Studies in Synthetic Chemistry
Vol.2 No.02(2014), Article ID:13662,8 pages
DOI:10.12677/SSC.2014.22005
Transition Metal Compounds as Efficient Catalysts in Aerobic Oxidation of Biomass-Derived 5-Hydroxymethylfurfural to 2,5-Diformylfuran
School of Energy Research, College of Energy, Xiamen University, Xiamen
Email: joseph201x@icloud.com, *lulin@xmu.edu.cn
Copyright © 2014 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received: Apr. 30th, 2014; revised: May 26th, 2014; accepted: Jun. 1st, 2014
ABSTRACT
2,5-diformylfuran (DFF) is one of the important platform molecules derived from 5-hydroxymethylfurfural (HMF) or other bio-based carbohydrates, which has lots of potential applications in many fields of organic synthesis industry. This compound has become a research focus in recent years due to its relatively high price and immature synthesis technology. To realize the industrial production of DFF in the future, many methods have been attempted to achieve this product efficiently. In this research, some transition metal compounds and lanthanides, including Nb2O5, V2O5, C3F9O9S3Yb·xH2O, Na3VO4, KVO3 and MnO2, were adopted as plausible high-efficiency catalysts in aerobic oxidation of biomass-derived model molecule 5-Hydroxymethylfurfural (HMF) with the purpose of achieving high value-added DFF. The results from gas chromatographic analysis (GC) and gas chromatography-mass spectrometer (GC-MS) showed that Nb2O5 and Na3VO4 seem to have no obvious catalytic activity in this reaction. Moreover, no DFF was formed with C3F9O9S3Yb·xH2O and MnO2, other oxidation products such as 5-acetoxymethyl-2-furaldehyde were formed; KVO3 and V2O5 were found to be efficient catalysts for the aerobic selective oxidation reaction from HMF to DFF.
