Journal of Organic Chemistry Research
Vol.
09
No.
02
(
2021
), Article ID:
43175
,
6
pages
10.12677/JOCR.2021.92002
电化学催化芳基甲酸脱羧合成多取代三氮唑化合物
许甲喆,周烨,曾东文,何永辉*
云南民族大学民族医药学院,云南 昆明

收稿日期:2021年4月14日;录用日期:2021年6月10日;发布日期:2021年6月17日

摘要
三氮唑类化合物具有抗炎、抗肿瘤等多种重要的生理活性,合成该类化合物一直是研究热点。本文以四丁基碘化铵为媒介,在电化学条件下实现了芳基甲酸脱羧活化,并与1,3-二取代-1,2,4-三氮唑类化合物发生脱氢交叉偶联反应,以45%~65%的产率得到了1,3,5-三取代-1,2,4-三氮唑类化合物。采用核磁共振和质谱等手段对产物结构进行表征,并提出了可能的反应机理。
关键词
三氮唑,电催化,脱羧

Electrochemical Oxidative Decarbonylation of Aromatic Formic Acid for Synthesis of 1,3,5-Trisubstituted 1H-1,2,4-Triazoles
Jiazhe Xu, Ye Zhou, Dongwen Zeng, Yonghui He*
School of Ethnic Medicine, Yunnan Minzu University, Kunming Yunnan

Received: Apr. 14th, 2021; accepted: Jun. 10th, 2021; published: Jun. 17th, 2021

ABSTRACT
Triazoles have many important physiological activities such as anti-inflammatory and anti-tumor. Thus, synthesis of these compounds has been a research hotspot. Triazoles have many important physiological activities such as anti-inflammatory and anti-tumor. Thus, synthesis of these compounds has been a research hotspot. The electrocatalytic decarbonylation of benzoic acid to construct 1,3,5-trisubstituted-1,2,4-triazoles with 1,3-bisubstituted-1,2,4-triazoles was realized in 45%~65% yield at room temperature and without oxidant. The products were confirmed by 1H NMR, 13C NMR, MS and HRMS. The reaction mechanism is proposed.
Keywords:Triazoles, Electrocatalysis, Decarbonylation

Copyright © 2021 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
1,2,4-三氮唑化合物为含三个氮原子的五元杂环结构,具有抗菌、抗炎、抗肿瘤、抗结核等重要的生理活性 [1] [2] [3] [4] [5]。同时,也是合成药物(马拉维若、三唑仑等)的重要砌块 [6] [7]。除此之外,三氮唑化合物还在配位化学、材料化学和天然产物中有广泛的应用。比如,最近的研究表明,1,2,4-三氮唑作为配体,与Ir3+配位后以高的量子产率发射蓝光,显示在有机发光二极管中,有较好的应用潜力 [8]。
合成1,2,4-三氮唑一直是研究热点,合成方法包括传统的佩利扎里反应、过渡金属催化脱氢偶联和过氧化物催化等 [9] [10] [11] [12]。最近Habtamu等人以四丁基碘化铵为媒介,在高温条件下,利用氧化剂叔丁基过氧化氢成功地实现了芳酮脱羰基活化,进攻1,3-二取代三唑,得到了1,3,5-三取代-1,2,4-三氮唑化合物 [13]。然而,这些报道的催化体系存在使用化学当量的氧化剂、过渡金属及苛刻的反应条件等不足。
电化学催化反应利用电子作为催化剂,避免使用氧化剂、金属等,在温和条件下,化学家们开发出了许多利用碳氢活化策略实现的电化学催化反应 [14] [15] [16]。本课题组采用电化学催化方法,实现了碳氢活化成功合成咪唑等杂环化合物 [17]。本文采用电化学催化芳基羧酸脱羧活化,成功实现了三氮唑杂环的碳氢活化交叉偶联反应,高选择性的构建了1,3,5-三取代的-1,2,4-三氮唑化合物。该方法具有操作简单,绿色环保等特点。
2. 实验部分
2.1. 主要仪器与试剂
Bruker-400型核磁共振仪(CDCl3为溶剂,TMS为内标)、BrukermicroTOF-Q II型高分辨质谱仪(国布鲁克公司),电化学合成仪ElectraSyn2.0 (德国IKA)。
