Material Sciences
Vol.08 No.05(2018), Article ID:24788,9
pages
10.12677/MS.2018.85048
Progress on the Fabrication of Rough Surface for Superhydrophobicity and Its Application
Yin He1, Yongmao Hu2, Shuhong Sun1, Yan Zhu1*
1Kunming University of Science and Technology, Kunming Yunnan
2Dali University, Dali Yunnan

Received: Mar. 20th, 2018; accepted: Apr. 30th, 2018; published: May 7th, 2018

ABSTRACT
The superhydrophobic surface has attracted attentions in the field of industry and scientific research due to its features such as self-cleaning, drag reduction and dust-repellent properties. This article reviews the progress on the preparation of rough surface for superhydrophobicity and its application. Meanwhile, the disadvantages of superhydrophobic surfaces are evaluated and their development directions are discussed.
Keywords:Superhydrophobic Surface, Micro and Nano Structures, Rough Surface
超疏水表面粗糙结构的构造 及其应用研究进展
贺胤1,胡永茂2,孙淑红1,朱艳1*
1昆明理工大学,云南 昆明
2大理大学,云南 大理

收稿日期:2018年3月20日;录用日期:2018年4月30日;发布日期:2018年5月7日

摘 要
超疏水表面具有自清洁、减阻、防尘等多种特性,在生产生活及科研领域备受瞩目。本文综述了超疏水表面制备方法的研究进展及其在不同领域的应用进展。最后简要讨论了超疏水表面存在的不足,并对其发展方向进行了展望。
关键词 :超疏水表面,微纳结构,表面粗糙结构

Copyright © 2018 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
在雨水、日照或风吹等自然外力作用下即可自动清除或降解表面污垢灰尘的自清洁表面自问世以来即受到广泛关注。过去的十几年中,受荷叶效应的启发,拥有超疏水特性的自清洁表面成为了发展的主流 [1] [2] [3] [4] 。超疏水材料依靠在表面构建微纳结构从而获得稳定的气–液层(Cassie-Baxter体系) [5] ,使得水滴无法附着。一般来说,超疏水表面通常指水接触角大于150˚,同时滚动角小于10˚的表面 [6] 。
自二十世纪九十年代中期以来,超疏水一直是一个活跃的科研领域。一般来说,适用于微米结构和纳米结构构造的技术如光刻、化学刻蚀、沉积和自组装等,都已经被广泛应用于超疏水表面粗糙结构的构造(表1)。尤为有趣的是,通过结合荷叶效应与X射线辐射、动态效应、光学效应 [6] [7] [8] [9] 等方式,超疏水表面可以直接由亲水材质表面改性得到。
获得超疏水表面的两个主要关键因素是低表面能及合适的粗糙度 [1] 。基于此,超疏水表面的制备方法也分为两种:第一,将原本就疏水的材料通过构造粗糙表面得到超疏水性能;第二,通过降低粗糙的亲水表面的表面能或在其上施加疏水材料来得到超疏水特性 [10] 。值得注意的是,粗糙度通常比低表面能更为关键,因为适度疏水和超疏水的材料在表面粗糙时都表现出相似的润湿行为。所以本文总结了粗糙度的构造方法,从刻蚀法、沉积法、铸造法等几个大类的最新研究进展进行了综述。
Table 1. Regular method for constructing a rough surface
表1. 构造粗糙表面的一般方法
2. 刻蚀法制备粗糙结构
在所有刻蚀法中,光刻技术能够构建出最为均匀的粗糙结构,一般用于创建大面积的周期性微/纳米结构。Bhushan和Jung [34] 利用激光刻蚀在硅表面构建了均匀的微米阵列,使表面达到疏水状态,接着利用气相沉积法将1,1-2,2-全氟十二烷基三氯硅烷(PF3)沉积在样品表面从而获得了一个接触角高达170˚的超疏水表面。Martines及其团队 [35] 利用电子束光刻技术制备出有序排列的纳米坑和纳米柱阵列,组合成一个尖顶面状结构,再使用十八烷基三氯硅烷(OTS)修饰后获得了静态接触角为164˚、接触角滞后只有1˚的超疏水表面。
