Bioprocess
Vol.
08
No.
03
(
2018
), Article ID:
26685
,
6
pages
10.12677/BP.2018.83007
Construction and Identification of Eukaryotic Expression Vector of Human Programmed Death Receptor 1
Yuling Mu, Xiao Yang, Yujia Kang, Jinlei Shi*
School of Life Science and Technology, Shanghai Tech University, Shanghai

Received: Aug. 12th, 2018; accepted: Aug. 27th, 2018; published: Sep. 4th, 2018

ABSTRACT
Objective: To construct a eukaryotic expression vector containing human EGFP-tagged human programmed death receptor (hPD-1), which lays a foundation for subsequent gene transfer experiments. Methods: The hPD-1 gene coding frame was amplified by PCR. The PD-1 and pEGFP-N1 plasmids were digested and ligated, and the recombinant vector pEGFP-N1-PD-1 was constructed. The recombinant plasmid was digested and sequenced for identifying. The recombinant plasmid was transfected into HEK293T cells, and the protein was extracted for Western blot analysis. Results: The recombinant eukaryotic expression vector pEGFP-N1-PD-1 was analyzed by restriction endonuclease. The bands after double digestion were consistent with the theoretical values. No nucleotide variation was observed in the sequencing results. The total protein of the transfected cells was extracted. Western blot can detect the target band. Conclusion: The eukaryotic expression vector pEGFP-N1-PD-1 was successfully constructed and expressed in 293T cells, which laid a foundation for the subsequent study of the function of PD-1 gene.
Keywords:PD1, Recombinant Plasmid, EGFP; Western Blot, Transfection
人PD1基因真核表达载体构建与鉴定
穆宇灵,杨霄,康宇佳,石金磊*
上海科技大学 生命科学与技术学院,上海

收稿日期:2018年8月12日;录用日期:2018年8月27日;发布日期:2018年9月4日

摘 要
目的:构建含EGFP标签的人程序性死亡受体(hPD-1)的真核表达载体,为后续基因转移实验奠定基础。方法:PCR扩增hPD-1基因编码框,将PD-1和pEGFP-N1质粒双酶切后进行连接转化,构建重组载体pEGFP-N1-PD-1,酶切重组质粒并测序鉴定,将构建好的重组质粒转染HEK293T细胞,并提取蛋白进行Western blot检测。结果:重组真核表达载体pEGFP-N1-PD-1经限制性内切酶分析,双酶切之后的条带与理论值相符,测序结果未见碱基变异,提取转染后细胞的总蛋白进行Western blot可以检测到目标条带。结论:成功构建了pEGFP-N1-PD-1真核表达载体,并且能够在293T细胞中正常表达,为后续研究PD-1基因的功能奠定基础。
关键词 :PD1,重组质粒,EGFP,Western Blot,转染

Copyright © 2018 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
1992年,PD-1受体基因(programmed cell death 1 receptor gene)在程序性凋亡的T细胞杂交瘤中被检测出 [1] 。对小鼠Pdcd1-/-突变体的自身免疫表型研究揭示了PD-1具有重要的负调控功能 [2] [3] 。在2000年和2001年,鉴定得到了PD-1的配体,从而在理解PD-1及其配体的功能方面取得了长足的进展 [4] [5] [6] [7] 。