Keywords:Catalytic Oxidation, 5-Hydroxymethylfurfural, 2,5-Diformylfuran, Biomass, Chemicals

过渡金属化合物为高效催化剂有氧氧化生物质衍生5-羟甲基糠醛生成2,5-二甲酰基呋喃的研究
张盛强,李维烽,林 鹿*
厦门大学,能源学院能源研究院,厦门
Email: joseph201x@icloud.com, *lulin@xmu.edu.cn
收稿日期:2014年4月30日;修回日期:2014年5月26日;录用日期:2014年6月1日

摘 要
2,5-二甲酰基呋喃(DFF)是一种由5-羟甲基糠醛或其他生物基碳水化合物衍生的重要平台分子,它在有机合成工业的众多领域中具有许多潜在用途。鉴于其目前较高的价格以及合成技术的不成熟性,对该化合物的研究近年来已然成为研究热点。为了将来能够实现DFF工业化生产,已开发了一些技术用于该产品的高效生产。在本研究中,一些过渡金属化合物以及镧系化合物被用作有氧催化氧化生物质模型化合物5-羟甲基糠醛(5-HMF)获取高附加值DFF产品转化反应过程的催化剂,这些催化剂包括Nb2O5,V2O5,C3F9O9S3Yb·xH2O,Na3VO4,KVO3以及MnO2。由气相色谱分析(GC)以及气质分析(GC-MS)的结果可知:Nb2O5和Na3VO4对该转化反应没有明显的催化活性。此外,当以C3F9O9S3Yb·xH2O和MnO2为催化剂时,没有DFF生成,但生成了其他氧化产物比如5-乙酰氧基甲基-2-呋喃醛。其中,发现KVO3和V2O5在有氧选择性催化氧化5-HMF生成DFF的反应中是两种高效的催化剂。
关键词
催化氧化,5-羟甲基糠醛,2,5-二甲酰基呋喃,生物质,化学品

1. 引言
现今的化工行业越来越依赖石油作为生产各种化学品及能源的主要资源。受全球经济迅猛发展的影响,未来的化工行业无疑对石油资源的依赖性将逐渐增强。面对这种对能源需求的增长以及能源储备的不断减少,我们亟需开发新型可持续资源能源用于各种燃料及大宗化学品的生产。生物质资源现今已然成为一种生产各种化学品及燃料的重要的可再生资源,并且已有多种方法可用于由生物质碳水化合物生产出各种高经济附加值的化学品[1] 。由碳水化合物催化脱水获得的5-羟甲基糠醛(HMF)[2] ,已成为联系生物质和精细化学品的一种重要的平台分子[3] [4] 。迄今为止,人们也已开发出很多方法用于由生物质出发生产得到HMF [5] -[8] 。此外,精细化学品也可经由HMF的进一步转化得到[1] [9] -[11] 。在由HMF衍生得到的化学品中,2,5-二甲酰基呋喃(DFF)是一种具有广阔应用前景的有机中间体,近年来受到人们的广泛关注。它不仅可以用作生产各种多功能材料的单体[12] [13] ,而且在各种聚合席夫碱[14] -[16] ,药物[17] ,大环配体[18] [19] ,抗真菌剂[20] ,荧光材料[13] ,树脂[21] 等的合成中也可作为初始材料。由HMF出发制取DFF的转化途径被视为未来工业化生产DFF产品可行的方法之一。尽管研究人员在该转化途径上倾注了大量工作并取得了较好的生产收率[13] ,然而为了未来能够更好地实现DFF的工业化生产,进一步研究工作还是十分必要的。
获取DFF的转化过程需要对HMF分子中的羟甲基进行选择性氧化,而不进攻更具活性的不饱和甲酰基,否则将形成其他氧化产物。尽管近年来人们开发出了很多用于生产DFF的方法,但设计并开发出适宜催化剂用于选择性生成DFF的研究仍具有挑战性。常见的化学催化氧化法通常是各种采用均相、非均相催化剂以及辅以更为绿色的分子氧进行氧化的催化方法[13] [22] -[28] 。从可持续性及经济性的角度考虑,分子氧辅助催化转化HMF生成DFF的方法近些年来已成为一种主流方法。已有报道称贵金属催化剂是一类适合于催化氧化反应的良好催化剂,但其成本高,不符合未来DFF大规模工业化生产的要求[29] 。