Hans Journal of Nanotechnology
Vol.
09
No.
04
(
2019
), Article ID:
33369
,
9
pages
10.12677/NAT.2019.94014
Advances in the Study of Conducting Polymers Doped with Spherical Polyelectrolyte Brushes
Na Su
Shanghai Publishing and Printing College, Shanghai

Received: Nov. 13th, 2019; accepted: Dec. 3rd, 2019; published: Dec. 10th, 2019

ABSTRACT
Since the first discovery of conducting polymers in 1977, the field of conducting polymers has become one of the most active fields in materials science due to its special structure and excellent physical and chemical properties. The antenna of intelligence label, namely Radio Frequency Identification (RFID) printed mainly by conductive ink consisting of conductive polymers displays the merits of environmental protection, efficient and low cost. In past decades, considerable efforts have been devoted to improving the electrical conductivity of conducting polymers by the method of “doping”. With the advantages of spherical symmetry or quasi-symmetric structure, high grafting density and high charge density, spherical polyelectrolyte brushes (SPB) are expected to be novel dopant of conducting polymers. In this article, the trends, doping mechanism and development prospects of conducting polymers doped with spherical polyelectrolyte brushes are described on the basis of an overview of the general doping system of conducting polymers.
Keywords:Spherical Polyelectrolyte Brushes, Conducting Polymers, Doping, RFID
球型聚电解质刷掺杂导电高分子 研究进展
苏娜
上海出版印刷高等专科学校,上海

收稿日期:2019年11月13日;录用日期:2019年12月3日;发布日期:2019年12月10日

摘 要
基于导电高分子自身特殊的结构及优异的物化性能,自发现之日起就成为材料学的研究热点。在智能标签(即无线射频系统,RFID)天线制印中,使用主要由导电聚合物组成导电油墨印刷RFID天线具有环保、高效和低成本的特点。而通过掺杂使导电聚合物具有高导电性无疑是研究的核心。球形聚电解质刷状物以其高度规整的对称或准对称结构、较高的接枝密度、较高的电荷密度等特性,有望成为一类新型高效的导电高分子掺杂剂。本文在概述了导电高分子一般的掺杂体系的基础上,重点介绍了球型聚电解质刷掺杂导电高分子的研究概况,并讨论二者间相互作用的机理。
关键词 :球型聚电解质刷,导电高分子,掺杂,RFID

Copyright © 2019 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
