Journal of Organic Chemistry Research
Vol.
08
No.
02
(
2020
), Article ID:
36520
,
9
pages
10.12677/JOCR.2020.82002
Synthesis of Spirooxindole-Chromeno[2,3-b]Indole Derivatives Promoted by Immobilized Solid Lewis Acid Catalyst FeCl3
Xuesong Liu, Shunming Yu*
Shanghai R&D Center, Anshan Hifichem Co. Ltd., Shanghai Gengcai New Material Technology Co. Ltd., Shanghai

Received: Jun. 28th, 2020; accepted: Jul. 8th, 2020; published: Jul. 15th, 2020

ABSTRACT
Polymer-supported Lewis acid catalysts is dominion over traditional ones in a number of aspects for instance; it retains its original catalytic activity, mild reaction conditions are required, which integrate remarkable advantages in catalyst regeneration, low corrosiveness of equipment, easy achievement of continuous production, environment-friendly aspects, etc. since there are great prospects of wide range of applications in the pharmaceutical and fine chemical industry. In this work, an immobilized solid Lewis acid catalyst (PS-FeCl3) was prepared, which catalyzed the multi-component reaction casecade of isatin, phenol and 2-haloindole in one-pot manner to provide diverse spirooxindole-chromeno[2,3-b]indole derivatives with high efficiency.
Keywords:Lewis Acid, Immobilized Solid Catalyst, PS-FeCl3, Spirooxindole-Chromeno[2,3-b]Indole, Multi-Component Reaction

固载化FeCl3催化合成螺环苯并吡喃[2,3-b]吲哚类衍生物
刘雪松,于顺明*
上海庚彩新材料科技有限公司,鞍山七彩化学股份有限公司上海研发中心,上海

收稿日期:2020年6月28日;录用日期:2020年7月8日;发布日期:2020年7月15日

摘 要
高分子固载Lewis酸催化剂相比传统Lewis酸催化剂,在基本保留其原有催化活性、反应条件更温和等特点的同时,还具有对设备低腐蚀性、易于实现连续化、环境友好等显著优点,因而在医药化工领域具有十分广阔的产业化应用前景。本文利用一种固载FeCl3催化剂,即将FeCl3与聚苯乙烯反应制成负载型氯化铁催化剂(PS-FeCl3),催化靛红、苯酚以及2-卤代吲哚化合物的多组分串联环化反应“一锅法”高效合成螺环苯并吡喃[2,3-b]吲哚类衍生物。
关键词 :路易斯酸,固载催化剂,三氯化铁,螺环苯并吡喃[2,3-b]吲哚,多组分串联反应

Copyright © 2020 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