PD-1是CD28家族的含有288个氨基酸的I型跨膜糖蛋白,由一个免疫球蛋白(Ig)超家族结构域,一个约20个氨基酸残基,一个跨膜结构域和一个约95个残基的胞内结构域组成的,含288个氨基酸的跨膜蛋白。PD-1中含有免疫受体酪氨酸抑制性基序(ITIM),以及一个酪氨酸基开关基序(ITSM) [8] 。
PD-1主要表达于活化的T细胞、B细胞、自然杀伤细胞、单核细胞以及间充质干细胞 [9] 。PD-L1和PD-L2是PD1的配体,是免疫反应中重要的负性调控因子。在功能上,PD-1及其配体PD-L1和PD-L2传递抑制性信号,调节T细胞活化,耐受性和免疫病理学之间的平衡。对外来抗原和自身抗原的免疫应答,需要特异和平衡的反应来清除病原体和肿瘤,同时也需要保持耐受。诱导和维持T细胞耐受则需要PD-1,其在非造血细胞上的配体PD-L1可限制效应T细胞应答并保护组织免受免疫介导的组织损伤 [8] 。
目前,由于PD-1在多种肿瘤细胞表面高度表达,靶向PD1/PD-L1单克隆抗体类药物的研发已成为肿瘤治疗领域的研究热点 [10] 。
本研究拟构建hPD-1基因的真核表达载体,采用融合EGFP标记的方法,检测其在HEK293T细胞中的表达。
2. 材料与方法
2.1. 主要试剂、质粒
pEGX-4T-1-hPD1质粒、pEGFP-N1质粒由本实验室保存。质粒抽提试剂盒、胶回试剂盒购自NucleoSpin公司。限制性内切酶和T4连接酶购自宝生物公司。Q5 High-Fidelity 2X Master Mix购自NEB公司。大肠杆菌TOP10感受态细胞和卡那抗生素购自康为世纪公司。2 × Taq PCR试剂盒购自天根公司。DNA Marker购自全式金公司,其他试剂为国产分析纯,引物合成与测序由上海铂尚生物技术有限公司完成。
2.2. 引物设计
根据人PD-1基因序列,应用Snapgene软件设计合适引物, PD1-F:5’-GCGAATTCATGCAGATCCCACAGGCG-3’和 PD1-R:5’-ATCCGCGGCAGGGGCCAAGAGCAGT-3’ (下划线为EcoR I和Sac II酶切位点,全长864 bp)。
2.3. 人PD-1基因的扩增
以pEGX-4T-1-PD1质粒为模板,以PD1引物扩增PD-1基因片段,PCR反应条件:98℃预变性30 s,98℃变性10 s,60℃退火15 s,72℃延伸30 s,35个循环,72℃延伸2 min,最后4℃保温,PCR产物经1%琼脂糖凝胶电泳后进行切胶回收。
2.4. 真核表达载体pEGFP-N1-PD-1的构建
用EcoR I、Sac II分别对凝胶回收得到的PD1片段和pEGFP-N1质粒进行双酶切,酶切反应体系25 μl:PD1 6.5 μl/pEGFP-N1 2 μl、EcoR I 0.5μl、Sac II 0.5 μl、10 × buffer 2.5 μl、去离子水补加到25 μl。反应条件: 37℃,30 min,琼脂糖凝胶电泳后回收。连接反应体系15 μl,载体与插入片段体积比为1:5 (载体2 μl)、10X快速连接缓冲液1.5 μl、T4-DNA ligase 1 μl、去离子水补加到15 μl、反应条件:16℃连接过夜。
2.5. 真核表达载体pEGFP-N1-PD-1的鉴定
将10 μl的连接产物通过热激法转入到TOP10感受态细胞,涂布于含卡钠的LB固体培养基上,37℃过夜培养,挑取抗性单菌落,进行PCR阳性克隆检测,随后,提取质粒进行双酶切和测序验证。
2.6. 重组质粒的真核表达鉴定
选取测序正确的质粒转染进293T细胞系中,表达至少24 h后,收集细胞悬液,用预冷PBS洗涤2次,加入RIPA裂解液于冰上匀浆细胞,4℃、12,000 rpm离心10 min,收集蛋白上清液备用,检测总蛋白浓度,将蛋白样品中加入4倍体积的上样缓冲液,100℃煮沸5 min变性蛋白样品。经10% SDS-PAGE电泳分离后进行western blot实验,使用GFP抗体检测在细胞系中该质粒的表达情况,同时,检测内参β-actin的表达水平作为样本量对照。
3. 结果
3.1. 目的片段PD1的获取
PCR得到的PD1片段,经1%琼脂糖凝胶电泳检测,与预期大小一致,PD1为864 bp,见图1。将目的片段进行切胶回收并纯化。
3.2. PD1和pEGFP-N1双酶切结果
PD1PCR回收片段和pEGFP-N1质粒经Sac II和EcoR I双酶切后,分别可见864 bp和4700 bp大小条带,见图2,将酶切产物凝胶回收并纯化,采用T4 DNA连接酶进行连接反应。
3.3. 阳性克隆PCR检测和双酶切鉴定结果
将过夜连接产物转化大肠杆菌长出单菌落后,采用PCR扩增方法检测PD1基因,菌落PCR结果经1%琼脂糖凝胶电泳检测,如图3所示,随机挑取的6个单克隆都可以检测到864 bp大小的条带,分别抽取1~4号质粒进行双酶切验证,如图4所示,可以看到在864 bp处有目的条带出现,初步证明pEGFP-N1-PD-1重组质粒构建成功,随后,我们将鉴定好的重组质粒送测序,发现无移码和碱基突变,pEGFP-N1-PD-1重组质粒构建成功。
3.4. 重组质粒在真核细胞系中的表达鉴定结果