Qian和Shen [17] 通过简单的化学刻蚀法在金属基底构造出超疏水表面(见图1)。首先,对铝等多种金属进行位错选择性化学腐蚀,再利用氟硅烷进行修饰,最终,被蚀刻的金属表面表现出了超疏水特性。
3. 形变法在制备粗糙结构
拉伸法也被用于制备超疏水表面。Zhang [36] 及其团队通过拉伸聚四氟乙烯膜使其转变为在表面具有很多空隙的纤维状晶体,从而使其表面粗糙度增大得到超疏水特性(如图2)。
4. 沉积法制备粗糙结构
Shibuichi [37] 等人将烷基乙烯酮二聚体(AKD)涂抹于玻璃板上,使其自发在玻璃表面形成分形结构。在没有任何氟化处理的情况下,他们获得了一个接触角大于170˚的超疏水表面。Klein及其团队 [38] 将基板简单的浸泡入含有纳米二氧化硅微球的浆液中,通过低温加热处理基板让微球自动吸附其上,氟化后的表面便具备了超疏水特性,并且疏水效果随球体面积减小而增大。Ma等人 [39] 通过静电纺丝法,以四氢呋喃和二甲基甲酰胺溶液制备出直径范围在150~400纳米的纤维嵌段共聚物,其水接触角为163˚。Shiu [12] 及其团队利用旋涂法将聚苯乙烯微球溶液涂抹于基板表面得到单分散的涂层。Huang及其团队 [40] 通过化学气相沉积法控制碳纳米管阵列的生长,制备出具有双层粗糙度的表面(如图3所示)。Zhao等人 [41] 通过化学气相沉积法在硅衬底上由铁和铝作为催化剂合成垂直排列的多壁碳纳米管(CNTs)阵列,氟化修饰后得到超疏水表面。Bormashenko等人 [42] 采用蒸镀法,将聚苯乙烯(PS)、聚碳酸酯(PC)和聚甲基丙烯酸甲酯(PMMA)等聚合物溶解在二氯甲烷(CH2Cl2)、氯仿(CHCl3)等氯化溶剂,通过结构间的自组装获得粗糙结构,从而制备超疏水表面。
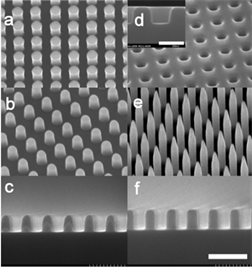
Figure 1. SEM images of (a-c) structure before hydrophobization; (d-f) structure after hydrophobization [17]
图1. 表面结构的SEM图像:(a-c) 疏水化前的表面结构;(d-f) 疏水化后的表面结构 [17]
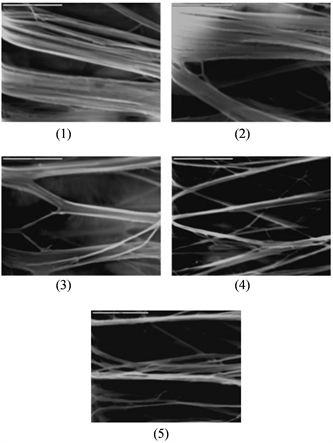
Figure 2. SEM images of the Teflon tape at different extension ratios: (1) Ɛ = 5 ± 5%; (2) Ɛ = 35 ± 5%; (3) Ɛ = 90 ± 5%; (4) Ɛ = 140 ± 5%; (5) Ɛ = 190 ± 5% [36]
图2. Teflon胶带在不同延伸比下的SEM图像。(1) Ɛ = 5 ± 5%;(2) Ɛ = 35 ± 5%;(3) Ɛ = 90 ± 5%;(4) Ɛ = 140 ± 5%;(5) Ɛ = 190 ± 5% [36]
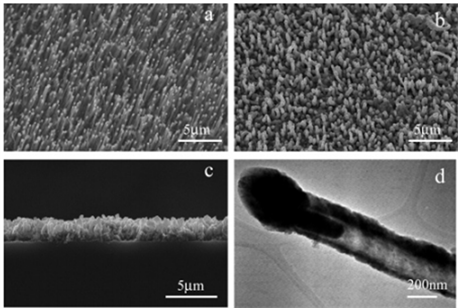
Figure 3. SEM images of (a) as-grown aligned CNT template, (b) topography of ZnO-coated CNTs, (c) cross-sectional view of ZnO-coated CNTs, and (d) TEM image of an individual ZnO-coated CNT [40]
图3. SEM图,(a) CNT模板,(b) ZnO涂覆的CNT的形貌,(c) ZnO涂覆的CNT的截面图,以及(d) ZnO涂覆CNT的TEM图像 [40]
5. 铸造法制备粗糙结构
Yabu和Shimomura等人 [43] 在潮湿环境下利用含氟嵌段聚合物溶液,制备出了多孔超疏水性透明薄膜。透明性的实现是因为蜂窝状图案化薄膜的孔径在亚波长范围。Sun及其团队 [44] 报道了一种纳米模板法构造超疏水PDMS表面的方法。他们首先使用荷叶作为原始模板制作了一个阴面PDMS模板,然后使用阴面模板倒膜获得含有荷叶结构的PDMS模板。Zhao及其团队 [45] 基于气相诱导相分离和PDMS表面富集的方法,在潮湿环境中浇铸共聚物,形成超疏水表面(如图4所示)。Lee及其团队 [46] 利用纳米多孔阳极氧化铝作为复制模板,在温度及压力的驱动下,采用纳米压印技术,获得垂直排列的聚苯乙烯纳米纤维。随着聚苯乙烯纳米纤维长径比的增加,纳米纤维不再垂直生长,而是弯曲向下,互相扭曲形成三维粗糙结构,静态水接触角大约为155˚。
6. 超疏水表面的应用