因而,一些低成本化的过渡金属氧化物可作为贵金属催化的替代用于催化氧化反应。采用其他诸如传统氧化剂、含卤素的化合物、高分子化合物时,在某些方面也存在一些不足,即使用其为氧化反应催化剂时能在较高HMF转化下获得较高收率的DFF[13] [23] -[26] [30] 。此外,目前对于诸如镧系化合物等稀土金属化合物在催化转化HMF生成DFF反应的催化性能研究还比较少。因此,本研究选择了一些过渡金属氧化物以及镧系化合物用于催化转化HMF生成DFF的反应,旨在寻找一些适宜而高效的催化剂。
2. 实验
2.1. 原料及化学试剂
5-羟甲基糠醛(HMF, >98%)购自安耐吉化学有限公司;2,5-二甲酰呋喃(DFF, >98%)购自TCI东京化成工业株式会社;偏钒酸钠(NaVO3,98.0%~102.0%)购自国药集团化学试剂有限公司;偏钒酸钾(KVO3, 98%)购自阿拉丁工业公司;五氧化二钒(V2O5,分析纯)购自上海试剂三厂;二氧化锰(MnO2,分析纯)购自西陇化工股份有限公司;三氟甲烷磺酸镱水合物(C3F9O9S3Yb·xH2O,(CF3SO3)3Yb·xH2O) (>99.9% metals basis)购自阿拉丁工业公司。钒盐直接作为氧化反应催化剂使用。其他化学试剂均为分析纯,购自国药集团化学试剂有限公司,并且无需预处理直接使用。
2.2. 实验方法
5-羟甲基糠醛(HMF)的氧化反应是在美国进口Parr圆柱形不锈钢高压反应釜(容积为50 mL或100 mL)中进行的。将催化剂,HMF和一种有机溶剂称重定量加至烧杯等容器混合均匀,然后加入到高压反应釜中,密闭反应釜,通入一定压力氧气,通氧气时可先用该气体对反应釜进行排气处理3次左右,充满一定压力氧气后关闭气瓶。反应体系在搅拌(转速800 rpm)下升温至预定温度,反应一定时间后,迅速将反应釜冷却至室温,减压后,取样并离心分离催化剂,对液相产物进行样品检测。
2.3. 样品分析方法
反应后样品的定性分析采用日本岛津公司GC-MS设备(型号为QP2010SE)进行分析操作。样品中的HMF和DFF采用Agilent 7890系列设备进行分析检测,其安装有HP-5毛细管柱(30.0 m × 320 μm × 0.25 μm) 和氢火焰离子化检测器(FID),操作温度为270℃。载气为N2,其流速为1.0 mL·min−1。进样口温度为250℃。柱温箱温度控制程序如下:升温至100℃并保温2 min然后逐渐升温至250℃,其升温速度为15℃·min−1,然后在250℃下保温2 min。HMF和DFF的定量采用外标法,分析误差控制在±5%的范围之内。基于反应物和产物的摩尔量,初始原料转化率,产品收率及选择性采用如下公式进行计算:
HMF转化率(%) =
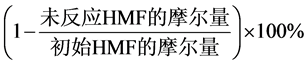 (1)
(1)
DFF收率(%) =
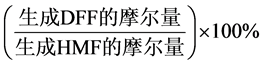 (2)
(2)
DFF选择性(%) =
 =
=
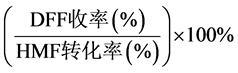 (3)
(3)
3. 结果与讨论
研究中,将一种新型催化剂用于有氧催化氧化HMF生成DFF的反应。该催化剂的俗名为三氟甲烷磺酸镱水合物(或称为三氟甲磺酸钇水合物),其化学式为C3F9O9S3Yb·xH2O或(CF3SO3)3Yb·xH2O。其结构如图1所示。它是一种路易斯酸催化剂,用于硅烷化烯醇醚的醇醛反应;也是酰化反应、苷化反应和环氧化物的加成反应的催化剂。
先对不同催化剂催化氧化HMF转化后的样品进行GC检测,催化剂包括五氧化二铌(Nb2O5)、五氧化二钒(V2O5)、甲酰化试剂(如上说明)、正钒酸钠(Na3VO4·nH2O, n = 1~16)、过氧化锰(MnO2)、偏钒酸钾(KVO3)。由GC检测(如表1所示)可知:1) 甲酰化试剂催化下,除溶剂峰外,还包括HMF和2个未知产物峰;2) 过氧化锰催化下,除了溶剂峰外,还包括HMF和1个未知产物峰;3) 偏钒酸钾催化下,除溶剂峰外,还包括HMF和多个未知产物峰;4) 五氧化二钒催化下,除溶剂峰外,还包括HMF和1个未知产物峰。在相同反应条件下,其他催化剂似乎对该反应无明显催化活性,GC检测未检测到可能是氧化产物的峰形。
