Advances in Microbiology
Vol.
08
No.
03
(
2019
), Article ID:
31380
,
7
pages
10.12677/AMB.2019.83013
The Impact of Strong Promoter Psod on Pyc Gene Expression in Corynebacterium glutamicum
Zhicheng Zou, Jiaxi Jiang, Junhui Ding, Baoyi Qiao, Yijia Cao, Wei Zhou*, Jun Chen*
College of Chemistry and Chemical Engineering, Wuhan University of Science and Technology, Wuhan Hubei

Received: Jul. 2nd, 2019; accepted: Jul. 16th, 2019; published: Jul. 23rd, 2019

ABSTRACT
In order to obtain a strain with high yield of L-malic acid, the wild-type C. glutamicum was modified with gene editing. The superoxide dismutase gene promoter (Psod) was inserted ahead of pyruvate carboxylase gene (pyc), a key gene in the oxaloacetate anaplerotic reaction, to promote the yield of L-malic acid in a flow of pyruvate to oxaloacetate, then to L-malic acid. This strain was named as CZM03. The experimental results showed that the PYC activity was upregulated about 2.2-fold in CZM03 compared with the wild-type C. glutamicum. There was no significant difference in the growth rate and the maximum biomass at the plateau stage between the two strains. This indicates that the knock-in of the Psod promoter had no significant effect on the growth of the bacteria, but the activity of PYC increased significantly, which was beneficial to the accumulation of the target products.
Keywords:L-Malic Acid, C. glutamicum, Gene Editing, Pyruvate Carboxylase, Superoxide Dismutase Gene Promoter (Psod)
强启动子Psod对谷氨酸棒状杆菌pyc基因 表达的影响
邹志成,蒋佳稀,丁俊辉,乔宝艺,曹懿佳,周卫*,陈俊*
武汉科技大学化学与化工学院,湖北 武汉

收稿日期:2019年7月2日;录用日期:2019年7月16日;发布日期:2019年7月23日

摘 要
为了获得L-苹果酸高产的谷氨酸棒状杆菌(C. glutamicum),通过基因编辑,在草酰乙酸回补途径的关键酶丙酮酸羧化酶基因(pyc)前面,敲入超氧化物歧化酶基因启动子(Psod),重组菌株命名为CZM03,通过强启动子Psod增强PYC酶活性,促进丙酮酸→草酰乙酸→苹果酸,利于苹果酸的生成。结果表明:与野生型C. glutamicum相比,重组菌株CZM03的PYC活性上调了约2.2倍;到平台期,两者的生长速率和最大生物量没有显著差异。这表明Psod强启动子的敲入,对菌的生长没有明显影响,但是PYC的酶活却有大幅度提高,这对于目的产物的积累是有利的。
关键词 :L-苹果酸,谷氨酸棒状杆菌,基因编辑,丙酮酸羧化酶,超氧化物歧化酶基因启动子Psod