柱分离用200~300目硅胶、溶剂和试剂均为市售分析纯;乙腈(分析纯,南京化学试剂股份有限公司);试验所用芳基甲酸1,3-二取代1,2,4-三氮唑类化合物、四丁基碘化铵均购自北京伊诺凯科技有限公司。
2.2. 实验方法
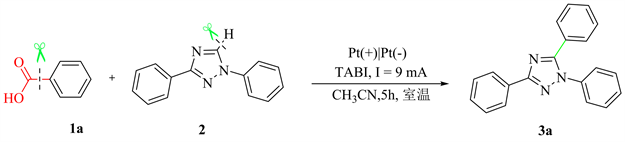
于25 mL的三颈圆底烧瓶中加入22 mg苯甲酸1 (0.2 mmol),44 mg三氮唑2 (0.2 mmol),36.9 mg四丁基碘化铵(0.1 mmol),15 ml乙腈,混合均匀,15 mm × 15 mm铂片为正极和负极,室温下以9 mA电流通电5小时。反应完全后,混合物减压脱溶,得残余物,柱层析(V(石油醚):V(乙酸乙酯) = 60:1) 纯化得化合物3。

1,3,5-三苯基-1-氢-1,2,4-三氮唑(3a):淡黄色固体,65%收率。1H NMR (400 MHz, CDCl3) δ: 8.27 (d, J = 7.2 Hz, 2H), 7.57~7.60 (m, 2H), 7.48~7.53 (m, 2H), 7.43~7.47 (m, 6H), 7.43~7.45 (m, 1H), 7.36~7.39 (m, 2H); 13C NMR (100 MHz, CDCl3) δ: 161.9, 154.7, 138.3, 130.7, 130.0, 129.3, 129.0, 128.9, 128.8, 128.5, 128.0, 126.6, 125.4; LRMS (EI 70 eV) m/z (%): 297 (M+, 100); HRMS m/z (ESI) calcd for C20H16N3 (M + H)+ 298.1338, found 298.1335。
1,3-二苯基-5-(对-甲苯基)-1-氢-1,2,4-三氮唑(3b):淡黄色固体,62%收率。1H NMR (400 MHz, CDCl3) δ: 8.27 (t, J = 6.8 Hz, 2H), 7.42~7.48 (m, 10H), 7.16 (d, J = 8.0 Hz, 2H), 2.36 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 161.8, 154.8, 140.1, 138.4, 130.8, 129.3, 129.2, 128.9, 128.8, 128.7, 128.5, 126.5, 125.4, 125.1, 21.3; LRMS (EI 70 eV) m/z (%): 311 (M+, 100); HRMS m/z (ESI) calcd for C21H18N3 (M + H)+ 312.1495, found 312.1488。
5-(对-甲氧苯基)-1,3-二苯基-1-氢-1,2,4-三氮唑(3c):淡黄色固体,60%收率;1H NMR (400 MHz, CDCl3) δ: 8.21~8.23 (m, 2H), 7.38~7.48 (m, 10H), 6.83 (d, J = 9.2 Hz, 2H), 3.75 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 160.7, 159.8, 152.6, 136.4, 130.8, 129.4, 128.3, 128.2, 127.6, 127.5, 125.5, 124.4, 118.3, 112.9, 54.2; HRMS m/z (ESI) calcd for C21H18N3O (M + H)+ 328.1444, found 328.1449。
5-(4-氯苯基)-1,3-二苯基-1-氢-1,2,4-三氮唑(3d):淡黄色固体,收率55%。1H NMR (400 MHz, CDCl3) δ: 8.23 (dd, J = 8.0 Hz, 1.6 Hz, 2H), 7.40~7.52 (m, 10H), 7.32~7.35 (m, 2H); 13C NMR (100 MHz, CDCl3) δ: 162.0, 153.7, 138.1, 136.2, 130.5, 130.2, 129.5, 129.5, 129.0, 128.8, 128.6, 126.5, 126.4, 125.4; LRMS (EI 70 eV) m/z (%): 331 (M+, 100); HRMS m/z (ESI) calcd for C20H15ClN3 (M + H)+ 332.0949, found 332.0955。
5-(4-氟苯基)-1,3-二苯基-1-氢-1,2,4-三氮唑(3e),淡黄色固体,54%收率;1HNMR (400 MHz, CDCl3) δ: 8.13 (dd, J = 8.4 Hz, 1.6 Hz, 2H), 7.45 (dd, J = 8.8 Hz, 5.2 Hz, 2H), 7.30~7.37 (m, 8H), 6.95 (t, J = 8.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 164.8 (d, J = 249.7 Hz), 162.2, 154.1, 138.4, 131.3, 131.2, 130.9, 129.7 (d, J = 3.3 Hz), 129.2, 128.8, 126.8, 125.7, 124.4 (d, J = 3.5 Hz), 116.1 (d, J = 21.8 Hz); LRMS (EI 70 eV) m/z (%): 315 (M+, 100); HRMS m/z (ESI) calcd for C20H15FN3 (M + H)+ 316.1244, found 316.1249。