螺环吲哚酮骨架是天然产物及药物活性分子中的一类十分重要的结构单元 [1] [2] [3]。螺环吲哚酮类化合物被发现具有十分广谱的生物活性,如抗癌、抗菌、降糖、降血压、抗HIV、抗结核、抗疟疾等 [4] [5]。同时,螺环吲哚酮类衍生物一般含有多个手性中心,拥有复杂的立体化学结构。因此,发展高效的合成方法来构建这类骨架化合物成为近些年有机合成化学和药物化学研究的热点领域 [6]。
其中,苯并吡喃[2,3-b]吲哚是一类同时具有吲哚和苯并吡喃骨架的杂环化合物 [7]。许多含有苯并吡喃[2,3-b]吲哚结构的化合物被发现具有良好的生物活性,如抗疟疾 [8]、抗癌 [9] 和抗增殖 [10] 活性等。但是到目前为止,关于该类化合物合成方法的报道还是非常少,而且基本都是通过多步反应实现的 [11]。因而,发展更加高效的合成方法来获得具有全新的苯并吡喃[2,3-b]吲哚类化合物具有重要意义。FeCl3是廉价易得、环境友好的重要Lewis酸,能溶于多种有机溶剂,广泛用做有机合成的催化剂。FeCl3经过活性炭、SiO2、高分子材料和离子交换树脂等材料负载后可以作为Lewis酸催化剂促进多种有机反应。固载FeCl3具有稳定性高、易于回收重复使用等优点,因而受到广泛关注。最近,我们也报道了FeCl3促进的2-卤代吲哚与3-羟基-3-(2-羟基苯基)-2-吲哚酮经多步反应,最后环化反应生成复杂的苯并吡喃[2,3-b]吲哚结构的方法 [12]。
本文通过反应条件筛选优化,发现了路易斯酸FeCl3可催化靛红、苯酚及2-卤代吲哚类化合物的多组分串联环化反应。文中参考文献方法制备了聚苯乙烯负载型FeCl3催化剂(PS-FeCl3)。在PS-FeCl3的促进下,我们以靛红、苯酚以及2-卤吲哚类化合物为起始物料,通过多组分“一锅法”反应,高效合成了一系列螺环苯并吡喃[2,3-b]吲哚类衍生物。该反应具有条件温和,操作简便,催化剂性能稳定,底物适用性广泛等特点。
2. 结果与讨论
2.1. 反应条件筛选与优化
首先,我们以N-甲基靛红1a (1.0 equiv)、4-甲基苯酚2a (1.0 equiv)以及N-乙酰基-2-氯吲哚3a (1.2 equiv)为起始原料,筛选各种酸(包括路易斯酸、质子酸及固载路易斯酸等)为催化剂进行反应的条件筛选及优化(表1)。筛选了一系列Lewis酸,如FeCl3、FeBr3、Fe(OTf)3、Sc(OTf)3、Cu(OTf)2、Zn(OTf)2、In(OTf)3、BF3·OEt2以及质子酸CF3SO3H和CF3CO2H等,发现大部分酸都能在室温下促进该反应发生,但反应进展均比较缓慢,转化率也较低。而且,随着延长反应时间或升高反应温度,2-氯吲哚3a均有部分分解,导致反应产率降低。FeCl3催化该三组分反应的效率最好,转化速度较快,但同样3a分解的进程也会加快。最后,当使用固载FeCl3(PS-FeCl3,3.0 eq.)时,虽然反应速度较为缓慢(24 h),但反应较为温和,3a的分解明显减少,最终可以较高产率(80%)获得环化产物4a (表1)。

Table 1. Screening of reaction conditions [a],[b]
表1. 反应条件筛选及优化[a],[b]
2.2. 底物拓展
在PS-FeCl3催化的反应条件下,我们考察了不同性质取代基及不同位置取代的靛红、苯酚与2-卤代吲哚的反应情况。从图1中结果可以看出,对于带不同取代基(R3为富电子、电中性或缺电子取代基)的酚类化合物2,反应均可顺利进行,以60-92%的产率生成相应地化合物4a-j;电中性或缺电子基团取代的氧化吲哚酮(R2)可以顺利进行反应,以68-78%的产率生成相应的目标产物4k-l;N-烷基取代(R1 = Bn)的氧化吲哚酮,并没有观察到明显的位阻效应,并以78%的产率得到化合物4k。通过该三组分“一锅法”反应能高效制备螺苯并吡喃[2,3-b]吲哚类化合物,底物的适应性非常广,带有不同性质取代基的反应底物,均可顺利参与该反应,并以较高产率获得目标化合物。
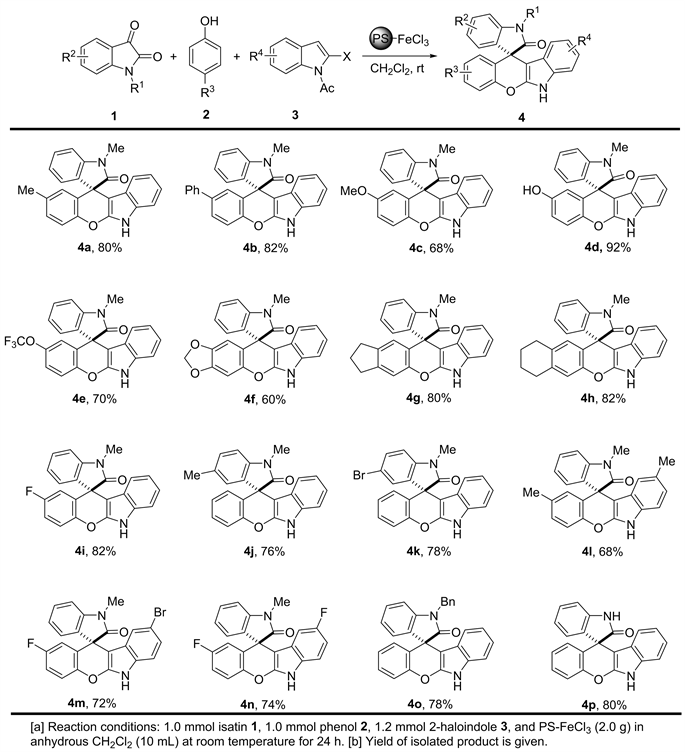
Figure 1. PS-FeCl3 promoted synthesis of spirooxindole-chromeno[2,3-b]indole derivatives [a],[b]
图1. PS-FeCl3促进合成螺苯并吡喃[2,3-b]吲哚类化合物[a],[b]
2.3. 化合物4d的晶体结构