Copyright © 2019 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
L-苹果酸是一种重要的四碳化合物,已被美国能源部列为十二大生物基平台化合物之一,在食品、医药、化工等行业有着广泛的应用。目前苹果酸的合成主要有化学合成法、酶法和发酵法 [1] 。化学合成法虽然成本低廉,但合成的苹果酸为DL型,不易吸收,且有一定的毒性,不宜在食品与医药工业中应用。酶转化法生产L-苹果酸主要以富马酸酶转化为主,依赖高纯度的富马酸为原料,而富马酸价格昂贵。发酵法合成苹果酸,目前通常采用曲霉属菌株作为生产菌株 [2] [3] [4] [5] ,产量较高,但是生产周期长、产杂酸多、且难以对其进行遗传改造。
C. glutamicum是经典的食品性安全菌,遗传背景清晰,已广泛用于化学品的生物制造 [6] [7] [8] 。C. glutamicum在厌氧条件下,丙酮酸经过还原TCA (rTCA)途径合成苹果酸,理论上的糖酸转化率为200%,是目前所有已知L-苹果酸合成途径中最高的 [9] 。本研究采用系统生物学方法对C. glutamicum中L-苹果酸代谢途径进行分析,通过基因编辑,对野生型C. glutamicum进行代谢途径改造,在草酰乙酸回补途径的关键酶丙酮酸羧化酶基因(pyc)前面,敲入超氧化物歧化酶基因启动子(Psod),增强PYC酶活性,促进丙酮酸→草酰乙酸→苹果酸,利于目标产物的积累。
2. 材料与方法
2.1. 材料
本研究所用到的菌株和质粒见表1。
野生型C. glutamicum ATCC13032菌株、E. coli DH5α由本实验室保存;质粒DNA提取试剂盒和琼脂糖凝胶DNA回收试剂盒均购自Omega Bio-Tek公司;限制性内切酶购自Thermo scientific;T4 DNA ligase购自TaKaRa;fast Pfu PCR Mix DNA聚合酶购自北京佳兰生物科技有限公司;引物均由江苏金唯智生物科技有限公司合成;其它试剂均为国产分析纯。
用于扩增pyc基因上游同源臂的引物为CPyr-U1和CPyr-U2;用于扩增pyc基因下游同源臂的引物
Table 1. Lists of strains and plasmids used for this study
表1. 本研究所用菌株、质粒
为CPyr-D1和CPyr-D2;用于扩增Psod启动子的引物分别CPs 1和CPs 2;Psod敲入后的检测引物为OPs1和Ops2。具体引物序列组成见表2。
Table 2. Primers used in this study
表2. 本研究所用的引物
注:引物CPyr-D 2与OPs 2共用。
发酵种子液培养基(g/L):葡萄糖20,蛋白胨5,酵母提取物2.5,氯化钠5,NaAce 2.5。
发酵培养基(g/L):葡萄糖20,KHCO3 20,NaAce 5,KH2PO4 0.75,K2HPO4 0.75,(NH4)2SO4 5,MgSO4∙7H2O 0.5,CaCl2 0.2,MnSO4∙4H2O 0.02,FeSO4∙7H2O 0.005,VH 0.2 mg/L,VB1 0.2 mg/L。
2.2. 方法
2.2.1. 基因敲出载体构建
Psod启动子的敲入载体pK19ms-Psod-pyc构建:先扩增用于同源重组的上、下游同源臂片段,再扩增Psod启动子片段,然后利用Soeing PCR,获得上游同源臂、Psod启动子、下游同源臂三者的融合片段;将融合片段以双酶切的方式克隆至pK19mobsacB,获得Psod启动子的敲入载体pK19ms-Psod-pyc。
2.2.2. 敲入载体电转化C. glutamicum
制备C. glutamicum感受态细胞,制备方法参照文献 [10] 进行。敲入载体电转化C. glutamicum感受态细胞的参数设置为:1.8 kV,4 mS。具体操作步骤参照文献 [10] 进行。
2.2.3. 酶活测定
丙酮酸羧化酶酶活测定参照文献 [11] 进行。
2.2.4. 生长曲线绘制
每隔3 h取样,于OD600处测菌液浓度,并绘制生长曲线。
2.2.5. 残糖测定
取0. 5 mL培养好的菌液于2 mL的离心管中,加入0. 5 mL的DNS,在沸水中孵育5 min立即置于冰水浴冷却,然后在A540测OD值,再根据标准曲线计算出残糖,具体参考文献 [12] 。
3. 结果与分析
3.1. 敲入载体pK19ms-Psod-pyc的构建
以野生型C. glutamicum基因组DNA为模板,扩增目的片段,参照方法1.2.1,构建敲入载体pK19ms-Psod-pyc。结果见图1,图1(a)的1号为pyc基因上游同源臂(约500 bp),2号为Psod启动子片段(约200 bp),3号pyc基因下游同源臂(约500 bp),4号为上游同源臂、Psod启动子、下游同源臂的融合片段(大小约为1200 bp)。

