Material Sciences
Vol.
11
No.
04
(
2021
), Article ID:
41624
,
7
pages
10.12677/MS.2021.114043
Co2+掺杂Zn0.5Cd0.5S固溶体的制备及其光催化CO2还原性能研究
李安1*,臧创奇2,臧琰1
1中南大学材料科学与工程学院,湖南 长沙
2桂林理工大学环境科学与工程学院,广西 桂林
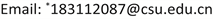
收稿日期:2021年3月18日;录用日期:2021年4月12日;发布日期:2021年4月19日

摘要
通过水热法制备了Zn0.5Cd0.5S固溶体和掺杂Co2+分别为0.5%、1%和2% (摩尔百分比)的Zn0.5Cd0.5S/Co掺杂型光催化剂。采用了X射线衍射(XRD)、扫描电子显微镜(SEM)、紫外–可见光分光光度计(UV-vis)和光致发光(PL)对材料进行了表征,并测试了可见光下光催化CO2还原活性。结果表明:Co2+掺杂后的催化剂结构与形貌没有发生显著变化,但是能带结构发生了明显调整;其中Zn0.5Cd0.5S/Co-1%催化剂在K2SO3/KHCO3液相体系,可见光辐射(λ > 420 nm)下CO2还原活性明显优于初始Zn0.5Cd0.5S材料。为高效光催化剂的构建提供了一种简易的方法。
关键词
光催化剂,CO2还原反应,Co2+掺杂,Zn0.5Cd0.5S固溶体,可见光

Synthesis and Photocatalytic CO2 Reduction Performance of Co2+ Doped Zn0.5Cd0.5S Solid Solution
An Li1*, Chuangqi Zang2, Yan Zang1
1School of Materials Science and Engineering, Central South University, Changsha Hunan
2School of Environmental Science and Engineering, Guilin University of Technology, Guilin Guangxi

Received: Mar. 18th, 2021; accepted: Apr. 12th, 2021; published: Apr. 19th, 2021

ABSTRACT
Zn0.5Cd0.5S solid solution and Co2+ doped Zn0.5Cd0.5S photocatalysts were successfully synthesized by hydrothermal method. The concentrations of Co2+ are 0.5 mol%, 1 mol% and 2 mol%, respectively. X-ray diffraction (XRD), scanning electron microscope (SEM), ultraviolet-visible spectrophotometer (UV-vis) and photoluminescence (PL) were used to characterize, and the photocatalytic CO2 reduction performance was measured under visible light. The results showed that the structure and morphology of Zn0.5Cd0.5S/Co did not change significantly, but the energy band structure was remarkably adjusted. Under the K2SO3/KHCO3 liquid system and visible light radiation (λ > 420 nm), the Zn0.5Cd0.5S/Co-1 sample presented better CO2 reduction performance than the pristine Zn0.5Cd0.5S. It provides a simple method for the construction of high-efficiency photocatalytic systems.
Keywords:Photocatalyst, CO2 Reduction Reaction, Co2+ Doping, Zn0.5Cd0.5S Solid Solution, Visible Light