图2为化合物4d的单晶结构,表明此方法合成的一系列螺苯并吡喃[2,3-b]吲哚类化合物具有很好的立体选择性。
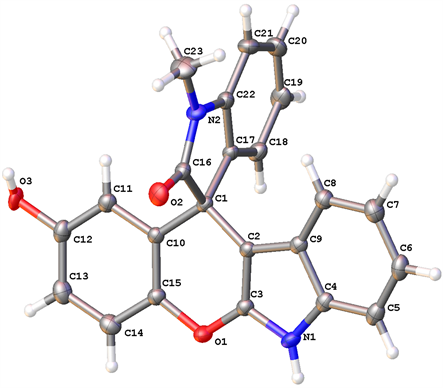
Figure 2. X-ray structure of 4d
图2. 化合物4d的晶体结构
2.4. 反应机理推测
根据氧化吲哚与酚类化合物的反应 [11] 及以上的实验结果,我们推测出如下所示的反应机理(图3)。首先,氧化吲哚酮1在路易斯酸Fe(III)的作用下与苯酚2发生Friedel-Crafts反应得到中间体叔醇I;然后叔醇I在路易斯酸的促进下脱水异构化形成中间体II;随后,2-卤代吲哚C3位对该中间体发生Michael加成得到中间体III;随后发生芳构化得到中间体产物IV。该中间体IV在Fe(III)作用下,发生分子内的亲核加成(C2-aryloxylation)得到中间体V;最后,卤原子(X)在Fe(III)的促进下消除同时脱除氮乙酰基即得到C-O环化产物4。
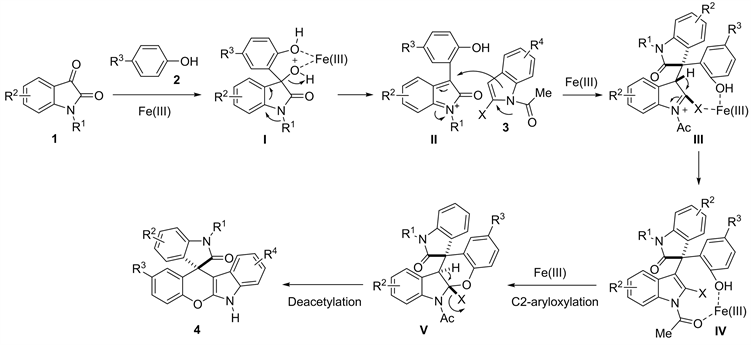
Figure 3. Plausible mechanism
图3. 反应机理推测
3. 实验部分
3.1. 仪器和试剂
所有化合物的1H NMR和13C NMR是用Bruker Avance IIIHD(500 MHz)型号的核磁测定的。核磁实验所用溶剂为CDCl3或DMSO-d6。1H化学位移以TMS(δ 0.00)或者CDCl3(δ 7.26)为参考,13C化学位移以CDCl3(δ 77.16)为参考,所有的化学位移单位为ppm。谱图中峰的多重性如下(s为单峰,d为双峰t为三重峰,m为多重峰或者未解析的峰,brs为宽峰,耦合常数以Hz为单位)。所有化合物的高分辨质谱都是用Agilent Techologies 6230 TOF LC/MS型高分辨质谱仪测定的。
3.2. PS-FeCl3催化剂的制备
PS-FeCl3催化剂按照文献方法制备 [13]:向装有回流冷凝管、温度计以及电动搅拌器的三口瓶中加入聚苯乙烯粉末及甲苯,在搅拌器加入FeCl3,回流反应。反应过程中,有沉淀生成。冷却后,过滤,固体经水、丙酮及甲基叔丁基醚洗涤,固体经真空干燥,保存待用。PS-FeCl3用等离子体发射光谱法(ICP)测定,FeCl3的负载量约为1.6 mmol/g。
3.3. PS-FeCl3催化的三组分“一锅法”合成螺环苯并吡喃[2,3-b]吲哚类化合物
向10 mL反应管中,加入靛红1 (1.0 mmol)、苯酚2 (1.0 mmol)和2-卤代吲哚3 (1.2 mmol)以及PS-FeCl3(2.0 g)以及无水二氯甲烷(10 mL),所得混合物置于室温下,振荡反应约24小时,并用薄层色谱(TLC)跟踪反应进程至2-卤代吲哚3完全转化。反应混合物经硅藻土过滤,滤饼用二氯甲烷洗涤,合并有机滤液后浓缩,粗产品经硅胶柱层析快速纯化得相应的目标化合物4a-o。
3.4. 实验数据
化合物4a:m.p. > 300˚C.1H NMR (400 MHz, CDCl3) δ 8.63 (s, 1H), 7.39 (ddd, J = 8.5, 5.4, 3.6 Hz, 1H), 7.09 (d, J = 7.8 Hz, 1H), 7.02 (d, J = 3.7 Hz, 3H), 6.96 (s, 1H), 6.90 (t, J = 8.6 Hz, 2H), 6.79 (t, J = 7.5 Hz, 1H), 6.47 (d, J = 2.2 Hz, 1H), 6.36 (d, J = 7.8 Hz, 1H), 3.44 (s, 3H), 2.18 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 178.9, 149.0, 146.5, 143.6, 134.9, 133.8, 131.0, 129.5, 128.7, 128.0, 125.5, 124.3, 123.7, 121.1, 120.6, 120.1, 117.6, 116.6, 110.8, 108.1, 86.3, 51.1, 26.8, 20.7. HRMS (ESI-TOF) m/z: calcd for C24H19N2O2 [M+H]+ 367.1447, found 367.1445.