超疏水表面最基本的应用即来源于其自清洁能力,水滴滚落或滑落的同时带走表面附着的污染物。在液体流动过程中,为了达到自清洁的功能及较低的阻力,除了接触角大之外,超疏水表面还应该具有非常低的水接触角滞后(CAH)。前进角(contact advancing angle)和后退角(contact receding angle)是两个静态值,它们之间的差值便被称为接触角滞后 [47] 。它是由于表面粗糙度和表面不均匀性而产生的。接触角滞后反映出了润湿/去湿循环的不可逆性,它是液滴沿固体表面流动时能量耗散的一种量度。具有低接触角滞后的表面具有低的水倾斜角度,它表示水滴滚落时表面必须倾斜的最小角度。水倾斜角越小,越有利于颗粒污染物的去除。通常情况下,我们将接触角滞后小于10º的表面称为自清洁表面。自清洁表面在各种应用中都广泛出现,包括自清洁窗户、挡风玻璃、建筑、船舶、外墙涂料用具、屋顶瓦片、纺织品(如图5所示)、太阳能电池板等。并且,在某些需要减小流体阻力的领域,如排水管、微/纳通道等处也有超疏水的应用机会 [48] 。

Figure 4. SEM images of (a) a two-dimensional PS microsphere array, (b) a PDMS negative replica [45]
图4. SEM图像,(a) 二维PS微球阵列,(b) PDMS阴面模板 [45]

Figure 5. Water droplets on (i) untreated woven cotton sheet, (ii) CNT treated woven cotton sheet and (iii) poly (butyl acrylate)-CNT-treated woven cotton sheet [48]
图5. 液滴在(i) 未处理的棉片,(ii) CNT处理后的棉片,(iii) 聚丙烯酸丁酯-CNT处理后的棉片 [48]
另外,超疏水表面的最新进展使得其被用于节约能源 [49] 与能源转换 [50] [51] 成为可能。首先,一个表面的疏水/亲水性能会显著影响毛细粘附力,反过来,也会影响固体表面滑动接触过程中摩擦和能量的耗散。选择合适的超疏水表面就可以降低能量的耗散。第二,超疏水和疏油表面,可以降低燃油运输过程中的损耗。第三,最近发现的可逆超疏水表面为新的能量转换提供了潜在可能,如微型毛细管发动机 [51] 。
湿润可能导致在静态或滑动接触过程中亲水性的固体之间的界面形成的凹形半月板 [52] 。这些半月板会产生负的压力进而导致一种内部吸引力,从而增加粘附力和摩擦力。在某些情况下,湿摩擦力可大于干摩擦力,而这通常是不利的 [53] 。另一方面,某些应用中,高黏附力则是有好处的,例如磁带和细胞生物材料表面的粘附。因此,增强润湿性在这些应用中是有利的 [54] 。在磁存储设备和微/纳机电系统(MEMS/NEMS)中,需要极小的黏附力甚至没有粘附力和摩擦力。随着这些设备的尺寸减小,往往会由表面力来支配体积力,因此,如何减小粘附力和摩擦力成为了这些设备正常运行的一个挑战。以上原因使得非粘性超疏水表面的发展对许多新兴应用起到至关重要的作用 [55] [56] 。
7. 总结及展望
受限于气–液层自身的不稳定性及微纳结构的脆弱性,超疏水材料的实用性大打折扣。在实际应用中,在海洋或输水管道等拥有高压、水流连续冲击或微生物附着的环境下,外界液体很容易渗透进微纳结构中破坏气–液层从而使表面丧失疏液效果。因此,开发出结构强度更高、拥有自修复能力的超疏水表面是接下来研究者的共同目标。例如,通过更合理的结构排列方式来提高材料的整体强度。另一方面,通过加热、紫外辐射等方式来实现聚合物超疏水表面的自修复 [57] 。另外,如何实现超疏水表面环保、经济的工业化生产也值得进一步研究。
基金项目
国家自然科学基金(No.11764003)。
文章引用
贺 胤,胡永茂,孙淑红,朱 艳. 超疏水表面粗糙结构的构造及其应用研究进展
Progress on the Fabrication of Rough Surface for Superhydrophobicity and Its Application[J]. 材料科学, 2018, 08(05): 429-437. https://doi.org/10.12677/MS.2018.85048
参考文献
- 1. Genzer, J. and Efimenko, K. (2000) Creating Long-Lived Superhydrophobic Polymer Surfaces through Mechanically Assembled Monolayers. Science, 290, 2130-2133.
https://doi.org/10.1126/science.290.5499.2130 - 2. Roach, P., Shirtcliffe. N.J. and Newton, M.I. (2008) Progess in Superhydrophobic Surface Development. Soft Matter, 4, 224-240.
https://doi.org/10.1039/B712575P - 3. Yang, Z., Wang, L., Sun, W., et al. (2017) Superhydrophobic Epoxy Coating Modified by Fluorographene Used for Anti-Corrosion and Self-Cleaning. Applied Surface Science, 401.