导电高分子最早是在1977年由美国科学家Heeger、MacDiarmid和日本科学家白川英树(Shirakawa)合作发现的 [1],三位科学家因此而获得了2000年的诺贝尔化学奖。之后,一系列新型的导电高聚物相继问世。常用的导电高分子有聚吡咯(PPy)、聚苯胺(PANI)、聚噻吩及其衍生物等。由于导电聚合物自身优异的物化性能,其在国民经济、工业生产等领域具有极大的应用价值 [2] [3] [4]。在印刷包装领域中,导电高分子同样具有广泛的应用。如在全新、多功能、有良好防伪效果的智能标签(即无线射频系统,RFID)制印中,导电油墨用于印刷智能标签的天线,替代传统的压箔法或腐蚀法制作的金属天线,具有低成本及高效快速的特点 [5] ;在印刷电路板(PCB)工业中,应用导电聚合物能良好地实现PCB板的孔直接金属化 [6]。进而取代污染环境的化学镀铜工艺;另外,一种在继续发展着的所谓“智能标志”可用来代替目前普遍存在于包装产物上的条形码。
本征态导电高分子中π共扼结构刚性分子链的存在,导致其不易溶于常用的有机溶剂、电导率不高、机械延展性较差。同时,由于导电高分子粒子难溶于反应体系,其链结构的测定非常困难,且聚合过程中极易交联聚集成颗粒状,影响其导电性和稳定性等性能。人们主要通过“掺杂”来解决这些问题。如聚吡咯的电导率一般为10−8 S/cm,掺杂后的电导率最高可达103 S/cm。因掺杂阴离子种类的不同,其电导率往往可以相差若干个数量级。但在实际应用中,由于导电高分子各个性能相互制约,找到一种低成本高效的掺杂剂使导电高分子在具有高导电性的同时,提高其综合性能(如溶解性、热稳定性、加工性能等)无疑是导电高分子领域的研究热点。
球型聚电解质刷(SPB)以其高度规整的对称或准对称结构、较大的体积等特性,设计引入具有一定功能基团的刷状物后有望成为一类新型高效导电高分子掺杂剂。本论文首先总结了现阶段流行使用的导电高分子掺杂剂,然后以聚吡咯(PPy)和聚苯胺(PANI)为例,概述了SPB掺杂聚吡咯、SPB掺杂聚苯胺以及SPB掺杂吡咯–苯胺共聚体系及其表征方法,并讨论了可能的掺杂机理,为球型聚电解质刷成为新型导电高分子掺杂剂提供科学依据。
2. 导电高分子掺杂剂
目前,应用比较广泛的掺杂剂是芳香族磺酸衍生物及有机磺酸盐,如十二烷基苯磺酸(DBSA)、对甲苯磺酸(TsOH) [7]、对甲苯磺酸钠(TsONa)等。Lee等 [8] 在1995年开发了一种具有表面活性性质的DBSA作为阴离子添加物,在过硫酸铵(APS)水溶液中,通过对吡咯氧化聚合,可实现聚吡咯的溶剂化。后来的研究进一步指出,在APS水溶液中聚合吡咯时,加入其它大的类表面活性磺酸化合物,也可引起添加物离子诱导聚吡咯的溶剂化 [9] [10] [11]。另外,导电聚合物的力学性能及加工性能在实际应用中也是很重要的考虑因素。研究表明,在合成或后加工的过程中,将导电聚合物与基体如聚苯乙烯(PS) [12]、聚甲基丙烯酸甲酯(PMMA) [13] 等聚合、共混或进行“分子复合”,可获得既具有一定导电性又具有良好力学性能的复合材料。对无机纳米粒子进行改性后再与聚吡咯复合可获得加工性能更好的复合材料,如PPy/Fe3O4 [14]、PPy/SiO2 [15] [16] 等。
近年来,采用聚合物电解质掺杂导电聚合物获得了成功 [17]。Wu等 [18] 通过使用不同浓度水溶性高分子电解质聚苯乙烯磺酸盐(PSS)的掺杂来提高聚吡咯的导电性。这可能是由于聚合物电解质分子尺寸较大,掺杂离子进入大分子链之间后不易脱杂,因此所得导电聚合物的导电性能较为稳定。同时,聚合物电解质的柔性长链带来的缠结效应能够有效地阻碍聚吡咯链的增长,使得生成的聚吡咯由于分子量较小而能够溶于有机相中。
3. 球型聚电解质刷掺杂导电高分子体系
聚合物刷是指高密度地将聚合物分子链的一端联接于各种界面或聚合物链上而形成的一种特殊均聚或共聚高分子体系 [19] [20] [21]。依据聚合物分子链联接对象的不同,聚合物刷通常分为联接于各种界面包括固态基体表面、液–液界面、液–气界面等 [22] 的界面刷及联接于各种聚合物分子主链上而形成的聚合物分子刷两类。聚电解质刷状物自1991年Pincus [23] 及Borisov [24] 首次合成后,人们用多个理论模型 [25] [26] [27] 对其进行深入系统研究。若聚电解质链连接在球体表面,即形成球形聚电解质刷(SPB)。由于具有球型对称或准对称结构,较高接枝密度、较高的电荷密度等特点,球型聚电解质刷已广泛应用于多个领域,如稳定胶体 [28]、纳米催化 [29] [30]、生物医药 [31] 等。目前,关于球型聚电解质刷掺杂导电聚合物的研究很少,公开的成果有Wang等 [32] 于2009年及Mpoukouvalas等 [33] 于2010年发表的聚吡咯掺杂体系及Korovin等 [34] 于2011年发表的聚苯胺掺杂体系,以上研究都是将SPB作为导电聚合物的载体来合成具有核–壳结构的复合粒子。