化合物4b:m.p. 288˚C - 290˚C. 1H NMR (400 MHz, CDCl3) δ9.47 (s, 1H), 7.46 - 7.34 (m, 6H), 7.31 (dt, J = 6.3, 3.1 Hz, 1H), 7.14 (d, J = 7.8 Hz, 1H), 7.07 (d, J = 7.2 Hz, 1H), 7.03 (t, J = 7.3 Hz, 1H), 6.92 - 6.88 (m, 1H), 6.84 (dd, J = 11.2, 5.1 Hz, 3H), 6.78 (d, J = 8.0 Hz, 1H), 6.41 (d, J = 7.1 Hz, 1H), 3.51 (s, 3H). 13C NMR (100 MHz, CDCl3) δ179.4, 150.5, 146.5, 143.5, 140.2, 137.3, 134.9, 131.3, 128.8, 128.7 (2C), 127.5, 127.1, 127.0 (2C), 126.4, 125.6, 124.1, 124.0, 121.6, 120.6, 119.8, 118.6, 116.4, 111.1, 108.2, 85.8, 51.4, 26.9. HRMS (ESI-TOF) m/z: calcd for C29H21N2O2 [M+H]+ 429.1603, found 429.1602.
化合物4c:m.p. 294˚C - 295˚C. 1H NMR (400 MHz, CDCl3) δ8.92 (s, 1H), 7.40 (ddd, J = 8.4, 6.2, 2.8 Hz, 1H), 7.11 (d, J = 7.8 Hz, 1H), 7.04 (q, J = 4.1, 3.4 Hz, 2H), 6.96 - 6.86 (m, 3H), 6.85 - 6.76 (m, 2H), 6.38 (d, J = 7.8 Hz, 1H), 6.23 (d, J = 2.9 Hz, 1H), 3.68 (s, 3H), 3.46 (s, 3H). 13C NMR (100 MHz, CDCl3) δ178.9, 155.9, 146.9, 145.3, 143.5, 134.5, 131.2, 128.8, 125.5, 124.3, 123.8, 122.2, 120.5, 120.0, 118.7, 116.5, 113.9, 112.8, 110.9, 108.1, 85.6, 55.6, 51.4, 26.8. HRMS (ESI-TOF) m/z: Calcd for C24H19N2O3 [M+H]+ 383.1396, found 383.1393.
化合物4d:1H NMR (400 MHz, CDCl3) δ 8.54 (s, 1H), 7.43 - 7.38 (m, 1H), 7.09 (d, J = 7.8 Hz, 1H), 7.07 - 7.99 (m, 3H), 6.99-6.92 (m, 2H), 6.83 - 6.72 (t,J = 6.0 Hz, 1H), 6.42-6.31 (m, 2H),3.42 (s, 3H). 13C NMR (101 MHz, CDCl3) δ166.9, 154.4, 152.9, 145.5, 143.8. 138.0, 136.5, 134.9, 129.4, 127.4, 126.9, 126.4, 124.8, 121.7, 119.8, 118.8, 117.5, 115.9, 114.7, 111.6, 109.8, 59.9, 36.4. HRMS (ESI-TOF) m/z: calcd for C26H16N2O3 [M+H]+369.1239, found 369.1240.
化合物4e:m.p. 290˚C - 291˚C.1H NMR (400 MHz, CDCl3) δ9.61 (s, 1H), 7.47-7.35 (m, 1H), 7.13 (d, J = 7.9 Hz, 1H), 7.02 (ddd, J = 13.6, 7.4, 4.8 Hz, 3H), 6.88 - 6.75 (m, 2H), 6.76 - 6.69 (m, 1H), 6.67 (d, J = 9.0 Hz, 1H), 6.52 (d, J = 2.9 Hz, 1H), 6.42 - 6.30 (m, 1H), 3.49 (s, 3H). 13C NMR (100 MHz, CDCl3) δ179.0, 149.3, 146.3, 144.9 (q, JC-F = 1.8 Hz), 143.2, 134.2, 131.3, 129.2, 125.5, 124.2, 123.8, 122.7, 121.3, 120.8, 120.4, 120.3 (q, JC-F = 85.2 Hz), 120.0, 119.4, 116.5, 111.1, 108.5, 85.2, 51.4, 27.0. HRMS (ESI-TOF) m/z: calcd for C24H16F3N2O3 [M+H]+ 437.1113, found 437.1106.