1,3-二苯基-5-(2-噻吩)-1-氢-1,2,4-三唑(3f):白色油状物,收率61%。1H NMR (400 MHz, CDCl3) δ: 8.21 - 8.23 (m, 2H), 7.51 (s, 5H), 7.40~7.47 (m, 3H), 7.36 (dd, J = 4.8 Hz, 0.8 Hz, 1H), 7.05 (dd, J = 3.6 Hz, 0.8 Hz, 1H), 6.93 (dd, J = 5.2 Hz, 4.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 161.8, 150.1, 137.9, 130.5, 129.8, 129.5, 129.4, 128.8, 128.7, 128.5, 127.5, 126.6, 126.6, 125.3; LRMS (EI 70 eV) m/z (%): 303 (M+, 100); HRMS m/z (ESI) calcd for C18H14N3S (M + H)+ 304.0902, found 304.0907。
5-(naphthalen-1-yl)-1,3-二苯基-1-氢-1,2,4-三氮唑(3g):棕红色油状物,收率45%; 1H NMR (400 MHz, CDCl3) δ: 8.31~8.33 (m, 2H), 7.94 (dd, J = 12.8 Hz, 8.0 Hz, 2H), 7.87 (dd, J = 6.8 Hz, 2.0 Hz, 1H), 7.39~7.51 (m, 7H), 7.28~7.30 (m, 2H), 7.20~7.23 (m, 3H); 13C NMR (100 MHz, CDCl3) δ: 162.0, 153.7, 137.8, 133.6, 131.5, 130.7, 130.6, 129.4, 129.0, 128.9, 128.6, 128.3, 128.0, 127.2, 126.6, 126.5, 126.0, 125.3, 124.9, 123.9; LRMS (EI 70 eV) m/z (%): 347 (M+, 100); HRMS m/z (ESI) calcd for C24H18N3 (M + H)+ 348.1495, found 348.1490。
3. 结果与讨论
3.1. 反应条件的筛选
以苯甲酸1a (0.2 mmol)和三氮唑2 (0.2 mmol)为底物,以四丁基碘化铵(0.1 mmol)为催化媒介的反应为模型,我们探讨了催化媒介的类型、电流的大小、溶剂的种类以及反应温度对模型反应产率的影响(表1)。
首先探讨催化媒介对反应的影响(表1,entries 1~5)。从表中可以看出,无催化媒介条件下不能发生反应;使用碘化铵、溴化铵均不如四丁基碘化铵的产率高;增加催化媒介的量不能提高反应产率。对溶剂进行了筛选,发现溶剂甲醇、乙醇和DMSO均会使产率有所下降(表1,entries 6~8)。随后对电流进行筛选,研究表明没有电流时,反应不能发生;电流为6mA和12mA时,产率均有所下降,说明9 mA电流为最合适的电流(表1,entries 9~11)。最后,对反应温度进行了考察(表1,entries 12~13),结果表明提高反应温度不能使产率提高。因此,此反应的最优反应条件为:以四丁基碘化铵为媒介、9 mA电流、乙腈为溶剂,室温下反应5小时。
Table 1. Optimization of reaction conditions
表1. 反应条件的优化
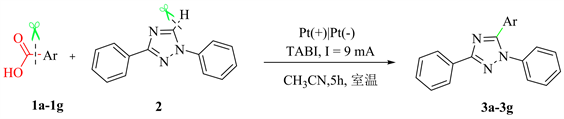
3.2. 目标产物的普适性研究
根据上述建立的最优反应条件(表1,Entries 2),得到了中等产率的目标化合物3a-3g,我们对该反应的底物普适性进行了研究,结果如表2所示。从表中可以看出,芳基羧基1a-1e的苯环上无论带有强吸电子基团还是给电子基团,都能很好的发生脱羧活化,实现三氮唑杂环的碳氢活化交叉偶联反应,以中等产率得到目标化合物。此外,芳环扩展到噻吩或者萘环,反应均能较好的发生。因此,该反应体系中芳基羧酸具有较好的底物普适性。
Table 2. Investigation of substrate scope
表2. 底物普适性研究
3.3. 反应机理分析
以模型反应为研究对象,在最优反应条件下对反应机理进行探讨。当反应中加入0.2 mmol自由基抑制剂2,2,6,6-四甲基哌啶氮氧化物时,反应受到抑制。根据文献 [13] 提出图1所示的反应机理:电化学条件下,碘负离子在阳极被氧化成碘自由基,该自由基进攻苯甲酸,导致苯甲酸脱氢,并进一步脱羰基,生成苯基自由基。然后,苯基自由基进攻二取代–三氮唑上的碳氢键,生成自由基中间体,经单电子转移并脱氢后得到目标化合物3a。