Figure 1. Construction of the knock-in vector pK19ms-Psod-pyc. (a) Amplification of the target fragments. 1: the up-stream arm of pycgene, 2: the Psod promoter fragment, 3: the down-stream arm of pycgene; (b) Identification of the knock-in vector by restriction enzyme digestion. 1: the recombinant vector pK19ms-Psod-pyc, 2: digestion the recombinant vector pK19ms-Psod-pyc by Hind III and BamH I
图1. 敲入载体pK19ms-Psod-pyc的构建。(a) 敲入片段的扩增:1:pyc基因上游同源臂,2:Psod启动子片段,3:pyc基因下游同源臂;(b) 敲入载体的酶切鉴定:1:重组质粒pK19ms-Psod-pyc,2:重组质粒pK19ms-Psod-pyc用Hind III和BamH I双酶切
将此融合片段克隆(酶切位点为Hind III和BamH I)至pK19mobsacB载体(大小为5719 bp),得到重组载体pK19ms-Psod-pyc。重组质粒进行双酶切鉴定,结果见图1(b),所得条带大小与预期相一致(1200 bp),说明上游同源臂、Psod启动子、下游同源臂的融合片段已成功克隆到pK19mobsacB载体上,重组载体命名为pK19ms-Psod-pyc。
3.2. 重组菌株CZM03的鉴定
将所构建的敲入质粒载体,电转化谷氨酸棒状杆菌,筛选阳性重组菌株并鉴定,结果见图2。M为DNA分子量标记Marker,1号和2号分别以野生型C. glutamicum和重组菌株CZM03的基因组DNA为模板,以上游同源臂的上游引物CPyr-U1和下游同源臂的下游引物CPyr-D2,检测Psod启动子的敲入情况,Psod启动子大小为200 bp,上、下游同源臂大小约为500 bp,由图2(a)可以看出,Psod启动子已成功敲入。
同时,在Psod内部设计选取引物为OPs 1,然后分别以野生型C. glutamicum和重组菌株CZM03的基因组DNA为模板,用OPs 1和CPyr-D 2 (OPs 2)进行扩增检测,结果见图2(b),泳道3为重组菌株CZM03的基因组DNA为模板扩增结果,有特异条带扩增出来,泳道4为野生型C. glutamicum的基因组DNA为模板扩增结果,无特异条带出现,这进一步证实Psod已成功插入到pyc基因上游。

Figure 2. Identification the recombinant strains
图2. 重组菌株的鉴定
3.3. 重组菌株的生长特性
野生型C. glutamicum (WT)、重组菌株CZM03的生长情况,结果见图3。WT、CZM03在接种后5 h左右进入对数生长期,对数生长约15 h后,进入平台期,平台期维持约10 h左右,进入衰亡期。两株菌的生长速率和平台期达到的最大生物量,没有明显差异。这表明Psod强启动子的敲入,对菌的生长没有明显影响,但是PYC的酶活有显著提高(见图4),这对于目的产物的积累是有利的。
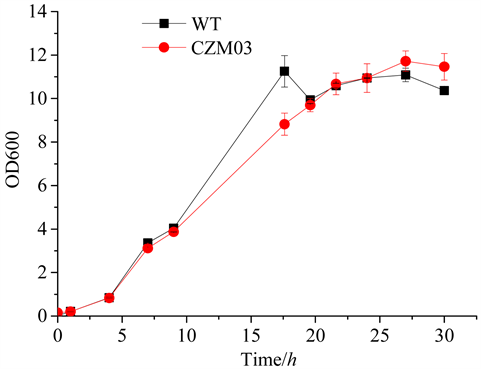
Figure 3. Growth curve of different strains
图3. 不同菌株的生长曲线
3.4. 重组菌株PYC酶活分析
丙酮酸至苹果酸的厌氧代谢通路为:丙酮酸→草酰乙酸→苹果酸。丙酮酸至草酰乙酸的转化步骤由PYC催化,通过Psod增强PYC酶活,可以加速丙酮酸向草酰乙酸的转化,进而利于苹果酸的生成。为此,我们检测了CZM03菌株的PYC酶活性,实验结果表明,敲入Psod后,C03菌株的PYC酶活较野生型上调了约2.2倍。