化合物4f:m.p. 276˚C - 277˚C. 1H NMR (400 MHz, DMSO- d6) δ11.67 (s, 1H), 7.39 (t, J = 7.6 Hz, 1H), 7.25 (d, J = 8.0 Hz, 2H), 7.07 - 6.86 (m, 4H), 6.75 (t, J = 7.5 Hz, 1H), 6.30 (d, J = 7.8 Hz, 1H), 6.11 (s, 1H), 6.02 (d, J = 5.7 Hz, 2H), 3.30 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ177.2, 147.5, 146.1, 145.5, 144.2, 143.4, 134.3, 130.9, 128.8, 124.4, 123.5, 123.0, 120.2, 119.5, 116.1, 113.3, 110.9, 108.9, 105.8, 101.9, 98.7, 85.6, 50.4, 26.4. HRMS (ESI-TOF) m/z: calcd for C24H16N2NaO4Na [M+Na]+ 419.1008, found 419.1003.
化合物4g:m.p. > 300˚C. 1H NMR (400 MHz, CDCl3) δ8.81 (s, 1H), 7.46-7.31 (m, 1H), 7.09 (d, J = 8.0 Hz, 1H), 7.01 (q, J = 5.0, 4.6 Hz, 2H), 6.87 (d, J = 6.6 Hz, 2H), 6.83 - 6.72 (m, 2H), 6.50 (s, 1H), 6.37 (d, J = 7.8 Hz, 1H), 3.44 (s, 3H), 2.85 (q, J = 6.9 Hz, 2H), 2.73 (qt, J = 15.3, 6.4 Hz, 2H), 2.03 (ddt, J = 13.7, 10.0, 6.1 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ179.65, 149.75, 146.86, 145.31, 143.54, 140.20, 135.40, 131.25, 128.59, 125.53, 124.40, 123.79, 122.77, 120.13, 119.79, 118.84, 116.41, 113.85, 111.03, 108.07, 85.86, 51.40, 32.77, 32.13, 26.88, 25.86. HRMS (ESI-TOF) m/z: calcd for C26H21N2O2 [M+H]+ 393.1603, found 393.1596.
化合物4h:m.p. 282˚C - 283˚C. 1H NMR (400 MHz, CDCl3) δ9.20 (s, 1H), 7.37 (ddd, J = 7.8, 5.5, 3.5 Hz, 1H), 7.09 (d, J = 7.8 Hz, 1H), 7.04 - 6.95 (m, 2H), 6.85 - 6.79 (m, 1H), 6.76 (ddd, J = 9.3, 7.3, 2.3 Hz, 2H), 6.48 (s, 1H), 6.34 (d, J = 6.8 Hz, 2H), 3.46 (s, 3H), 2.68 (q, J = 5.4 Hz, 2H), 2.56 (qd, J = 16.4, 8.1 Hz, 2H), 1.81 - 1.64 (m, 4H).13C NMR (100 MHz, CDCl3) δ179.7, 148.7, 146.8, 143.6, 138.0, 135.3, 133.1, 131.2, 128.6, 127.6, 125.5, 124.4, 123.8, 120.1, 119.7, 118.5, 117.8, 116.3, 111.1, 108.1, 85.9, 51.1, 29.2, 28.7, 26.9, 23.2, 22.9. HRMS (ESI-TOF) m/z: calcd for C27H23N2O2 [M+H]+ 407.1760, found 407.1755.
化合物4i:m.p. > 300˚C. 1H NMR (400 MHz, CDCl3) δ9.18 (s, 1H), 7.46 - 7.35 (m, 1H), 7.11 (d, J = 7.8 Hz, 1H), 7.06 - 6.98 (m, 2H), 6.90 (ddd, J = 8.9, 7.5, 3.0 Hz, 1H), 6.85 (dd, J = 6.9, 1.5 Hz, 1H), 6.84 - 6.79 (m, 2H), 6.79 - 6.74 (m, 1H), 6.39 (dd, J = 9.0, 3.1 Hz, 1H), 6.36 (d, J = 7.4 Hz, 1H), 3.46 (s, 3H). 13C NMR (100 MHz, CDCl3) δ178.7, 158.8 (d, JC-F= 241.2 Hz), 147.2 (d, JC-F = 1.1 Hz), 146.5, 143.4, 134.2, 131.3, 129.1, 125.5, 124.0, 122.8 (d, JC-F = 3.6 Hz), 120.8, 120.1, 119.4, 119.3, 116.6, 115.8 (d, JC-F = 11.8 Hz), 113.5 (d, JC-F = 11.9 Hz), 111.0, 108.4, 85.3, 51.5, 26.9. HRMS (ESI-TOF) m/z: calcd for C23H16FN2O2 [M+H]+ 371.1196, found 371.1186.