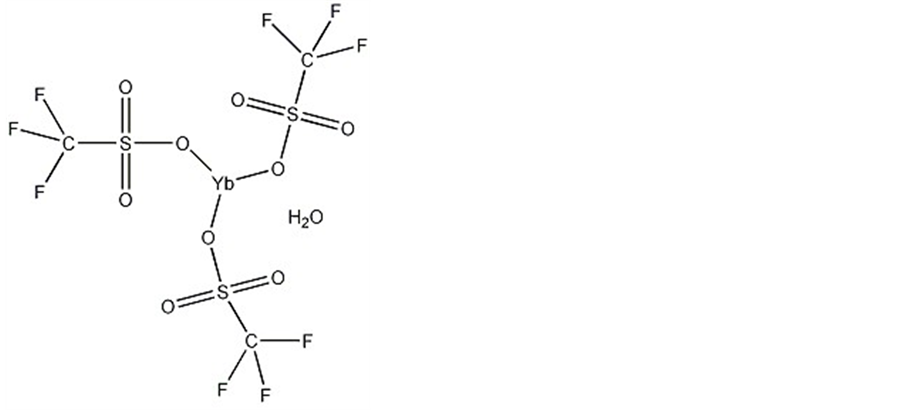
Figure 1. The structural formula of ytterbium trifluoromethanesulfonate hydrate
图1. 三氟甲烷磺酸镱水合物的结构式
Table 1. GC detection results from the experiments of the aerobic oxidation of HMF to DFF with transition metal compounds and lanthanides as catalysts
表1. 采用过渡金属氧化物及镧系化合物为催化剂催化有氧氧化HMF生成DFF实验的GC检测结果
接着就以上四种催化剂催化下的产物进行GC-MS检测,其结果如表2和表3所示。
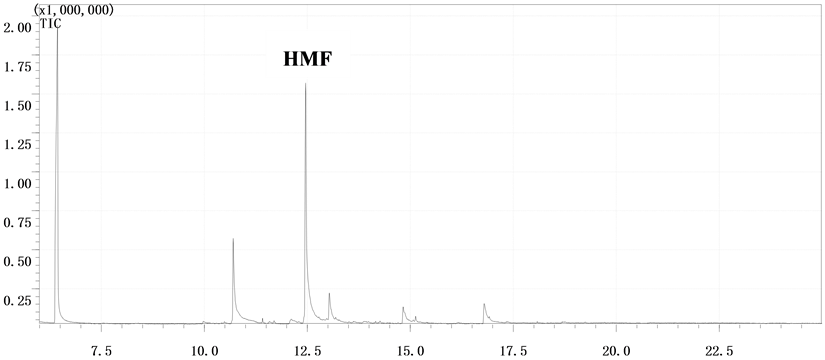
由表2和表3可知,GC-MS能够很好地将DFF检测出来,特别是以氧化钒为催化剂时的两次结果,都能检测到DFF,产物稳定性较好。该结果能够通过图2得以证实。此外,DFF产品的定性可以通过市场购置DFF和实验中转化生成DFF的对比图谱得以确认,如图3所示。
为了进一步确认上述催化剂的效果,选择以下3种催化剂进行氧化实验。并对不同催化剂下有氧催化氧化HMF转化生成DFF 的样品进行了GC检测,在相同GC检测条件下,相关样品的检测结果如图4(a)~(e)所示。
从以上实验可以大致推测V2O5和KVO3对HMF氧化生成DFF的反应有效,可采用GC-MS检测再次确认其效果。此外,所用MnO2可能对所研究的催化反应不起作用,这与一些文献报道不一致[22] [27] ,这可能是因为未选择好晶体形态合适的锰化合物,普通MnO2有可能对该转化反应不起作用。
4. 结论
本研究选取了几种过渡金属化合物以及镧系化合物(Nb2O5, V2O5, C3F9O9S3Yb·xH2O, Na3VO4, KVO3, 及MnO2)作为有氧氧化生物质衍生模型化合物HMF反应的催化剂以期获得高附加值产品DFF。其中,Nb2O5和Na3VO4对该氧化反应不具有明显的反应活性。另外还发现,当以C3F9O9S3Yb·xH2O和MnO2为催化剂时,没有DFF生成,但生成了其他氧化产物比如5-乙酰氧基甲基-2-呋喃醛。但发现KVO3和V2O5在有氧选择性催化氧化HMF生成DFF的反应中是两种高效的催化剂。经进一步反应工艺条件优化后它们可望用于未来DFF的工业化生产。
Table 2. GC-MS detection results from the experiments of the aerobic oxidation of HMF to DFF with transition metal compounds and lanthanides as catalysts for the first time
表2. 采用过渡金属氧化物及镧系化合物为催化剂催化有氧氧化HMF生成DFF实验的第一次GC-MS检测结果
Table 3. GC-MS detection results from the experiments of the aerobic oxidation of HMF to DFF with transition metal compounds and lanthanides as catalysts for the second time
表3. 采用过渡金属氧化物及镧系化合物为催化剂催化有氧氧化HMF 生成DFF实验的第二次GC-MS检测结果
√: existence; ×: Inexistence.