3.1. 球型聚电解质刷掺杂聚吡咯
聚吡咯因其合成容易、电导率高、环境友好等优点,在导电聚合物中占有重要地位。Wang等报道了关于球型聚阴电解质刷掺杂聚吡咯应用于有机电子器件上的研究。涉及的球型聚阴电解质刷可通过两步法制备。首先采用苯乙烯和二乙烯基苯共聚生成表带有乙烯基团的核,然后加入苯乙烯磺酸钠单体,通过热引发剂偶氮二异丁腈(AIBN)引发聚合反应,生成球型聚阴电解质刷。研究发现球型聚电解质刷作为导电聚合物载体可使导电油墨具有良好的成膜性能,其电导率随着PPy含量的增加而增大。同时,与PEDOT/PSS体系相比,球型聚电解质刷掺杂的聚吡咯具有低的泄露电流,为其在电化学领域的应用提供了有利条件。球型聚阴电解刷掺杂聚吡咯的反应原理如图1所示 [35]。
Mpoukouvalas等对聚吡咯–聚苯乙烯磺酸盐PPy/PSSx (x = H+, Li+, Na+, Cs+)核–壳纳米粒子进行了深入研究。结果表明在PPy的体积分数相同的条件下,不同的阳离子对该体系的化学行为影响不同。尤其在温度高于400 K时,H+与Na+的化学行为和Li+与Cs+的化学行为差异很大,这可能是由于离子电导率的影响所致。且对于PPy/PSSx粒子而言,恒温直流电导率随着反离子原子半径的增加而降低(Li原子除外)。该研究为掺杂体系的选择提供了方向。
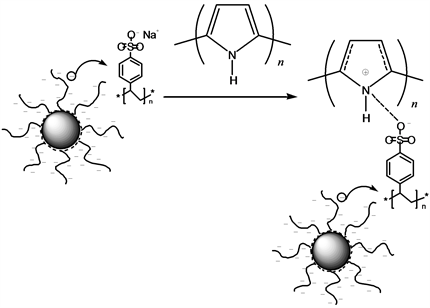
Figure 1. The reaction scheme of PPy doped by ASPB [35]
图1. 球型聚电解质刷掺杂聚吡咯反应原理图 [35]
3.2. 球型聚电解质刷掺杂聚苯胺
聚苯胺具有原料廉价易得,结构多样化,环境稳定性良好等优点,现已成为最具有应用前景的导电聚合物之一。聚苯胺的化学性质与其制备条件、电解液pH值密切相关。研究发现,酸性体系更有利于聚苯胺化学行为的发生,而在碱性和中性体系中,聚苯胺会发生去质子化而去掺杂。其与SPB的相互作用原理如图2所示。

Figure 2. The reaction scheme for PANI doped by ASPB
图2. 球型聚阴电解质刷掺杂聚苯胺反应原理图
Ke等 [36] 用聚苯胺包覆的聚合物微球(PANI-PS)悬浮液进行了循环伏安测试。结果表明该微球悬浮液出现两个氧化峰,其中0.5 V的氧化峰可归因于交联聚苯胺的氧化 [36]。同时发现,经离心分离纯化后得到的微球悬浮液的氧化峰电流和电势有明显的差别。且在合成过程中,由于微球表面聚苯胺的链长分布、交联程度不完全均一,因此每次伏安测试的氧化还原电流大小和电势位置的重现性较差,这给研究工作带来困难。因此,合成出表面具有更好氧化还原活性物质的微球对探索导电聚合物掺杂机理至关重要。
Korovin等 [37] 将球型聚阴电解质刷(PS核,PSS刷)作为模板,APS为氧化剂,在0.01 M盐酸水溶液中合成SPB-PANI复合粒子。结果表明合成的复合粒子在NaCl溶液浓度为10−5 M~10−1 M范围内的zeta电位为−40~−80 mV,表明其具有较好的胶体稳定性。同时,在相同条件下,SPB-PANI复合粒子的电导率高于PS-PANi复合粒子的电导率。可见球型聚电解质刷掺杂的导电聚合物导电性能更好。
Su [38] 以过硫酸铵(APS)为氧化剂,用原位化学氧化聚合法合成了ASPB掺杂的导电聚苯胺复合粒子(PANI/ASPB),并通过多种表征方法(FTIR、UV-Vis、XRD等)进行表征。结果表明,掺杂剂ASPB的加入可提高导电复合材料的热稳定性、溶解度及电导率。同时考察掺杂剂种类(ASPB、CSPB、SiO2、PSS)、ASPB的用量、聚合反应时间及温度对复合材料导电性能的影响。当单体用量为10%,聚合时间为6小时,ASPB的分子量为2000 g/mol时,电导率最高可达19.3 S/cm,而未掺杂ASPB时,PANI的电导率仅为7.10 S/cm。
3.3. 球型聚电解质刷掺杂苯胺–吡咯共聚体系
较之于导电聚合物聚吡咯、聚苯胺的大量报道,苯胺–吡咯共聚体系的研究显得非常不足,尤其对共聚物掺杂体系的研究更少。由于共聚材料可克服单一均聚体系中单一π电子的缺点,得到性能更优异的复合材料 [39]。因此,对于苯胺–吡咯共聚体系的研究逐渐引起人们的关注。
迄今为止,对于此类的研究大多数集中在使用各种模板来合成具有纳米结构的各单体共聚物 [40]。如用一步电化学法合成涂布在碳纤维上的PANI-PPy复合材料 [41] 及用化学共聚法使用模板制备吡咯/苯胺共聚纳米纤维 [42]。一般而言,苯胺与吡咯的共聚过程可用两种竞争过程加以描述。一种为共聚单体之间的初级相互作用。另一种为纯活性体苯胺与纯活性体吡咯及苯胺–吡咯型活性体与吡咯–苯胺活性体的次级相互作用。