https://doi.org/10.1016/j.apsusc.2017.01.009 - 4. Zhang, X., Shi, F., Niu, J., et al. (2008) Superhydrophobic Surfaces: From Structural Control to Functional Application. Journal of Materials Chemistry, 18, 621-633.
https://doi.org/10.1039/B711226B - 5. Gene, W., Edward, B. and Tamir, S. (2008) The Rigorous Derivation of Young, Cassie-Baxter and Wenzel Equations and the Analysis of the Contact Angle Hysteresis Phenomenon. Chemical Physics Letters, 450, 355-359.
https://doi.org/10.1016/j.cplett.2007.11.033 - 6. Polizos, G., Tuncer, E., Qiu, X., et al. (2011) Nonfunctionalized Polydimethyl Siloxane Superhydrophobic Surfaces Based on Hydrophobic-Hydrophilic Interactions. Langmuir, 27, 2953-2957.
https://doi.org/10.1021/la1042712 - 7. Jung, Y.C. and Bhushan, B. (2009) Dynamic Effects Induced Transition of Droplets on Biomimetic Superhydrophobic Surfaces. Langmuir, 25, 9208-9218.
https://doi.org/10.1021/la900761u - 8. Bhushan, B. (2012) Fabrication Techniques Used for Structures with Superhydrophobicity, Self-Cleaning, Low Adhesion/Low Drag with Antifouling Properties.
- 9. Nakajima, A., Fuji-shima, A., Hashimoto, K., et al. (2000) ChemInform Abstract: Preparation of Transparent Superhydrophobic Boehmite and Silica Films by Sublimation of Aluminum Acetylacetonate. Cheminform, 31.
https://doi.org/10.1002/chin.200001251 - 10. Nosonovsky, M. (2007) Multiscale Roughness and Stability of Su-perhydrophobic Biomimetic Interfaces. Langmuir, 23, 3157-3161.
https://doi.org/10.1021/la062301d - 11. Accardo, A., Gentile, F., Mecarini, F., et al. (2010) In Situ X-Ray Scattering Studies of Protein Solution Droplets Drying on Micro- and Nanopatterned Superhydrophobic PMMA Surfaces. Langmuir, 26, 15057-15064.
https://doi.org/10.1021/la102958w - 12. Shiu, J., Kuo, C., Chen, P., et al. (2004) Fabrication of Tunable Super-hydrophobic Surfaces by Nanosphere Lithography. Chemistry of Materials, 16, 561-564.
https://doi.org/10.1021/cm034696h - 13. Accardo, A., Burghammer, M., Di, C.E., et al. (2011) Calcium Carbonate Mineralization: X-Ray Microdiffraction Probing of the Interface of an Evaporating Drop on a Superhydrophobic Surface. Langmuir, 27, 8216-8222.