转染了pEGFP-N1-PD-1重组质粒的细胞总蛋白样品表达了EGFP-PD-1融合蛋白,条带大小与预期相符,未转染的对照组则无条带。实验结果表明,该重组质粒可以在真核细胞系中准确表达,见图5。
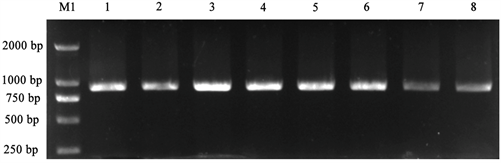 M1:Trans2K® DNA Marker;1~8:PD1 PCR片段
M1:Trans2K® DNA Marker;1~8:PD1 PCR片段
Figure 1. Target fragment PCR amplification results
图1. 目的片段PCR扩增结果
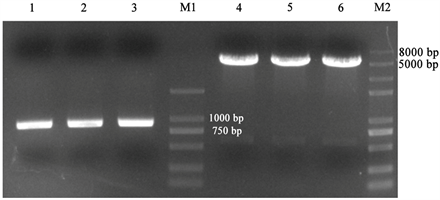 M1:Trans2K® DNA Marker;M2:Trans 2K® Plus II DNA Marker;1~3:双酶切后的PD1、4~6:双酶切后的载体;
M1:Trans2K® DNA Marker;M2:Trans 2K® Plus II DNA Marker;1~3:双酶切后的PD1、4~6:双酶切后的载体;
Figure 2. Double enzyme digestion electrophoresis results of vector and target fragment
图2. 载体和目的片段双酶切电泳结果
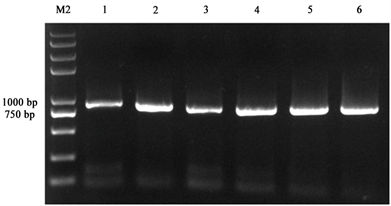 M2:Trans 2K® Plus II DNA Marker;1~6:阳性克隆PCR结果
M2:Trans 2K® Plus II DNA Marker;1~6:阳性克隆PCR结果
Figure 3. Detection of recombinant vector positive clone PCR
图3. 重组载体阳性克隆PCR检测
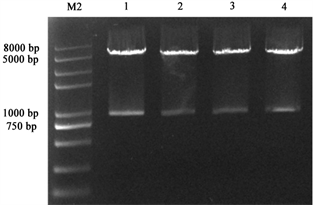 1~4:双酶切后的重组质粒;M2:Trans 2K® Plus II DNA Marker
1~4:双酶切后的重组质粒;M2:Trans 2K® Plus II DNA Marker
Figure 4. Results of double enzyme digestion of recombinant plasmid
图4. 重组质粒双酶切验证结果
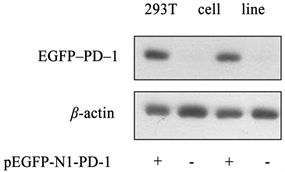 +:转染了pEGFP-N1-PD-1的阳性细胞;-:为未转染质粒的对照组
+:转染了pEGFP-N1-PD-1的阳性细胞;-:为未转染质粒的对照组
Figure 5. Detection of EGFP-PD-1 protein expression in 293T cell line by Western blot
图5. Western blot检测293T细胞系中EGFP-PD-1蛋白的表达
4. 讨论
PD1和PD1的配体PD-L1、PD-L2,是B7家族的细胞表面I型跨膜蛋白,作为一种重要的免疫抑制分子,表达在肿瘤细胞表面的PD-L1,与T细胞表面PD1结合,下调肿瘤特异性T细胞活性,或灭活T细胞,阻断免疫应答,使肿瘤细胞产生免疫逃逸 [11] [12] 。而PD1/PD-L1靶向药物是针对PD1/PD-L1的抗体蛋白,阻断这一通路,两种蛋白不能结合,使得T细胞可以继续杀伤肿瘤细胞。
近年来,针对PD1/PD-L1的单克隆抗体靶向药物在非小细胞肺癌和黑色素瘤等实体肿瘤治疗中均取得了较好的疗效,并迅速应用到其他类型肿瘤,其中包括乳腺癌、肝癌、胰腺癌、消化道肿瘤、妇科肿瘤、泌尿系统肿瘤以及骨髓瘤和淋巴瘤等多种恶性肿瘤,显著改善了部分肿瘤患者的临床进展和不良预后 [13] [14] 。Koh等人发现PD1的表达水平与经典型霍奇金淋巴瘤患者的不良预后有关,降低PD1的表达水平可提高患者的总体生存率 [15] 。
本实验通过克隆人PD1基因的编码区,重组到真核质粒pEGFP-N1中,成功的在293T细胞中观察到绿色荧光,并且通过Western blot检测证实其确有表达,为进一步研究PD1的功能奠定了基础。
基金项目
上海科技大学生命科学与技术学院本科生实验室运行经费2018A0202-405-07。
文章引用
穆宇灵,杨 霄,康宇佳,石金磊. 人PD1基因真核表达载体构建与鉴定
Construction and Identification of Eukaryotic Expression Vector of Human Programmed Death Receptor 1[J]. 生物过程, 2018, 08(03): 55-60. https://doi.org/10.12677/BP.2018.83007
参考文献
- 1. Ishida, Y., Agata, Y., Shibahara, K. and Honjo, T. (1992) Induced Expression of PD-1, a Novel Member of the Immunoglobulin Gene Superfamily, upon Programmed Cell Death. EMBO Journal, 11, 3887-3895.