 (a)
(a)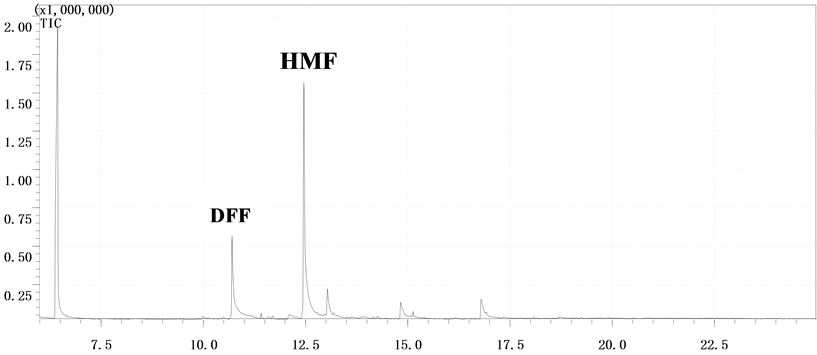 (b)
(b)
Figure 2. GC-MS detection of products over V2O5 catalyst: (a) The first time; (b) The second time
图2. 以V2O5为催化剂时GC-MS检测样品结果:(a) 第一次;(b) 第二次
 (a)
(a) (b)
(b)
Figure 3. The qualitative analysis of DFF product: (a) Commercially available DFF; (b) Experimentally obtained DFF
图3. DFF产品定性分析:(a) 市场购置的 DFF;(b) 实验室获得的DFF
 (a)
(a)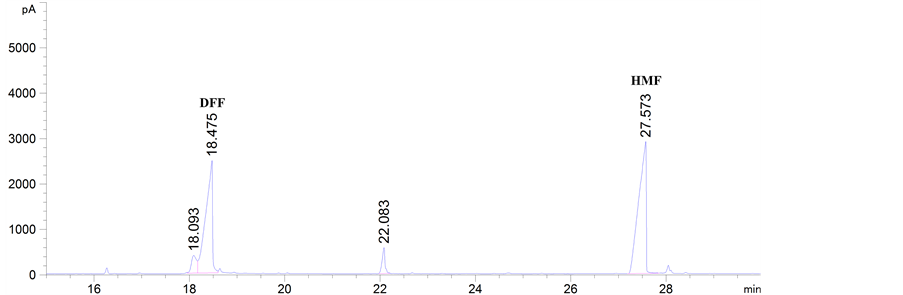 (b)
(b)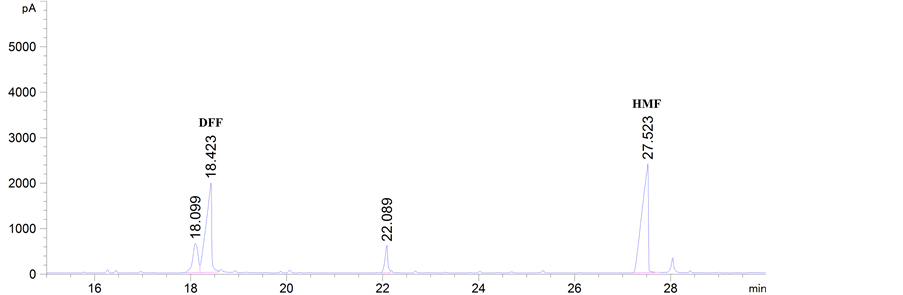 (c)
(c)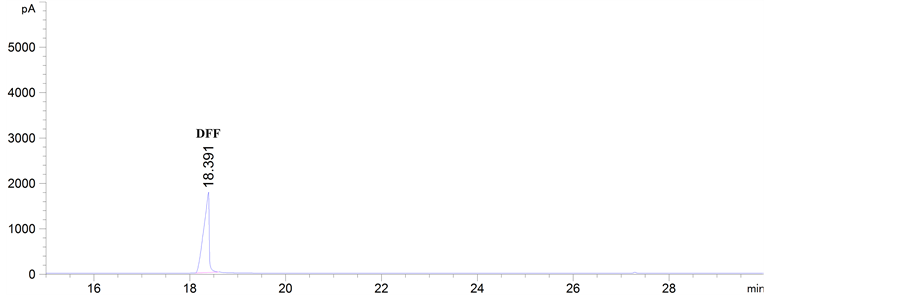 (d)
(d)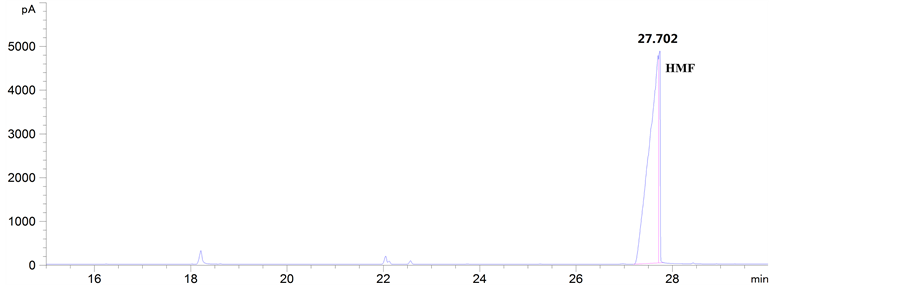 (e)
(e)
Figure 4. GC analysis of the samples from the aerobic catalytic oxidation of HMF with different catalysts: (a) MnO2, (b) V2O5, (c) KVO3; and GC analysis of the standard substance of DFF and HMF under the same given determination conditions: (d) DFF, (e) HMF
图4. 由不同催化剂催化有氧氧化HMF获得样品的GC分析结果:(a) MnO2,(b) V2O5,(c) KVO3;以及在相同检测条件下DFF和HMF标品的GC分析结果:(d) DFF,(e) HMF
致 谢
厦门市科技局重大科技平台项目(3502Z20131016)、973计划项目(2010CB732201)、国家自然科学基金项目(21106121)和福建省科技厅产学研重大项目(2013N5011)。
参考文献 (References)
- [1] Antonyraj, C.A., Jeong, J., Kim, B., Shin, S., Kim, S., Lee, K.Y. and Cho, J.K. (2013) Selective oxidation of HMF to DFF using Ru/γ-alumina catalyst in moderate boiling solvents toward industrial production. Journal of Industrial and Engineering Chemistry, 19, 1056-1059.