- 14. Darmanin, T., De Givenchy, E.T., Amigoni, S., et al. (2013) Superhydrophobic Surfaces by Electrochemical Processes. Advanced Materials, 25, 1378-1394.
https://doi.org/10.1002/adma.201204300 - 15. Bae, G.Y., Jang, J., Jeong, Y.G., et al. (2010) Superhydrophobic PLA Fabrics Prepared by UV Photo-Grafting of Hydrophobic Silica Particles Possessing Vinyl Groups. Journal of Colloid and Interface Science, 344, 584-587.
https://doi.org/10.1016/j.jcis.2010.01.024 - 16. Balamurali, B., Breedveld, V. and Hess, D.W. (2008) Fabrication of Roll-Off and Sticky? Superhydrophobic Cellulose Surfaces via Plasma Processing. Langmuir, 24, 4785-4790.
https://doi.org/10.1021/la703766c - 17. Qian, B. and Shen, Z. (2005) Fabrication of Superhydrophobic Surfaces by Dislocation-Selective Chemical Etching on Aluminum, Copper, and Zinc Substrates. Langmuir the ACS Journal of Surfaces & Colloids, 21, 9007-9009.
https://doi.org/10.1021/la051308c - 18. Lomga, J., Varshney, P., Nanda, D., et al. (2017) Fabrication of Durable and Regenerable Superhydrophobic Coatings with Excellent Self-Cleaning and Anti-Fogging Properties for Aluminium Surfaces. Journal of Alloys & Compounds, 702, 161-170.
- 19. Michels, A.F., Soave, P.A., Nardi, J., et al. (2016) Adjustable, (super)hydrophobicity by e-Beam Deposition of Nanostructured PTFE on Textured Silicon Surfaces. Journal of Materials Science, 51, 1316-1323.
https://doi.org/10.1007/s10853-015-9449-3 - 20. Feng, J., Huang, M. and Qian, X. (2009) Fabrication of Poly-ethylene Superhydrophobic Surfaces by Stretching-Controlled Micromolding. Macromolecular Materials & Engineering, 294, 295-300.
https://doi.org/10.1002/mame.200800331 - 21. Xu, L., Karunakaran, R.G., Guo, J., et al. (2012) Transparent, Superhydrophobic Surfaces from One-Step Spin Coating of Hydrophobic Nanoparticles. ACS Applied Materials & In-terfaces, 4, 1118-1125.
https://doi.org/10.1021/am201750h - 22. Mahadik, S.A., Parale, V., Vhatkara, R.S., et al. (2013) Superhydrophobic Silica Coating by Dip Coating Method. Applied Surface Science, 277, 67-72.
https://doi.org/10.1016/j.apsusc.2013.04.001 - 23. Vilar, I., Yage, J.L. and Borros, S. (2016) Superhydrophobic Copper Surfaces with Anti-Corrosion Properties Fabricated by Solventless CVD Methods. ACS Applied Materials & Interfaces, 9, 1057-1065.
https://doi.org/10.1021/acsami.6b12119 - 24. Simovich, T., Wu, A.H. and Lamb, R.N. (2015) Hierarchically Rough, Mechanically Durable and Superhydrophobic Epoxy Coatings through Rapid Evaporation Spray Method. Thin Solid Films, 589, 472-478.
- 25. Song, X., Zhai, J., Wang, Y., et al. (2005) Fabrication of Superhydrophobic Surfaces by Self-Assembly and Their Water-Adhesion Properties. Journal of Physical Chemistry B, 109, 4048-4052.
https://doi.org/10.1021/jp045152l - 26. Han, J.T., Zheng, Y., Cho, J.H., et al. (2005) Stable Superhydrophobic Organic-Inorganic Hybrid Films by Electrostatic Self-Assembly. Journal of Physical Chemistry B, 109, 20773-20778.