Copyright © 2021 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
通过光催化减少二氧化碳的太阳能转换是在未来能源转换组合的节能和可再生模式组件中产生碳质产品的一个备受瞩目的途径 [1] [2] [3]。CO2作为一种比较稳定的气体,通常需要在高温高压等苛刻环境下才会发生转化,光催化技术利用太阳能激发半导体材料产生电子–空穴对,在光催化剂表面诱导发生氧化还原反应,可以有效实现CO2转化为CO和其他高附加值的碳氢化合物(如CH4、HCOOH、CH3OH等) [4] [5]。但是,目前光催化CO2还原效率仍然处于较低水平,其中关键的要求是可见光响应的新型高效光催化材料的开发。
过渡金属硫化物半导体材料,如ZnS、CdS、ZnxCd1-xS、CuGaS2等材料由于其较负的导带位置,满足CO2还原发生的热力学条件,从而作为CO2还原光催化剂引起了广泛的研究兴趣 [6] [7] [8] [9]。为进一步优化过渡金属硫化物的光催化活性,目前研究的热点方向是通过各种方式对其进行改性,方法包括元素掺杂、表面修饰、引入空位、和其他半导体材料进行复合等等 [8] - [13]。在上述的途径中,元素掺杂是一种简易却行之有效的改性方式。本文选取可见光响应的Zn0.5Cd0.5S固溶体材料为研究对象,对其进行Co元素掺杂,以期改善Zn0.5Cd0.5S固溶体的光催化CO2活性。通过水热法制备了不同掺杂比例的Zn0.5Cd0.5S/Co,测试了不同掺杂比例对其光催化CO2还原活性的影响。
2. 实验
2.1. 催化剂制备
采用水热法制备Zn0.5Cd0.5S固溶体催化剂。首先取3.0 mmol氯化锌(ZnCl2),3.0 mmol四水合硝酸镉(Cd(NO3)2·4H2O)置于100 mL的聚四氟乙烯反应釜内,加入40 mL去离子水搅拌溶解,随后边搅拌边滴入30 mL 0.23 mol/L的硫化钠(Na2S)溶液,室温下磁力搅拌30 min后,转移到180℃烘箱中保温12 h,自然冷却到室温。将收集到的黄色粉末用去离子水离心清洗6遍,8000 rpm/s离心5 min,随后转移到60℃烘箱中干燥12 h,研磨后即得Zn0.5Cd0.5S催化剂粉末,样品标记为Zn0.5Cd0.5S。同理,分别向第一步得溶液中加入摩尔百分比为0.5%、1%、2%的六水合硝酸钴(Co(NO3)2·6H2O),即可制得不同掺杂比例的Zn0.5Cd0.5S/Co催化剂,标记为Zn0.5Cd0.5S/Co-0.5、Zn0.5Cd0.5S/Co-1和Zn0.5Cd0.5S/Co-2。
2.2. 材料表征与性能测试
1) 材料表征:催化剂材料的物相由日本株式会社理学公司型号Miniflex 600型X射线衍射仪测试鉴定,通过对Zn0.5Cd0.5S固溶体和Zn0.5Cd0.5S/Co掺杂型催化剂进行X射线衍射测试,得衍射峰的出峰位置和出峰强度,和XRD标准PDF卡片进行比对,得到样品的物相鉴定结果。衍射仪参数设置扫描范围5˚~ 80˚,步长0.02˚;形貌图片及成分鉴定信息由美国FE-SEM Quanta 200场发射扫描电镜采集;采用日本岛津公司UV-2600紫外–可见漫反射仪测定催化剂的紫外–可见光吸收谱;光致发光谱由日本JASCO FP-6500荧光光谱仪采集,激发波长350 nm。
2) 光催化CO2还原性能测试:使用上海博弈科学仪器公司Online-3光催化CO2还原评价系统对催化剂材料的CO2还原性能进行评价。首先将200 mg催化剂粉末的悬浮液中加入0.1 M K2SO3和0.5 M KHCO3,向反应器中加入新鲜去离子水,将液体体积固定在200 mL,随后将反应系统抽真空后,反复向系统中注入高纯CO2(99.999%)气体,直至气压计最终压力接近常压(101~102 KPa)。反应器采用300 W氙灯(北京泊菲莱Microsolar 300)和420 nm截止滤波片(L42, HOYA),在25℃恒温条件下进行辐照。反应过程中的产物CO、CH4等含碳物质使用日本岛津公司GC-2014 (装载火焰离子化检测器,N2做载气)气相色谱仪收集检测,产物H2由岛津GC-2014C (装载热导检测器,Ar做载气)在线气相色谱仪收集检测。
3. 结果与讨论
3.1. X射线衍射分析
图1为Zn0.5Cd0.5S固溶体、Zn0.5Cd0.5S/Co-0.5、Zn0.5Cd0.5S/Co-1和Zn0.5Cd0.5S/Co-2的XRD衍射图谱。结果表明:Zn0.5Cd0.5S是六方结构ZnS (JCPDS: PDF-75-1534)和六方结构CdS (JCPDS: PDF-10-0454)的固溶体。与未掺杂的材料相比,Zn0.5Cd0.5S/Co的衍射峰并没有发生明显移动,Co2+掺杂并没有影响Zn0.5Cd0.5S固溶体的结构,也没观察到有CoO或Co(OH)2的峰,可能是由于Co2+掺杂量较小,在固溶体中分散均匀,掺杂后未发生明显的结构或相改变。
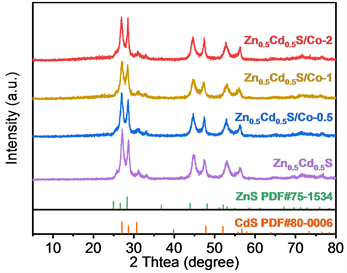
Figure 1. X-ray diffraction patterns of Zn0.5Cd0.5S, Zn0.5Cd0.5S/Co-0.5, Zn0.5Cd0.5S/Co-1 and Zn0.5Cd0.5S/Co-2
图1. Zn0.5Cd0.5S,Zn0.5Cd0.5S/Co-0.5,Zn0.5Cd0.5S/Co-1,Zn0.5Cd0.5S/Co-2的X射线衍射图谱
3.2. 扫描电镜结果分析
图2(a)、图2(b)分别为Zn0.5Cd0.5S和Zn0.5Cd0.5S/Co-1样品的SEM图,从(a)图中可以看出水热法合成的Zn0.5Cd0.5S为纳米级小颗粒团聚而成,伴随着少量尺寸100 nm左右大块状,表面较为光滑。从(b)图可以看出Co2+掺杂后的样品基本维持原形貌,表面状态没有显著改变。图2(c)~(f)为Zn0.5Cd0.5S/Co-1样品的EDS图,可以看出,Co元素在Zn0.5Cd0.5S分布均匀,与XRD结果相符。