- [2] De Souza, R.L., Yu, H., Rataboul, F. and Essayem, N. (2012) 5-hydroxymethylfurfural (5-HMF) production from hexoses: Limits of heterogeneous catalysis in hydrothermal conditions and potential of concentrated aqueous organic acids as reactive solvent system. Challenges, 3, 212-232.
- [3] Davis, S.E., Houk, L.R., Tamargo, E.C., Datye, A.K. and Davis, R.J. (2011) Oxidation of 5-hydroxymethylfurfural over supported Pt, Pd and Au catalysts. Catalysis Today, 160, 55-60.
- [4] Yang, Z.Z., Deng, J., Pan, T., Guo, Q.X. and Fu, Y. (2012) A one-pot approach for conversion of fructose to 2,5-diformylfuran by combination of Fe3O4-SBA-SO3H and K-OMS-2. Green Chemistry, 14, 2986-2989.
- [5] Simeonov, S.P., Coelho, J.A.S. and Afonso, C.A.M. (2012) An integrated approach for the production and isolation of 5-hydroxymethylfurfural from carbohydrates. ChemSusChem, 5, 1388-1391.
- [6] Ding, Z.D., Shi, J.C., Xiao, J.J., Gu, W.X., Zheng, C.G. and Wang, H.J. (2012) Catalytic conversion of cellulose to 5-hydroxymethyl furfural using acidic ionic liquids and Co-catalyst. Carbohydrate Polymers, 90, 792-798.
- [7] Dutta, S., De, S., Alam, M.I., Abu-Omar, M.M. and Saha, B. (2012) Direct conversion of cellulose and lignocellulosic biomass into chemicals and biofuel with metal chloride catalysts. Journal of Catalysis, 288, 8-15.
- [8] Tao, F.R., Song, H.L. and Chou, L.J. (2011) Catalytic conversion of cellulose to chemicals in ionic liquid. Carbohydrate Research, 346, 58-63.
- [9] Gallo, J.M.R., Alonso, D.M., Mellmer, M.A. and Dumesic, J.A. (2013) Production and upgrading of 5-hydroxymenthylfurfural using heterogeneous catalysts and biomass-derived solvents. Green Chemistry, 15, 85-89.
- [10] Alamillo, R., Tucker, M., Chia, M., Pagán-Torres, Y. and Dumesic, J. (2012) The selective hydrogenation of biomass-derived 5-hydroxymethylfurfural using heterogeneous catalysts. Green Chemistry, 14, 1413–1419.
- [11] Che, P., Lu, F., Zhang, J., Huang, Y., Nie, X., Gao, J. and Xu, J. (2012) Catalytic selective etherification of hydroxyl groups in 5-hydroxymethylfurfural over H4SiW12O40/MCM-41 nanospheres for liquid fuel production. Bioresource Technology, 119, 433-436.
- [12] Tong, X.L., Ma, Y. and Li, Y.D. (2010) Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes. Applied Catalysis A: General, 385, 1-13.
- [13] Ma, J.P., Du, Z.T., Xu, J., Chu, Q.H. and Pang, Y. (2011) Efficient aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran, and synthesis of a fluorescent material. ChemSusChem, 4, 51-54.
- [14] Hui, Z. and Gandini, A. (1992) Polymeric Schiff bases bearing furan moieties. European Polymer Journal, 28, 1461- 1469.