化合物4j:m.p. 298˚C - 300˚C. 1H NMR (400 MHz, DMSO- d6) δ 11.73 (s, 1H), 7.36 (d, J = 6.3 Hz, 2H), 7.27 (d, J = 8.0 Hz, 1H), 7.17 (q, J = 8.1 Hz, 2H), 7.12 - 7.03 (m, 1H), 6.97 (t, J = 7.7 Hz, 1H), 6.77 (d, J = 10.7 Hz, 2H), 6.69 (d, J = 7.8 Hz, 1H), 6.35 (d, J = 7.8 Hz, 1H), 3.30 (s, 3H), 2.13 (s, 3H). 13C NMR (100 MHz,DMSO-d6) δ 177.0, 150.4, 145.8, 140.9, 134.7, 132.1, 130.8, 128.9, 128.8, 128.2, 124.9, 124.5, 123.6, 121.8, 120.2, 119.5, 117.3, 116.1, 110.9, 108.5, 85.9, 50.2, 26.3, 20.3. HRMS (ESI-TOF) m/z: calcd for C24H19N2O2 [M+H]+ 367.1447, found 367.1446.
化合物4k:m.p. > 300˚C. 1H NMR (400 MHz, DMSO-d6) δ11.80 (s, 1H), 7.59 (dd, J = 8.3, 2.1 Hz, 1H), 7.46 - 7.33 (m, 2H), 7.28 (t, J = 8.3 Hz, 2H), 7.15 - 7.05 (m, 2H), 7.00 (t, J = 7.6 Hz, 1H), 6.81 (t, J = 7.5 Hz, 1H), 6.74 (d, J = 7.8 Hz, 1H), 6.37 (d, J = 7.8 Hz, 1H), 3.32 (s, 3H). 13C NMR (100 MHz,DMSO-d6) δ176.7, 150.4, 145.9, 142.7, 136.8, 131.6, 130.8, 129.1, 128.1, 127.1, 124.6, 123.4, 120.9, 120.3, 119.7, 117.5, 115.9, 114.8, 111.1, 111.0, 85.1, 50.3, 26.5. HRMS (ESI-TOF) m/z: calcd for C23H16BrN2O2 [M+H]+ 431.0395, found 431.0389.
化合物4l:m.p. > 300˚C. 1H NMR (400 MHz, DMSO-d6) δ11.55 (s, 1H), 7.40 (t, J = 7.7 Hz, 1H), 7.28 (d, J = 7.9 Hz, 1H), 7.23 (d, J = 8.5 Hz, 1H), 7.20 - 7.07 (m, 2H), 6.98 (t, J = 7.5 Hz, 1H), 6.90 (d, J = 7.4 Hz, 1H), 6.78 (d, J = 8.2 Hz, 1H), 6.46 (s, 1H), 6.07 (s, 1H), 3.33 (s, 3H), 2.14 (s, 3H), 2.11 (s, 3H). 13C NMR (100 MHz,DMSO-d6) δ177.2, 148.5, 146.1, 143.3, 134.6, 133.5, 129.6, 129.0, 128.7, 127.9, 127.8, 124.4, 123.8, 123.1, 121.4, 121.3, 117.1, 116.0, 110.7, 108.7, 85.5, 50.2, 26.4, 21.2, 20.1. HRMS (ESI-TOF) m/z: calcd for C25H21N2O2 [M+H]+ 381.1603, found 381.1596.
化合物4m:m.p. > 300˚C. 1H NMR (400 MHz, CDCl3) δ 9.71 (s, 1H), 7.44 (td, J = 7.7, 1.3 Hz, 1H), 7.30 (dd, J = 8.8, 2.4 Hz, 1H), 7.15 (d, J = 7.8 Hz, 1H), 7.07 (t, J = 7.5 Hz, 1H), 7.02 - 6.96 (m, 1H), 6.79 (d, J = 2.4 Hz, 1H), 6.63 (d, J = 8.8 Hz, 1H), 6.60 - 6.46 (m, 2H), 5.97 (dd, J = 9.5, 2.4 Hz, 1H), 3.51 (s, 3H). 13C NMR (100 MHz,CDCl3) δ 179.0, 157.9 (d, JC-F = 116.3 Hz), 150.0, 147.2, 143.1, 133.8, 131.8, 130.5, 129.4, 127.6, 125.5, 124.4, 124.2 (d, JC-F = 5.2 Hz), 123.2, 119.9, 116.5, 111.8 (d, JC-F = 4.8 Hz), 108.7, 108.3 (d, JC-F = 12.8 Hz), 102.0 (d, JC-F = 12.4 Hz), 85.7 (d, JC-F = 2.1 Hz), 51.1, 27.1. HRMS (ESI-TOF) m/z: calcd for C23H14BrFN2O2 [M+H]+ 449.0301, found 449.0297.