Figure 2. (a) (b) SEM images of Zn0.5Cd0.5Sand Zn0.5Cd0.5S/Co-1; (c)~(f) EDS of Zn0.5Cd0.5S/Co-1
图2. (a) (b) Zn0.5Cd0.5S和Zn0.5Cd0.5S/Co-1的扫描电镜图;(c)~(f) Zn0.5Cd0.5S/Co-1样品的EDS图
3.3. 紫外–可见光漫反射吸收谱
图3分别为Zn0.5Cd0.5S和不同Co掺杂比例的Zn0.5Cd0.5S/Co样品的UV-vis吸收谱图,从图中可以看出,所有样品在可见光区域都有很强的吸收,随着少量Co2+掺入后,两个样品Zn0.5Cd0.5S/Co-0.5和Zn0.5Cd0.5S/Co-1的吸收波长均发生了明显红移,表明了掺杂后的样品禁带宽度有所降低,对可见光吸收加强。当Co2+掺杂比例继续增加时,高掺杂比例样品Zn0.5Cd0.5S/Co-2吸收边发生了蓝移,表明了其禁带宽度的增加,可见光吸收降低,另外,Co元素掺杂之后的样品在730 nm处产生了一个明显的吸收峰,这是因为Co的掺入产生了局域表面等离子共振效应,所产生的一个共振吸收峰。
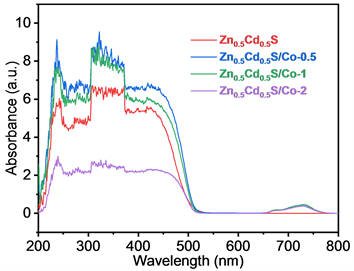
Figure 3. UV-vis diffuse reflectance spectra of Zn0.5Cd0.5S, Zn0.5Cd0.5S/Co-0.5, Zn0.5Cd0.5S/Co-1 and Zn0.5Cd0.5S/Co-2
图3. Zn0.5Cd0.5S,Zn0.5Cd0.5S/Co-0.5,Zn0.5Cd0.5S/Co-1,Zn0.5Cd0.5S/Co-2的UV-vis 漫反射吸收光谱
3.4. 光致发光谱
图4分别为Zn0.5Cd0.5S和不同Co掺杂比例的Zn0.5Cd0.5S/Co样品的光致发光谱,激发波长为350 nm,可以明显看出,在发光波长430 nm左右,掺杂Co2+样品的发光强度都低于初始的Zn0.5Cd0.5S样品,并且Zn0.5Cd0.5S/Co-1的强度最低,意味着其电子空穴对复合效率的降低,解释了在光催化性能上的差异。
Figure 4. PL spectra of Zn0.5Cd0.5S, Zn0.5Cd0.5S/Co-0.5, Zn0.5Cd0.5S/Co-1 and Zn0.5Cd0.5S/Co-2
图4. Zn0.5Cd0.5S,Zn0.5Cd0.5S/Co-0.5,Zn0.5Cd0.5S/Co-1,Zn0.5Cd0.5S/Co-2的光致发光谱
3.5. 光催化CO2还原活性
从图5(a)、图5(b)分别可以看出,光催化CO2还原性能测试中,原始Zn0.5Cd0.5S的CO和H2产量分别为3.58 μmol和1.68 mmol,由于反应介质为水,析氢反应成为主要的竞争反应 [5],Co2+的掺杂都导致了CO和H2产量的提升,并且Zn0.5Cd0.5S/Co-1产量提升最为明显,图5(c)、图5(d)分别展示了各个样品的CO和H2的平均生成速率,与原始样品相比,Zn0.5Cd0.5S/Co-1样品的CO和H2的生成速率分别提升了1.63和1.83倍。