- [15] Méalares, C. and Gandini, A. (1996) Polymeric Schiff bases bearing furan moieties 2. Polyazines and polyazomethines. Polymer International, 40, 33-39.
- [16] Xiang, T.F., Liu, X.M., Yi, P., Guo, M.M., Chen, Y.S., Wesdemiotis, C., Xu, J. and Pang, Y. (2013) Schiff base polymers derived from 2,5-diformylfuran. Polymer International, 62, 1517-1523.
- [17] Hopkins, K.T., Wilson, W.D., Bender, B.C., McCurdy, D.R., Hall, J.E., Tidwell, R.R., Kumar, A., Bajic, M. and Boykin, D.W. (1998) Extended aromatic furan amidino derivatives as anti-Pneumocystis carinii agents. Journal of Medical Chemistry, 41, 3872-3878.
- [18] Richter, D.T. and Lash, T.D. (1999) Oxidation with dilute aqueous ferric chloride solutions greatly improves yields in the “4+1” synthesis of sapphyrins. Tetrahedron Letters, 40, 6735-6738.
- [19] Adams, H., Bastida, R., De Blas, A., Carnota, M., Fenton, D.E., Macias, A., Rodriguez, A. and Rodriguez-Blas, T. (1997) Complexes of lead(II) and lanthanide(III) ions with a macrocyclic ligand containing a furan head unit. Crystal structure of a methanol inclusion compound of a novel macrocycle. Polyhedron, 16, 567-572.
- [20] Del Poeta, M., Schell, W.A., Dykstra, C.C., Jones, S.K., Tidwell, R.R., Kumar, A., Boykin, D.W. and Perfect, J.R. (1998) In vitro antifungal activities of a series of dication-substituted carbazoles, furans, and benzimidazoles. Antimicrobial Agents & Chemotherapy, 42, 2503-2510.
- [21] Amarasekara, A.S., Green, D. and Williams, L.D. (2009) Renewable resources based polymers: Synthesis and characterization of 2,5-diformylfuran-urea resin. European Polymer Journal, 45, 595-598.
- [22] Liu, B., Zhang, Z., Lv, K., Deng, K. and Duan, H. (2014) Efficient aerobic oxidation of biomass-derived 5-hydroxymethylfurfural to 2,5-diformylfuran catalyzed by magnetic nanoparticle supported manganese oxide. Applied Catalysis A: General, 472, 64-71.
- [23] Sádaba, I., Gorbanev, Y.Y., Kegnæs, S., Putluru, S.S.R., Berg, R.W. and Riisager, A. (2013) Catalytic performance of zeolite-supported Vanadia in the aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran. ChemCatChem, 5, 284-293.
- [24] Le, N.-T., Lakshmanan, P., Cho, K., Han, Y. and Kim, H. (2013) Selective oxidation of 5-hydroxymethyl-2-furfural into 2,5-diformylfuran over VO2+ and Cu2+ ions immobilized on sulfonated carbon catalysts. Applied Catalysis A: General, 464-465, 305-312.
- [25] Yoon, H.-J., Choi, J.-W., Jang, H.-S., Cho, J.K., Byun, J.-W., Chung, W.-J., Lee, S.-M. and Lee, Y.-S. (2011) Selective oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran by polymer-supported IBX amide. Synlett, 2011, 165-168.
- [26] Takagaki, A., Takahashi, M., Nishimura, S. and Ebitani, K. (2011) One-pot synthesis of 2,5-diformylfuran from carbohydrate derivatives by sulfonated resin and hydrotalcite-supported ruthenium catalysts. ACS Catalysis, 1, 1562-1565.
- [27] Amarasekara, A.S., Green, D. and McMillan, E. (2008) Efficient oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran using Mn(III)-Salen catalysts. Catalysis Communications, 9, 286-288.
- [28] Partenheimer, W. and Vladimir, V. (2001) Grushin, synthesis of 2,5-diformylfuran and furan-2,5-dicarboxylic acid by catalytic air-oxidation of 5-hydroxymethylfurfural. Unexpectedly selective aerobic oxidation of benzyl alcohol to benzaldehyde with metal=bromide catalysts. Advanced Synthesis & Catalysis, 343, 102-111.
- [29] Nie, J.F., Xie, J.H. and Liu, H.C. (2013) Efficient aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran on supported Ru catalysts. Journal of Catalysis, 301, 83-91.

NOTES
*通讯作者。