化合物4n:m.p. > 300˚C. 1H NMR (400 MHz, DMSO- d6) δ11.94 (s, 1H), 7.50 - 7.38 (m, 2H), 7.34 - 7.20 (m, 3H), 7.07 - 6.94 (m, 2H), 6.81 (td, J = 9.3, 2.6 Hz, 1H), 6.54 (dd, J = 9.0, 3.1 Hz, 1H), 5.93 (dd, J = 9.6, 2.6 Hz, 1H), 3.32 (s, 3H). 13C NMR (100 MHz, DMSO- d6) δ176.4, 158.3 (d, JC-F = 239.6 Hz), 156.9 (d, JC-F = 231.1 Hz),147.3, 146.8 (d, JC-F = 1.1 Hz), 143.4, 133.3, 129.2, 127.4, 124.5, 123.7 (d, JC-F = 3.7 Hz), 123.2, 123.1 (d, JC-F = 3.7 Hz), 119.2 (d, JC-F = 4.2 Hz,), 116.3 (d, JC-F = 12.8 Hz), 113.5 (d, JC-F = 12.8 Hz), 112.2 (d, JC-F = 4.8 Hz), 109.2, 107.9 (d, JC-F = 12.6 Hz), 101.3 (d, JC-F = 12.1 Hz), 85.8 (d, JC-F = 2.1 Hz), 50.3, 26.4. HRMS (ESI-TOF) m/z: calcd for C23H15F2N2O2 [M+H]+ 389.1102, found 389.1093.
化合物4o:m.p. 289˚C - 290˚C. 1H NMR (400 MHz, DMSO- d6) δ11.78 (s, 1H), 7.44 (d, J = 6.5 Hz, 2H), 7.41 - 7.37 (m, 2H), 7.35 (d, J = 7.5 Hz, 2H), 7.34 - 7.29 (m, 2H), 7.25 (dd, J = 17.5, 8.0 Hz, 2H), 7.09 (ddd, J = 8.3, 5.9, 2.7 Hz, 1H), 6.96 (d, J = 4.5 Hz, 3H), 6.67 (t, J = 7.9 Hz, 2H), 6.19 (d, J = 7.8 Hz, 1H), 5.08 (d, J = 15.4 Hz, 1H), 5.00 (d, J = 15.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ177.4, 150.5, 146.0, 142.4, 136.3, 134.4, 130.8, 129.0, 128.7, 128.5 (2C), 127.8, 127.7 (2C), 127.6, 124.7, 124.6, 123.5, 123.2, 121.7, 120.3, 119.3, 117.5, 116.4, 111.0, 109.4, 85.7, 50.2, 43.3. HRMS (ESI-TOF) m/z: calcd for C29H21N2O2 [M+H]+ 429.1603, found 429.1598.
化合物4p:m.p. > 300˚C. 1H NMR (400 MHz, DMSO-d6) δ11.71 (s, 1H), 10.74 (s, 1H), 7.41 - 7.32 (m, 2H), 7.32 - 7.24 (m, 2H), 7.10 (t, J = 8.1 Hz, 2H), 7.01 - 6.94 (m, 1H), 6.94 - 6.86 (m, 2H), 6.79 (t, J = 7.5 Hz, 1H), 6.74 (dd, J = 7.8, 1.5 Hz, 1H), 6.42 (d, J = 7.8 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ178.8, 150.5, 145.8, 141.9, 135.2, 130.8, 128.8, 128.7, 128.0, 124.8, 124.5, 123.78, 122.4, 121.9, 120.1, 119.4, 117.3, 116.1, 110.9, 109.7, 85.9, 50.6. HRMS (ESI-TOF) m/z: calcd for C22H15N2O2 [M+H]+ 339.1134, found 339.1126.