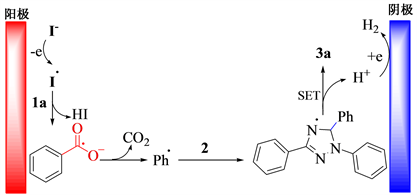
Figure 1. Plausible mechanism for synthesis of compound 3a
图1. 合成化合物3a可能的反应机理
4. 结论
采用碘盐媒介的电化学催化芳基脱羧活化,在室温下实现了1,3-二取代-1,2,4-三氮唑的碳氢活化交叉偶联反应,得到了1,3,5-三取代-1,2,4-三氮唑化合物。目标化合物用1HNMR、13CNMR、HRMS等进行了结构表征。以特征目标产物例,分析了其波谱数据及可能的反应机理。该反应具有操作简便和环境友好等特点。
文章引用
许甲喆,周 烨,曾东文,何永辉. 电化学催化芳基甲酸脱羧合成多取代三氮唑化合物
Electrochemical Oxidative Decarbonylation of Aromatic Formic Acid for Synthesis of 1,3,5-Trisubstituted 1H-1,2,4-Triazoles[J]. 有机化学研究, 2021, 09(02): 7-12. https://doi.org/10.12677/JOCR.2021.92002
参考文献
- 1. Gabor, M. (1986) Anti-Inflammatory and Anti-Allergic Properties of Flavonoids. Progress in Clinical and Biological Research, 213, 471-480.
- 2. Singh, G., Singh, L. and Ishar, M.P.S. (2002) 2-(N-Methylanilino)-3-Formylchromone—A Versatile Synthon for Incorporation of Chromone Moiety in a Variety of Heterocyclic Systems and Macrocycles through Reactions with Bifunctional Nucleophiles. Tetrahedron, 58, 7883-7890. https://doi.org/10.1016/S0040-4020(02)00908-0
- 3. Martens, S. and Mithofer, A. (2005) Flavones and Flavone Synthases. Phytochemistry, 66, 2399-2407. https://doi.org/10.1016/j.phytochem.2005.07.013
- 4. Chohan, Z.H., Shaikh, A.U., Rauf, A. and Supuran, C.T. (2006) Antibacterial, Antifungal and Cytotoxic Properties of Novel N-Substituted Sulfonamides from 4-Hydroxycoumarin. Journal of Enzyme Inhibition and Medicinal Chemistry, 21, 741-748. https://doi.org/10.1080/14756360600810340
- 5. Djemgou, P.C., Gatsing, D., Tchuendem, M., Ngadjui, B.T., Tane, P., Ahmed, A.A., Gamal-Eldeen, A.M., Adoga, G.I., Hirata, T. and Mabry, T.J. (2006) Antitumor and Immunostimulatory Activity of Two Chromones and Other Constituents from Cassia Petersiana. Natural Product Communications, 1, 961-968. https://doi.org/10.1177/1934578X0600101109
- 6. Kuroda, M., Uchida, S., Watanabe, K. and Mimaki, Y. (2009) Chromones from the Tubers of Eranthis Cilicica and Their Antioxidant Activity. Phytochemistry, 70, 288-293. https://doi.org/10.1016/j.phytochem.2008.12.002
- 7. Sandhya, M.B. (2007) Synthesis, Characterization and Pharmacological Activities of Coumarin Devivatives. International Journal of Chemical and Pharmaceutical Sciences, 11, 16-25.
