产氢产氧反应动力学。
4. 结论
综上所述,以TaCl5为钽源,利用水热合成–高温氮化–光沉积联合技术,成功制备Co3(PO4)2/Ta3N5异质结纳米花。当Co3(PO4)2修饰量为2.0 wt%时,样品光电流强度高达0.561 μA∙cm−2,是未修饰Ta3N5 (0.1 μA∙cm−2)的5.61倍。构建Co3(PO4)2/Ta3N5异质结,明显增强样品在紫外光区到可见光区的光吸收能力,有效抑制光生载流子复合,降低HER和OER过电位,提高表面催化反应动力学。在模拟太阳光照射下,光催化解水产氢活性为417.6 μmol∙g−1∙h−1,远高于未修饰的Ta3N5。本文为进一步开发新型高性能Ta3N5基光催化材料提供了实验技术支持。
文章引用
孟祥磊. Co3(PO4)2/Ta3N5纳米花的控制合成及光催化解水析氢性能
Controllable Synthesis and Photocatalytic Hydrogen Evolution of Co3(PO4)2/Ta3N5 Nanoflowers[J]. 材料科学, 2023, 13(04): 330-336. https://doi.org/10.12677/MS.2023.134037
参考文献
- 1. Xu, J.S., Jiang, H.P., Yu, X.H., Gao, J.S., Yang, J. and Liu, Q.Q. (2023) Progress and Challenges in Full Spectrum Photocatalysts: Mechanism and Photocatalytic Applications. Journal of Industrial and Engineering Chemistry, 119, 112-129. https://doi.org/10.1016/j.jiec.2022.11.057
- 2. Chen, P., Liu, F., Ding, H., Chen, S., Chen, L., Li, Y., Au, C. and Yin, S. (2019) Porous Double-Shell CdS@C3N4 Octahedron Derived by in Situ Supramolecular Self-Assembly for Enhanced Photocatalytic Activity. Applied Catalysis B: Environmental, 252, 33-40. https://doi.org/10.1016/j.apcatb.2019.04.006
- 3. Ge, L., Han, C., Xiao, X. and Guo, L. (2013) In Situ Synthesis of Cobalt-Phosphate (Co-Pi) Modified g-C3N4 Photocatalysts with Enhanced Photocatalytic Activities. Applied Catalysis B: Environmental, 142-143, 414-422. https://doi.org/10.1016/j.apcatb.2013.05.051
- 4. Cui, X., Gong, Y.H., Liu, Y.P., Yu, H.B., Qin, W.C. and Huo, M.G. (2022) Synthesis of a Z-Scheme Ternary Photocatalyst (Ta3N5/Ag3PO4/AgBr) for the Enhanced Photocatalytic Degradation of Tetracycline under Visible Light. Journal of Physics and Chemistry of Solids, 170, 110962. https://doi.org/10.1016/j.jpcs.2022.110962
- 5. Xiao, M., Luo, B., Thaweesak, S.H. and Wang, L.Z. (2018) No-ble-Metal-Free MoS2/Ta3N5 Heterostructure Photocatalyst for Hydrogen Generation. Progress in Natural Science: Mate-rials International, 28, 189-193. https://doi.org/10.1016/j.pnsc.2018.02.003
- 6. Shi, W.L., Li, M.Y., Huang, X.L., Ren, H.J., Yan, C. and Guo, F. (2019) Facile Synthesis of 2D/2D Co3(PO4)2/g-C3N4 Heterojunction for Highly Photocatalytic Overall Water Splitting under Visible Light. Chemical Engineering Journal, 19, 122960. https://doi.org/10.1016/j.cej.2019.122960
- 7. Di, T.G., Zhu, B.C., Zhang, J., Cheng, B. and Yu, J.G. (2016) Enhanced Photocatalytic H2 Production on CdS Nanorod Using Cobalt-Phosphate as Oxidation Coatalyst. Applied Sur-face Science, 389, 775-782. https://doi.org/10.1016/j.apsusc.2016.08.002
- 8. Lu, K.Q., Qi, M.Y., Tang, Z.R. and Xu, Y.J. (2019) Earth-Abundant MoS2 and Cobalt Phosphate Dual Cocatalysts on 1D CdS Nanowires for Boosting Photocatalytic Hy-drogen Production. Langmuir, 35, 11056-11065. https://doi.org/10.1021/acs.langmuir.9b01409
- 9. Li, L., Gao, H., Liu, G., Wang, S., Yi, Z., Wu, X. and Yang, H. (2022) Synthesis of Carnation Flower-Like Bi2O2CO3 Photocatalyst and Its Promising Application for Photoreduction of Cr (VI). Advanced Powder Technology, 33, 103481. https://doi.org/10.1016/j.apt.2022.103481
- 10. Guo, J.H., Shi, H.X., Huang, X.B., Shi, H.F. and An, Z.F. (2018) AgCl/Ag3PO4: A Sable Ag-Based Nanocomposite Photocatalyst with Enhanced Photocatalytic Activity for the Degrada-tion of Parabens. Journal of Colloid and Interface Science, 515, 10-17. https://doi.org/10.1016/j.jcis.2018.01.015