Hang [43] 等通过化学氧化聚合法制备了PANI-PPy/ASPB导电复合粒子,最终该导电复合粒子的室温电导率可达8.3 S/cm,高于未经掺杂的苯胺–吡咯共聚粒子。
4. 球型聚电解质刷掺杂导电高分子表征方法
根据Su、Schridffer和Heeger [44] 于1979年提出的SSH (Su-Schrieffer-Heeger)理论,导电高分子的载流子主要由极化子(polaron),双极化子(bipolaron),孤子(solition)组成。鉴于当前对球型聚电解质刷掺杂导电聚合物体系的研究较少,其相互作用机理还没有一套成熟的理论来解释,为此可以借鉴导电高分子其它掺杂体系的研究方法,以此来探究反应过程中球型聚电解质刷与导电高分子间的相互作用机理。目前广泛采用的研究手段有场发射扫描电镜(FE-SEM)、红外光谱(FTIR),拉曼光谱(Roman)及X射线光电子能谱(XPS)等。
4.1. 场发射扫描电镜
FE-SEM可清晰的观察出导电聚合物掺杂前后的形貌特征。以聚吡咯为例,未经掺杂聚吡咯的微观形貌往往呈菜花状或瘤状 [45],这是由于此时吡咯单体的α位和β位聚合能力相当,所以聚合时表现为三维生长。掺杂剂的加入可提供吡咯有序生长所需的空间因素,从而得到具有特殊微观形貌的聚吡咯,如树枝状 [46]、管状 [47] 等。
4.2. 红外光谱和拉曼光谱
红外光谱主要用于研究掺杂诱导的导电聚合物链结构的变化。Carrasco等 [42] 用FTIR研究了共轭长度与导电聚合物的电导率之间的关系。即以log (电导率)的值为纵坐标,红外光谱图中吡咯环的反对称伸缩振动吸收峰与对称伸缩振动吸收峰的峰面积比值(A1445/A1535)为横坐标做图,由Baughman–Shacklette theory理论 [48] 可知,斜率越大,共轭长度越小。
Yuan等 [7] 采用拉曼光谱对PPy,PPy-TsOH,PPy/C及PPy-TsOH/C的掺杂机理进行了分析。研究发现,在Roman谱图中,PPy及PPy-TsOH的特征吸收峰在PPy/C及PPy-TsOH/C的谱图中均有出现,同时与PPy/C相比,PPy-TsOH/C中双极化子(936,1084和1261 cm−1)相对于极化子(984和1051 cm−1)的比例增加,表明其具有相对较高的电导率。
4.3. X射线光电子能谱
X射线光电子能谱能给出5~10 nm近表面区域原子的成分和化学键信息,因此掺杂过程中的许多信息如掺杂程度N+/N、链交联程度、能态分布、原子价态等都可以通过XPS谱图解析出来,是研究导电聚合物的结构和掺杂状况最常用的手段。林薇薇等 [49] 采用XPS方法对不同的聚苯胺掺杂体系PANI-HCl,PANI-SDBS-HCl,PANI-DBSA进行了研究。结果表明PANI-HCl体系的聚苯胺在样品后处理过程中易发生脱掺杂行为;在SDBS和HCl共存体系下合成聚苯胺时,DBS-与带正电荷聚苯胺链结合,起到了掺杂和诱导聚苯胺可溶性的作用。同时,PANI-SDBS-HCl与PANI-DBSA的N1s和S2p谱图相似,但SDBS-HCl复合体系的N+/N和S−/N分别为0.46和1.14,掺杂程度更为理想。
Ruangchuay等 [50] 利用XPS研究了α-萘磺酸盐(α-NSA)掺杂的导电聚吡咯。比较了α-萘磺酸盐及PPy/α-NS-的C 1s, O 1s, N 1s 及S 2p谱图,并计算了PPy/α-NS-中各元素含量,同时提出了在延长X射线照射时间条件下,PPy去质子化及掺杂剂α-NS−脱磺酸基反应的可能机理,如图3所示 [45]。

Figure 3. Schematic representations of deprotonation of PPy and desulfonation of α-naphthalenesulfonate [45]
图3. 聚吡咯去质子化及α-萘磺酸盐脱磺酸基反应原理 [45]
5. 球型聚电解质刷与导电高分子相互作用机理探讨
在PANI/ASPB体系中,ASPB可起到模板的作用。主要是基于它能够通过静电作用捕获和控制刷层内的试剂量 [51]。图4描述了在SPB内带电试剂的具体分布。由于空间位阻原因,密集接枝的PSS刷提供了一个浓度梯度环。苯胺单体加入体系中导致其与钠离子交换,使得苯胺在PSS刷浓度梯度环中的有效浓度增加,且其分布遵循PSS链的密度梯度。因此苯胺单体浓度最高的区域位于SPB的近核附近,因此,聚合反应优先发生在刷层的最里面。苯胺单体聚合成聚苯胺导致了聚合阳离子的形成,这些聚阳离子,相当于PSS刷层内多价态反离子取代了单价离子的作用,使得PANI和PSS的络合作用不可逆,因此将聚苯胺固定在在刷层内反应,起到了模板作用。