- 27. Zhi, C., Feng, L., Hao, L., et al. (2011) One-Step Electrodeposition Process to Fabricate Cathodic Superhydrophobic Surface. Applied Surface Science, 258, 1395-1398.
https://doi.org/10.1016/j.apsusc.2011.09.086 - 28. Li, J., Jing, Z., Zha, F., et al. (2014) Facile Spray-Coating Process for the Fabrication of Tunable Adhesive Superhydrophobic Surfaces with Heterogeneous Chemical Composi-tions Used for Selective Transportation of Microdroplets with Different Volumes. ACS Applied Materials & Interfaces, 6, 8868-8877.
https://doi.org/10.1021/am5015937 - 29. Zhi, D., Lu, Y., Sathasivam, S., et al. (2017) Large-Scale Fabrication of Translucent and Repairable Superhydrophobic Spray Coatings with Remarkable Mechanical, Chemical Durability and UV Resistance. Journal of Materials Chemistry A, 5, 10622-10631.
- 30. Ma, M., Mao, Y., Gupta, M., et al. (2005) Superhydrophobic Fabrics Produced by Electrospinning and Chemical Vapor Deposition. Macromolecules, 38, 9742-9748.
https://doi.org/10.1021/ma0511189 - 31. Zhu, Y., He, Y., Zhang, J., et al. (2017) Preparation of Large Scale, Durable, Superhydrophobic PTFE Films using Rough Glass Templates. Surface & Interface Analysis, 49, 1422-1430.
- 32. Pozzato, A., Zilio, S.D., Fois, G., et al. (2006) Superhydrophobic Surfaces Fabricated by Nanoimprint Lithography. Microelectronic Engineering, 83, 884-888.
- 33. Peng, C.W., Chang, K.C., Weng, C.J., et al. (2013) Nano-Casting Technique to Prepare Polyaniline Surface with Biomimetic Superhydrophobic Structures for Anticorrosion Application. Electrochimica Acta, 95, 192-199.
https://doi.org/10.1016/j.electacta.2013.02.016 - 34. Bhushan, B. and Chae, J.Y. (2007) Wetting Study of Patterned Surfaces for Superhydrophobicity. Ultramicroscopy, 107, 1033-1041.
https://doi.org/10.1016/j.ultramic.2007.05.002 - 35. Martines, E., Seunarine, K., Morgan, H., et al. (2005) Su-perhydrophobicity and Superhydrophilicity of Regular Nanopatterns. Nano Letters, 5, 2097-2103.
https://doi.org/10.1021/nl051435t - 36. Zhang, J., Li, J. and Han, Y. (2010) Superhydrophobic PTFE Surfaces by Extension. Macromolecular Rapid Communications, 25, 1105-1108.
https://doi.org/10.1002/marc.200400065 - 37. Satoshi, S., Tomohiro, O., Naoki, S.A., et al. (1996) Super Wa-ter-Repellent Surfaces Resulting from Fractal Structure. Journal of Physical Chemistry, 100, 19512-19517.
https://doi.org/10.1021/jp9616728 - 38. Klein, R.J., Biesheuvel, P.M., Yu, B.C., et al. (2013) Producing Su-per-Hydrophobic Surfaces with Nano-Silica Spheres. Zeitschrift Fur Metallkunde, 94, 377-380.
https://doi.org/10.3139/146.030377 - 39. Ma, M., Hill, R.M., Lowery, J.L., et al. (2005) Electrospun poly(styrene-block-dimethylsiloxane) Block Copolymer Fibers Exhibiting Superhydrophobicity. Langmuir the ACS Journal of Surfaces & Colloids, 21, 5549-5554.
https://doi.org/10.1021/la047064y - 40. Huang, L., Lau, S.P., Yang, H.Y., et al. (2005) Stable Superhydrophobic Surface via Carbon Nanotubes Coated with a ZnO Thin Film. Journal of Physical Chemistry B, 109, 7746-7748.
https://doi.org/10.1021/jp046549s - 41. Zhao, N., Xie, Q., Weng, L., et al. (2005) Superhydrophobic Surface from Vapor-Induced Phase Separation of Copolymer Micellar Solution. Macromolecules, 38, 8996-8999.