- 2. Nishimura, H., Nose, M., Hiai, H., Minato, N. and Honjo, T. (1999) Development of Lupus-Like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity, 11, 141-151. https://doi.org/10.1016/S1074-7613(00)80089-8
- 3. Nishimura, H., Okazaki, T., Tanaka, Y., Nakatani, K., Hara, M., et al. (2001) Autoimmune Dilated Cardiomyopathy in PD-1 Receptor-Deficient Mice. Science, 291, 319-322. https://doi.org/10.1126/science.291.5502.319
- 4. Dong, H., Zhu, G., Tamada, K. and Chen, L. (1999) B7-H1, a Third Member of the B7 Family, Costimulates T-Cell Proliferation and Interleukin-10 Secretion. Nature Medicine, 5, 1365-1369. https://doi.org/10.1038/70932
- 5. Freeman, G.J., Long, A.J., Iwai, Y., Bourque, K., Chernova, T., et al. (2000) Engagement of the PD-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. Journal of Experimental Medicine, 192, 1027-1034. https://doi.org/10.1084/jem.192.7.1027
- 6. Latchman, Y., Wood, C.R., Chernova, T., Chaudhary, D., Borde, M., et al. (2001) PD-L2 Is a Second Ligand for PD-1 and Inhibits T Cell Activation. Nature Immunology, 2, 261-268. https://doi.org/10.1038/85330
- 7. Tseng, S.Y., Otsuji, M., Gorski, K., Huang, X., Slansky, J.E., et al. (2001) B7-DC, a New Dendritic Cell Molecule with Potent Costimulatory Properties for T Cells. Journal of Experimental Medicine, 193, 839-846. https://doi.org/10.1084/jem.193.7.839
- 8. Keir, M.E., et al. (2008) PD-1 and Its Ligands in Tolerance and Immunity. Annual Review of Immunology, 26, 677-704. https://doi.org/10.1146/annurev.immunol.26.021607.090331
- 9. Böger, C., Behrens, H.M., Mathiak, M., et al. (2016) PD-L1 Is an Independent Prognostic Predictor in Gastric Cancer of Western Patients. Oncotarget, 7, 24269-24283. https://doi.org/10.18632/oncotarget.8169
- 10. Iraolagoitia, X.L., Spallanzani, R.G., Torres, N.I., et al. (2016) NK Cells Restrain Spontaneous Antitumor CD8+ T Cell Priming through PD-1/PD-L1 Interactions with Dendritic Cells. Journal of Immunology, 197, 953-961. https://doi.org/10.4049/jimmunol.1502291
- 11. Leng, C., Li, Y., Qin, J., et al. (2016) Relationship between Expression of PDL1 and PD-L2 on Esophageal Squamous Cell Carcinoma and the Antitumor Effects of CD8+ T Cells. Oncology Report, 35, 699-708. https://doi.org/10.3892/or.2015.4435
- 12. Hughes, P.E., Caenepeel, S., Wu, L.C., et al. (2016) Targeted Therapyand Checkpoint Immunotherapy Combinations for the Treatment of Cancer. Trends in Immunology, 37, 462-476. https://doi.org/10.1016/j.it.2016.04.010
- 13. 谢丽叶, 付杰军, 卢奕, 张健. PD1/PD-L1激活促进癌症发生、发展和转移的研究进展[J]. 肿瘤防治研究, 2017, 44(6): 423-427.
- 14. 赵宇飞, 陈晓, 李薇. PD1/PD-L1抑制剂治疗肿瘤[J]. 中国肿瘤生物治疗杂志, 2017, 24(8): 904-911.
- 15. Koh, Y.W., Jeon, Y.K., Yoon, D.H., et al. (2016) Programmed Death 1expression in the Peritumoral Microenvironment Is Associated with a Poorer Prognosis in Classical Hodgkin Lymphoma. Tumor Biology, 37, 7507-7514. https://doi.org/10.1007/s13277-015-4622-5
NOTES
*通讯作者。