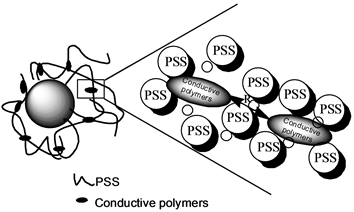
Figure 4. Schematic representations of the doping mechanism of ASPB [51]
图4. 掺杂机理图 [51]
6. 结束语
球形聚电解质刷由于具有更高的对称或准对称结构,更大的体积,设计引入含吸电子基团及具有一定功能基团的刷状物后,通过控制其结构参数(接枝密度、分子量、电荷密度等),不仅能够提高导电高分子的电导率,同时还可以改善其力学及加工性能。鉴于导电高分子本身的动态性质及球型聚电解刷体系的复杂性,显然目前的研究还远远不够,特别是关于球型聚电解刷的结构参数,如接枝密度等,与导电高分子性能的构效关系。总之,球型聚电解刷作为新一类导电高分子掺杂剂的应用展现出良好的前景,关于该方面的研究尚待进一步深入。
基金项目
本研究成果受国家新闻出版署“柔版印刷绿色制版与标准化”重点实验室招标课题资助(ZBKT201702)。
文章引用
苏 娜. 球型聚电解质刷掺杂导电高分子研究进展
Advances in the Study of Conducting Polymers Doped with Spherical Polyelectrolyte Brushes[J]. 纳米技术, 2019, 09(04): 113-121. https://doi.org/10.12677/NAT.2019.94014
参考文献
- 1. Shirakawa, H., Louis, E.L. and MacDiarmid, A.G. (1977) Synthesis of Electrically Conducting Organic Polymers: Halogen Derivatives of Polyacetylene, (CH)x. Journal of the Chemical Society, Chemical Communications, 16, 578-580. https://doi.org/10.1039/c39770000578
- 2. Sun, Y.Y., Jia, D.D., Zhang, A.T., et al. (2019) Synthesis of Polypyrrole Coated Melamine Foam by In-Situ Interfacial Polymerization Method for Highly Compressible and Flexible Supercapacitor. Journal of Colloid and Interface Science, 557, 617-627. https://doi.org/10.1016/j.jcis.2019.09.065
- 3. Hang, Y., Li, H.F., Wang, Z.F., et al. (2016) Nanostructured Polypyrrole as a Flexible Electrode Material of Supercapacitor. Nano Energy, 22, 422-438. https://doi.org/10.1016/j.nanoen.2016.02.047
- 4. Kandulna, R. and Choudhary, R.B. (2017) Robust Electron Transport Properties of PANI/PPY/ZnO Polymeric Nanocomposites for OLED Applications. Optik, 144, 40-48. https://doi.org/10.1016/j.ijleo.2017.06.094
- 5. Liu, C.F., Wang, Z.Y., Du, Y.B. and Liu, S.N. (2008) Research on Key Technologies of Tag Detection in Passive RFID System. IEEE Xplore, 11.
- 6. Meyer, H., Nichols, R.J., Schröer, D. and Stamp, L. (1994) The Use of Conducting Polymers and Colloid in the Though Hole Plating of Printed Circuit Boards. Elec-trochimica Acta, 39, 1325-1338.