- 8. Park, H.J., Kim, J.N., Yoo, H.-J., Wee, K.-R., Kang, S.O., Cho, D.W. and Yoon, U.C. (2013) Rational Design, Synthesis, and Characterization of Deep Blue Phosphorescent Ir(III) Complexes Containing (4’-Substituted-2’-Pyridyl)-1,2,4-Triazole Ancillary Ligands. The Journal of Organic Chemistry, 78, 8054-8064. https://doi.org/10.1021/jo4012514
- 9. Zhang, C., Liang, Z., Jia, X., Wang, M., Zhang, G. and Hu, M.-L. (2020) A Practical Base Mediated Synthesis of 1,2,4-Triazoles Enabled by a Deamination Annulation Strategy. Chemical Communications, 56, 14215-14218. https://doi.org/10.1039/D0CC05828A
- 10. Yan, M., Ma, R., Chen, R., Wang, L., Wang, Z. and Ma, Y. (2020) Synthesis of 1,2-Dihydro-1,3,5-Triazine Derivatives via Cu(ii)-Catalyzed C(sp3)–H Activation of N,N-Dimethylethanolamine with Amidines. Chemical Communications, 56, 10946-10949. https://doi.org/10.1039/D0CC03820B
- 11. Zhang, L., Tang, D., Gao, J., Wang, J., Wu, P., Meng, X. and Chen, B. (2016) Direct Access to 1,3,5-Trisubstituted 1H-1,2,4-Triazoles from N-Phenylbenzamidines via Copper-Catalyzed Diamination of Aryl Nitriles. Synthesis, 48, 3924-3930. https://doi.org/10.1055/s-0035-1562490
- 12. Liew, S.K., Holownia, A., Tilley, A.J., Carrera, E.I., Seferos, D.S. and Yudin, A.K. (2016) A Study of Boratriazaroles: An Underdeveloped Class of Heterocycles. The Journal of Organic Chemistry, 81, 10444-10453. https://doi.org/10.1021/acs.joc.6b01565
- 13. Agisho, H.A., Esatu, H., Hairat, S. and Zaki, M. (2020) TBHP/TBAI–Mediated Simple and Efficient Synthesis of 3,5-Disubstituted and 1,3,5-Trisubstituted 1H-1,2,4-Triazoles via Oxidative Decarbonylation of Aromatic Aldehydes and Testing for Antibacterial Activities. Tetrahedron Letters, 61, Article ID: 151989. https://doi.org/10.1016/j.tetlet.2020.151989
- 14. Li, C.-J. (2009) Cross-dehydrogenative coupling (CDC): Exploring C-C Bond Formations beyond Functional Group Transformations. Accounts of Chemical Research, 42, 335-344. https://doi.org/10.1021/ar800164n
- 15. Yan, M., Kawamata, Y. and Baran, P.S. (2017) Synthetic Organic Electrochemical Methods since 2000: On the Verge of a Renaissance. Chemical Reviews, 117, 13230-13319. https://doi.org/10.1021/acs.chemrev.7b00397
- 16. Xiong, P. and Xu, H.-C. (2019) Chemistry with Electrochemically Generated N-Centered Radicals. Accounts of Chemical Research, 52, 3339-3350. https://doi.org/10.1021/acs.accounts.9b00472
- 17. Zeng, L., Li, J., Gao, J., Huang, X., Wang, W., Zheng, X., Gu, L., Li, G., Zhang, S. and He, Y. (2020) An Electrochemical Oxidative Multicomponent Cascade Annulation of Ketones and Amines Used to Produce Imidazoles. Green Chemistry, 22, 3416-3420. https://doi.org/10.1039/D0GC00375A
NOTES
*通讯作者。