Figure 4. Assay of the enzyme activity of PYC & metabolic products
图4. PYC酶活测定和代谢产物分析
4. 结论
1) 构建强启动子Psod敲入载体,成功地在野生型C. glutamicum的丙酮酸羧化酶基因(pyc)前面敲入Psod启动子。
2) 强启动子Psod敲入后,所获得的重组菌株,与野生型C. glutamicum相比,生长速度和最大生物量无明显差异。
3) 与野生型C. glutamicum相比,重组菌株CZM03的PYC活性上调了约2.2倍。
致谢
感谢全国大学生创新创业大赛项目(201710488013)的资助;感谢武汉科技大学大学生创新创业大赛项目(201710488013)的资助;感谢湖北大学生物催化与酶工程国家重点实验室开放基金(SKLBEE2018002)的资助。
文章引用
邹志成,蒋佳稀,丁俊辉,乔宝艺,曹懿佳,周 卫,陈 俊. 强启动子Psod对谷氨酸棒状杆菌pyc基因表达的影响
The Impact of Strong Promoter Psod on Pyc Gene Expression in Corynebacterium glutamicum[J]. 微生物前沿, 2019, 08(03): 103-109. https://doi.org/10.12677/AMB.2019.83013
参考文献
- 1. Knuf, C., Nookaew, I., Remmers, I., et al. (2014) Physiological Characterization of the High Malic Acid Producing Aspergillus oryzae Strain 2103a-68. Applied Microbiology and Biotechnology, 98, 3517-3527. https://doi.org/10.1007/s00253-013-5465-x
- 2. Zelle, R.M., De Hulster, E., Van Winden, W.A., et al. (2008) Malic Acid Production by Saccharomyces cerevisiae: Engineering of Pyruvate Carboxylation, Oxaloacetate Reduction, and Malate Export. Applied & Environmental Microbiology, 74, 2766-2777. https://doi.org/10.1128/AEM.02591-07
- 3. Zhang, T., Ge, C., Deng, L., et al. (2015) C4-Dicarboxylic Acid Production by Overexpressing the Reductive TCA Pathway. FEMS Microbiology Letters, 362, pii: fnv052. https://doi.org/10.1093/femsle/fnv052
- 4. McCulloch, M., McFarland, S., Berry, A., et al. (2013) Metabolic Engineering of Aspergillusoryzae NRRL 3488 for Increased Production of l-Malic Acid. Applied Microbiology and Biotechnology, 97, 8903-8912. https://doi.org/10.1007/s00253-013-5132-2
- 5. Liu, J., Xie, Z., Shin, H.D., et al. (2017) Rewiring the Reductive Tricarboxylic Acid Pathway and L-Malate Transport Pathway of Aspergillus oryzae for Overproduction of L-Malate. Journal of Biotechnology, 253, 1-9. https://doi.org/10.1016/j.jbiotec.2017.05.011
- 6. Heider, S.A. and Wendisch, V.F. (2015) Engineering Microbial Cell Factories: Metabolic Engineering of Corynebacterium glutamicum with a Focus on Non-Natural Products. Biotechnology Journal, 10, 1170-1184. https://doi.org/10.1002/biot.201400590
- 7. Kim, H.T., Baritugo, K.A., Oh, Y.H., et al. (2018) Metabolic Engineering of Corynebacterium glutamicum for the High-Level Production of Cadaverineh. ACS Sustainable Chemistry & Engineering, 6, 5296-5305. https://doi.org/10.1021/acssuschemeng.8b00009
- 8. Kim, H.T., Khang, T.U., Baritugo, K.A., et al. (2019) Metabolic Engineering of Corynebacterium glutamicum for the Production of Glutaric Acid, a C5 Dicarboxylic Acid Platform Chemical. Metabolic Engineering, 51, 99-109. https://doi.org/10.1016/j.ymben.2018.08.007
- 9. Liu, J., Li, J., Shin, H.D., et al. (2017) Protein and Metabolic Engineering for the Production of Organic Acids. Bioresource Technology, 239, 412-421. https://doi.org/10.1016/j.biortech.2017.04.052
- 10. Van der Rest, M., Lange, C. and Molenaar, D. (1999) A Heat Shock Following Electroporation Induces Highly Efficient Transformation of Corynebacterium glutamicum with Xenogeneic Plasmid DNA. Applied Microbiology and Biotechnology, 52, 541-545. https://doi.org/10.1007/s002530051557
- 11. 仇爱梅, 窦文芳, 李会, 等. 磷酸烯醇式丙酮酸羧化酶基因的敲除对于谷氨酸棒杆菌V1生理代谢的影响[J]. 微生物学通报, 2012, 39(9): 1215-1224.
- 12. Kim, D.H., Lee, S.B. and Jeong, G.T. (2014) Production of Reducing Sugar from Enteromorpha intestinalis by Hydrothermal and Enzymatic Hydrolysis. Bioresource Technology, 161, 348-353. https://doi.org/10.1016/j.biortech.2014.03.078
NOTES
*通讯作者。