- 7. Yuan, X.X., Zeng, X., Zhang, H.J., Ma, Z.F. and Wang, C.Y. (2010) Improved Per-formance of Proton Exchange Membrane Fuel Cells with p-Toluenesulfonic Acid-Doped Co-PPy/C as Cathode Electrocatalyst. Journal of the American Chemical Society, 132, 1754-1755. https://doi.org/10.1021/ja909537g
- 8. Lee, J.Y., Kim, D.Y. and Kim, C.Y. (1995) Synthesis of Soluble Polypyrrole of the Doped State in Organic Solvents. Synthetic Metals, 74, 103-106. https://doi.org/10.1016/0379-6779(95)03359-9
- 9. Lee, J.Y., Song, K.T., Kim, S.Y., Kim, Y.C., Kim, D.Y. and Kim, C.Y. (1997) Synthesis and Characterization of Soluble Polypyrrole. Synthetic Metals, 84, 137-140. https://doi.org/10.1016/S0379-6779(97)80683-2
- 10. Oh, E.J., Jang, K.S., Suh, J.S., Kim, H., Kim, K.H., Yo, C.H. and Joo, J. (1997) UV-Vis./NIR and Transport Studies of Chemically Synthesized Soluble Polypyrrole. Synthetic Metals, 84, 147-148. https://doi.org/10.1016/S0379-6779(97)80686-8
- 11. Shen, Y. and Wan, M. (1998) In Situ Doping Polymerization of Pyrrole with Sulfonic Acid as a Dopant. Synthetic Metals, 96, 127-132. https://doi.org/10.1016/S0379-6779(98)00076-9
- 12. Liu, D., Guo, J.S., et al. (2015) Facile Mass Production of Semi-Conductive Polypyrrole/Polystyrene Composite with Enhanced Mechanical Strength via In-Situ Bulk Polymerization. Materials Letters, 139, 191-193. https://doi.org/10.1016/j.matlet.2014.10.062
- 13. Dey, S. and Kumar, A. (2019) Enhanced Photoluminescence through Forster Resonance Energy Transfer in Polypyrrole-PMMA Blends for Application in Optoelectronic Devices. Materials Science Semiconductor Processing, 103, Article ID: 104644. https://doi.org/10.1016/j.mssp.2019.104644
- 14. Sun, X.D., Lv, X.L., Li, X.P., et al. (2018) Fe3O4@SiO2 Nanoparticles Wrapped with Polypyrrole (PPy) Aerogel: A Highly Performance Material as Excellent Electromagnetic Absorber. Materials Letters, 221, 93-96. https://doi.org/10.1016/j.matlet.2018.03.079
- 15. Liu, T.S., Liu, N., Zhai, S.R., et al. (2019) Tailor-Made Core/Shell/Shell-Like Fe3O4@SiO2@PPy Composites with Prominent Microwave Absorption Performance. Journal of Alloys and Compounds, 779, 831-843. https://doi.org/10.1016/j.jallcom.2018.11.167
- 16. Sun, L.B., Jiang, L., Peng, S., et al. (2019) Preparation of Au Cata-lysts Supported on Core-Shell SiO2/Polypyrrole Composites with High Catalytic Performances in the Reduction of 4-Nitrophenol. Synthetic Metals, 248, 20-26. https://doi.org/10.1016/j.synthmet.2018.12.024
- 17. Pattananuwat, and Aht-ong, D. (2017) Controllable Morphology of Polypyrrole Wrapped Graphene Hydrogel Framework Composites via Cyclic Voltammetry with Aiding of Poly(Sodium 4-Styrene Sulfonate) for the Flexible Supercapacitor Electrode. Eleatrochimica Acta, 224, 149-160. https://doi.org/10.1016/j.electacta.2016.12.036
- 18. Wu, T.M., Chang, H.L. and Lin, Y.W. (2009) Synthesis and Cha-racterization of Conductive Polypyrrole with Improved Conductivity and Processability. Polymer International, 58, 1065-1070. https://doi.org/10.1002/pi.2634
- 19. Milner, S.T. (1991) Polymer Brushes. Science, 251, 905-914. https://doi.org/10.1126/science.251.4996.905
- 20. Eskandari, and Abousalman-Rezvani, Z. (2019) Polymer Grafting on Graphene Layers by Controlled Radical Polymerization. Advances in Colloid and Interface Science, 273, Article ID: 102021. https://doi.org/10.1016/j.cis.2019.102021
- 21. Yang, W.F. and Zhou, F. (2017) Polymer Brushes for Antibiofouling and Lubrication. Biosurface and Biotribology, 3, 97-114. https://doi.org/10.1016/j.bsbt.2017.10.001