4. 总结
在本文中,我们以靛红、苯酚以及2-卤吲哚类化合物为起始物料,在聚苯乙烯负载型氯化铁催化剂(PS-FeCl3)催化下,快速简便的合成了一系列螺环苯并吡喃[2,3-b]吲哚类化合物。在该反应过程中,我们实现了多组分“一锅法”合成策略;而且,利用PS-FeCl3做催化剂,反应条件温和,操作简便,催化剂性能稳定。该反应具有底物适用范围广、反应条件温和、操作简单等优点。最后,我们还对此反应提出了一个可能的反应机理,为构建复杂螺环吲哚类化合物供了重要的理论参考。
致谢
感谢中国科学院上海有机化学研究所冷雪冰教授在晶体结构分析上提供的帮助。
文章引用
刘雪松,于顺明. 固载化FeCl3催化合成螺环苯并吡喃[2,3-b]吲哚类衍生物
Synthesis of Spirooxindole-Chromeno[2,3-b]Indole Derivatives Promoted by Immobilized Solid Lewis Acid Catalyst FeCl3[J]. 有机化学研究, 2020, 08(02): 15-23. https://doi.org/10.12677/JOCR.2020.82002
参考文献
- 1. Reisman, S.E., Ready, J.M., Hasuoka, A., Smith, C.J. and Wood, J.L. (2006) Total Synthesis of (±)-Welwitindolinone A Isonitrile. Journal of the American Chemical Society, 128, 1448-1449. https://doi.org/10.1021/ja057640s
- 2. Zhou, X., Xiao, T., Iwama, Y., Qin, Y. (2012) Biomimetic Total Synthesis of (+)-Gelsemine. Angewandte Chemie International Edition, 51, 4909-4912. https://doi.org/10.1002/anie.201201736
- 3. Xu, J., Shao, L.-D., Li, D., Deng, X., Liu, Y.-C., Zhao, Q.-S. and Xia, C. (2014) Construction of Tetracyclic 3-Spi- rooxindole through Cross-Dehydrogenation of Pyridinium: Applications in Facile Synthesis of (±)-Corynoxine and (±)- Corynoxine B. Journal of the American Chemical Society, 136, 17962-17965. https://doi.org/10.1021/ja5121343
- 4. Galliford, C.V. and Scheidt, K.A. (2007) Pyrrolidinyl-Spirooxindole Natural Products as Inspirations for the Development of Potential Therapeutic Agents. Angewandte Chemie International Edition, 46, 8748-8758. https://doi.org/10.1002/anie.200701342
- 5. Girgis, A.S., Stawinski, J., Ismail, N.S.M. and Farag, H.E. (2012) Synthesis and QSAR Study of Novel Cytotoxic Spi-ro[3H-indole-3,2′(1′H)-pyrrolo[3,4-c]pyrrole]-2,3′,5′(1H,2′aH,4′H)-triones. Journal of Medicinal Chemistry, 47, 312-322. https://doi.org/10.1016/j.ejmech.2011.10.058
- 6. Ball-Jones, N.R., Badillo, J.J. and Franz, A.K. (2012) Strategies for the Enantioselective Synthesis of Spirooxindoles. Organic Biomolecular Chemistry, 10, 5165-5181. https://doi.org/10.1039/c2ob25184a
- 7. Momose, R., Tanaka, N., Fromont, J. and Kobayashi, J. (2013) Hyr-timomines A-C, New Heteroaromatic Alkaloids from a Sponge Hyrtios sp. Organic Letters, 15, 2010-2013. https://doi.org/10.1021/ol400687b
- 8. Peng, W., Świtalska, M., Wang, L., Mei, Z.-W., Edazawa, Y., Pang, C.-Q., El-Sayed, I.E.-T., Wietrzyk, J. and Inokuchi, T. (2012) Synthesis and in Vitro Anti-Proliferative Activity of New 11-Aminoalkylamino-Substituted Chromeno[2,3-b]indoles. European Journal Medicinal Chemistry, 58, 441-451. https://doi.org/10.1016/j.ejmech.2012.10.023
- 9. Graczol-Foerdos, E., Novak, T., Blasko, G., Fejes, I., Per-ron-Sierra, F. and Nyerges, M. (2013) Synthesis of Chromeno [2,3-b]indole Derivates. Heterocycles, 87, 2053-2069. https://doi.org/10.3987/COM-13-12780
- 10. Corey, E.J. and Guzman-Perez, A. (1998) The Catalytic Enan-tio-Selective Construction of Molecules with Quaternary Carbon Stereocenters. Angewandte Chemie International Edition, 37, 388-401. https://doi.org/10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V
- 11. Zhao, W.-X., Wang, Z.-B., Chu, B.-Y. and Sun, J.-W. (2015) Enantioselective Formation of All-Carbon Quaternary Stereocenters from Indoles and Tertiary Alcohols Bearing a Directing Group. Angewandte Chemie International Edition, 54, 1910-1913. https://doi.org/10.1002/anie.201405252
- 12. Luo, M.P., Zhu, X.L., Liu, R.F., Yu, S.-M. and Wei, W.G. (2020) FeCl3-Promoted Annulation of 2-Haloindoles: Switchable Synthesis of Spirooxindole-chromeno[3,2-b]indoles and Spirooxindole-chromeno[3,2-b]indoles. The Journal of Organic Chemistry, 85, 3638-3654. https://doi.org/10.1021/acs.joc.9b03300
- 13. Liu, F., Lv, Y. and Huang, H.M. (1990) Synthesis of Polymer Catalysts and Its Use for Addition and Esterification Reaction. Petrochem Technol, 19, 814-818. (In Chinese)
NOTES
*通讯作者。