https://doi.org/10.1021/ma051560r - 42. Bormashenko, E., Bormashenko, Y., Stein, T., et al. (2007) Environ-mental Scanning Electron Microscopy Study of the Fine Structure of the Triple Line and Cassie Wenzel Wetting Tran-sition for Sessile Drops Deposited on Rough Polymer Substrates. Langmuir, 23, 4378-4382.
https://doi.org/10.1021/la0634802 - 43. And, H.Y. and Masatsugu, S. (2005) Single-Step Fabrication of Trans-parent Superhydrophobic Porous Polymer Films. Chemistry of Materials, 17, 5231-5234.
https://doi.org/10.1021/cm051281i - 44. Sun, M., Luo, C., Xu, L., et al. (2005) Artificial Lotus Leaf by Nano-casting. Langmuir the ACS Journal of Surfaces & Colloids, 21, 8978-8981.
https://doi.org/10.1021/la050316q - 45. Zhao, Y., Li, M., Lu, Q., et al. (2008) Superhydrophobic Polyimide Films with a Hierarchical Topography: Combined Replica Molding and Layer-by-Layer Assembly. Langmuir, 24, 12651-12657.
https://doi.org/10.1021/la8024364 - 46. Lee, W., Jin, M.K., Woncheol, Y.A., et al. (2004) Nanostructuring of a Polymeric Substrate with Well-Defined Nanometer-Scale Topography and Tailored Surface Wet-tability. Langmuir the ACS Journal of Surfaces & Colloids, 20, 7665-7669.
https://doi.org/10.1021/la049411+ - 47. Li, W. and Amirfazli, A. (2005) A Thermodynamic Approach for De-termining the Contact Angle Hysteresis for Superhydrophobic Surfaces. Journal of Colloid and Interface Science, 292, 195-201.
https://doi.org/10.1016/j.jcis.2005.05.062 - 48. Truesdell, R., Mammoli, A., Vorobieff, P., et al. (2006) Drag Reduction on a Patterned Superhydrophobic Surface. Physical Review Letters, 97, Article ID: 44504.
https://doi.org/10.1103/PhysRevLett.97.044504 - 49. Nosonovsky, M. and Bhushan, B. (2009) Multiscale Effects and Capillary Interactions in Functional Biomimetic Surfaces for Energy Conversion and Green Engineering. Philo-sophical Transactions. Series A, Mathematical, Physical, and Engineering Sciences, 367, 1511-1539.
https://doi.org/10.1098/rsta.2009.0008 - 50. Nosonovsky, M. and Bhushan, B. (2008) Superhydrophobicity for Energy Conversion and Conservation Applications. Journal of Adhesion Science & Technology, 22, 2105-2115.
- 51. Nosonovsky, M. and Bhushan, B. (2009) Superhydrophobic Surfaces and Emerging Applications: Non-Adhesion, Energy, Green Engineering. Current Opinion in Colloid & Interface Science, 14, 270-280.
https://doi.org/10.1016/j.cocis.2009.05.004 - 52. Moore, D.F. (1975) Principles and Applications of Tribology. Pergamon Press, Oxford.
- 53. Gohar, R. and Rahnejat, H. (2002) Introduction to Tribology.
- 54. Bhushan, B. (2003) Adhesion and Stiction: Mechanisms, Measurement Techniques, and Methods for Reduction. Journal of Vacuum Science & Technology B Microelectronics & Nanometer Structures, 21, 2262-2296.
https://doi.org/10.1116/1.1627336 - 55. Bhushan, B. (2009) Introduction: Biomimetics: Lessons from Nature—An Overview. Philosophical Transactions, 367, 1445-1486.
https://doi.org/10.1098/rsta.2009.0011 - 56. Bharat, B. (2007) Adhesion of Multi-Level Hierarchical Attachment Systems in Gecko Feet. Journal of Adhesion Science & Technology, 21, 1213-1258.
- 57. Chen, K., Gu, K., Qiang, S., et al. (2017) Environmental Stimuli-Responsive Self-Repairing Waterbased Superhydrophobic Coatings. RSC Advances, 7, 543-550.
https://doi.org/10.1039/C6RA25135H
NOTES
*通讯作者。