- 22. Halperin, A., Tirrell, M. and Lodge, T.P. (1992) Tethered Chains in Polymer Microstructures. In: Macromolecules: Synthesis, Order and Advanced Properties, Advances in Polymer Science, Vol. 100, Springer, Berlin, 31-71. https://doi.org/10.1007/BFb0051635
- 23. Pincus, (1991) Colloid Stabilization with Grafted Polyelectrolytes. Macro-molecules, 24, 2912-2919. https://doi.org/10.1021/ma00010a043
- 24. Borisov, O.V., Birshtein, T.M. and Zhulina, E.B. (1991) Collapse of Grafted Polyelectrolyte Layer. Journal de Physique II, 1, 521-526. https://doi.org/10.1051/jp2:1991186
- 25. Csajka, F.S., Netz, R.R., Seidel, C. and Joanny, J.F. (2001) Collapse of Polyelectrolyte Brushes: Scaling Theory and Simulations. The European Physical Journal E, 4, 505-513. https://doi.org/10.1007/s101890170105
- 26. Sandberg, D.J., Carrillo Jan-Michael, Y. and Dobrynin, A.V. (2007) Molecular Dynamics Simulations of Polyelectrolyte Brushes: From Single Chains to Bundles of Chains. Langmuir, 23, 12716-12728. https://doi.org/10.1021/la702203c
- 27. Biesheuvel, M., Frans, A.M.L. and Cohen, S.M.A. (2006) Phase Behavior of Mixtures of Oppositely Charged Protein Nanoparticles at Asymmetric Charge Ratios. Physical Review E, 73, Article ID: 041408. https://doi.org/10.1103/PhysRevE.73.041408
- 28. Lu, Y. and Ballauff, M. (2016) Spherical Polyelectrolyte Brushes as Nanoreactors for the Generation of Metallic and Oxidic Nanoparticles: Synthesis and Application in Catalysis. Progress in Polymer Science, 59, 86-104. https://doi.org/10.1016/j.progpolymsci.2016.03.002
- 29. Lu, Y., Wittemann, A. and Ballauff, M. (2009) Supramolecular Structures Generated by Spherical Polyelectrolyte Brushes and Their Application in Catalysis. Macromolecules, 30, 806-815. https://doi.org/10.1002/marc.200800789
- 30. Sharma, G. and Ballauff, M. (2004) Cationic Spherical Polyelectrolyte Brushes as Nanoreactors for the Generation of Gold Particles. Macromolecules, 25, 547-552. https://doi.org/10.1002/marc.200300107
- 31. Garni, M., Wehr, R., Avsar, S.Y., et al. (2019) Polymer Membranes as Templates for Bio-Applications Ranging from Artificial Cells to Active Surfaces. European Polymer Journal, 112, 346-364. https://doi.org/10.1016/j.eurpolymj.2018.12.047
- 32. Wang, J.J., Sun, L., Mpoukouvalas, K., Fassbender, B., Bonac-curso, E., Brunklaus, G., Muehlebach, A., Rime, F., Butt, H.J. and Wegner, G. (2009) Facile Synthesis of Spherical Polye-lectrolyte Brushes as Carriers for Conducting Polymers to Be Used in Plastic Electronics. Macromolecules, 210, 1504-1509. https://doi.org/10.1002/macp.200900199
- 33. Mpoukouvalas, K., Wang, J.J. and Wegner, G. (2010) Conductivity of Poly(pyrrole)-Poly(styrene sulfonate) Core-Shell Nanoparticles. ChemPhysChem, 11, 139-148. https://doi.org/10.1002/cphc.200900643
- 34. Korovin, A.N., Sergeyev, V.G., Pyshkina, O.A., Hanske, C., Fery, A., Wittemann, A. and Tsarkova, L. (2011) Nanoreactor-Assisted Polymerization toward Stable Dispersions of Conductive Composite Particles. Macromolecular Rapid Communications, 32, 462-467. https://doi.org/10.1002/marc.201000626
- 35. Su, N., Li, H.B., Yuan, S.J., et al. (2012) Synthesis and Characterization of Polypyrrole Doped with Anionic Spherical Polyelectrolyte Brushes. eXPRESS Polymer Letters, 6, 697-705. https://doi.org/10.3144/expresspolymlett.2012.75
- 36. Aoki, K., Chen, J.Y., Ke, Q., et al. (2003) Redox Reactions of Polyaniline-Coated Latex Suspensions. Langmuir, 19, 5511-5516. https://doi.org/10.1021/la034314m
- 37. Genies, E.M., Lapkowski, M. and Penneau, J.F. (1988) Cyclic Voltammetry of Polyaniline: Interpretation of the Middle Peak. Journal of Electroanalytical Chemistry, 249, 97-107. https://doi.org/10.1016/0022-0728(88)80351-6
- 38. Su, N. (2015) Polyaniline-Doped Spherical Polyelectrolyte Brush Nanocomposites with Enhanced Electrical Conductivity, Thermal Stability, and Solubility Property. Polymers, 7, 1599-1616. https://doi.org/10.3390/polym7091473
- 39. Fusalba, F. and Bélanger, D. (1999) Electropolymerization of Polypyrrole and Polyaniline-Polypyrrole from Organic Acidic Medium. The Journal of Physical Chemistry B, 103, 9044-9054. https://doi.org/10.1021/jp9916790
- 40. Lu, M., Li, X.H. and Li, H.L. (2002) Synthesis and Characterization of Conducting Copolymer Nanofibrils of Pyrrole and 3-Methylthiophene Using the Template-Synthesis Method. Materials Science and Engineering: A, 334, 291-297. https://doi.org/10.1016/S0921-5093(01)01906-2
- 41. Rajagopalan, R. and Iroh, J.O. (2003) Characterization of Polyaniline-Polypyrrole Composite Coatings on Low Carbon Steel: A XPS and Infrared Spectroscopy Study. Applied Surface Science, 218, 58-69. https://doi.org/10.1016/S0169-4332(03)00579-8
- 42. Li, X.H., Zhang, X.G. and Li, H.L. (2001) Preparation and Characterization of Pyrrole/Aniline Copolymer Nanofibrils Using the Template-Synthesis Method. Journal of Applied Polymer Science, 81, 3002-3007. https://doi.org/10.1002/app.1750
- 43. Huang, Y., Su, N., Zhang, X.Z., et al. (2014) Controllable Synthesis and Cha-racterization of Poly(aniline-co-pyrrole) Using Anionic Spherical Polyelectrolyte Brushes as Dopant and Template. Polymer Composites, 35, 1858-1863. https://doi.org/10.1002/pc.22841
- 44. Joo, J., Lee, J.K., Baeck, J.S., Kim, K.H, Oh, E.J. and Epstein, J. (2001) Electrical, Magnetic, and Structural Properties of Chemically and Electrochemically Synthesized Polypyrroles. Synthetic Metals, 117, 45-51. https://doi.org/10.1016/S0379-6779(00)00537-3
- 45. Tamer, U., Kanbes, C. and Ertas, N. (2009) Branched Fibers of Conducting Polypyrrole: Synthesis and Characterization. International Journal of Polymer Analysis and Characterization, 14, 259-270. https://doi.org/10.1080/10236660802663522
- 46. Han, J., Yan, W. and Xu, Y.B. (2006) Synthesis of Long Polypyrrole Micro/Nanotubules with Rectangular Sections. Chemistry Letters, 35, 306. https://doi.org/10.1246/cl.2006.306
- 47. Carrasco, M., Grande, H.J., Cortazar, M., Alberdi, J.M., Areizaga, J. and Pomposo, J.A. (2006) Structure-Conductivity Relationships in Chemical Polypyrroles of Low, Medium and High Conductivity. Synthetic Metals, 156, 420-425. https://doi.org/10.1016/j.synthmet.2006.01.005
- 48. Baughman, R. and Shacklelette, L. (1989) Conductivity as a Func-tion of Conjugation Length: Theory and Experiment for Conducting Polymer Complexes. Physical Review B, 39, 5872. https://doi.org/10.1103/PhysRevB.39.5872
- 49. Lin, W.W., Nan, J.Y., Tian, Y.H. and Liang, H.J. (2000) XPS Study of Competing Doping Behavior of Polyaniline. Chinese Journal of Chemical Physics, 13, 592.
- 50. Ruangchuay, L., Schwank, J. and Sirivat, A. (2002) Surface Degradation of α-Naphthalene Sulfonate-Doped Polypyrrole during XPS Characterization. Applied Surface Science, 199, 128-137. https://doi.org/10.1016/S0169-4332(02)00564-0
- 51. Su, N. (2015) Improving Electrical Conductivity, Thermal Stability, and Solubility of Polyaniline-Polypyrrole Nanocomposite by Doping with Anionic Spherical Polyelectrolyte Brushes. Nanoscale Research Letters, 10, Article No. 301. https://doi.org/10.1186/s11671-015-0997-x