Figure 5. (a) CO evolution vs. irradiation time; (b) H2 evolution vs. irradiation time; (c) CO evolution rate; (d) H2 evolution rate over Zn0.5Cd0.5S and Zn0.5Cd0.5S/Co samples under visible light
图5. Zn0.5Cd0.5S和各个Zn0.5Cd0.5S/Co样品可见光照射下的(a) CO生成量和时间关系图,(b) H2生成量和时间关系图,(c) CO生成速率图,(d) H2生成速率图
3.6. 机理分析
结合以上结果,如图6所示,我们可以合理推断Co2+的掺入在Zn0.5Cd0.5S的能级结构中引入一个新的能级,从而使得光生电子转移到Co上,降低了半导体的禁带宽度,从而提升了半导体的光响应能力,同时提升了电子和空穴在空间上的分离,从而降低光生电子空穴对的复合效率,进而提高了Zn0.5Cd0.5S的光催化CO2还原活性。

Figure 6. Schematic band diagram of Zn0.5Cd0.5S/Co
图6. Zn0.5Cd0.5S/Co样品的能带示意图
4. 结论
以水热法合成了Zn0.5Cd0.5S固溶体和不同Co2+掺杂比例的Zn0.5Cd0.5S/Co掺杂型光催化剂,通过光催化CO2还原测试对其性能进行了评价,结果表明掺杂后的Zn0.5Cd0.5S样品较原始样品性能均有显著提升,在我们的研究范围内,Zn0.5Cd0.5S/Co-1样品性能提升最为明显,归因于Co2+的掺入使得半导体的禁带宽度发生了显著降低,改善了其可见光吸收能力,并在空间上提高了光生电子空穴对的分离效果,降低了其复合率,从而使得其CO2还原性能得到明显提升。
文章引用
李 安,臧创奇,臧 琰. Co2+掺杂Zn0.5Cd0.5S固溶体的制备及其光催化CO2还原性能研究
Synthesis and Photocatalytic CO2 Reduction Performance of Co2+ Doped Zn0.5Cd0.5S Solid Solution[J]. 材料科学, 2021, 11(04): 360-366. https://doi.org/10.12677/MS.2021.114043
参考文献
- 1. Fujishima, A. and Honda, K. (1972) Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature, 238, 37-38. https://doi.org/10.1038/238037a0
- 2. Cortright, R.D., Davda, R.R. and Dumesic, J.A. (2002) Hydrogen from Catalytic Reforming of Biomass-Derived Hydrocarbons in Liquid Water. Nature, 418, 964-967. https://doi.org/10.1038/nature01009
- 3. Tong, H., Ouyang, S., Bi, Y., Umezawa, N., Oshikiri, M. and Ye, J. (2012) Nano-Photocatalytic Materials: Possibilities and Challenges. Advanced Materials, 24, 229-251. https://doi.org/10.1002/adma.201102752
- 4. Fujiwara, H., Hosokawa, H., Murakoshi, K., Wada, Y. and Yanag-ida, S. (1998) Surface Characteristics of ZnS Nanocrystallites Relating to Their Photocatalysis for CO2 Reduction. Langmuir, 21, 5154-5159. https://doi.org/10.1021/la9801561
- 5. Kondratenko, E.V., Mul, G., Baltrusaitis, J., Larrazabal, O.G. and Pe-rez-Ramirez, J. (2013) CO2 Photo-Reduction: Insights into CO2 Activation and Reaction on Surfaces of Photocatalysts. Energy Environment Science, 6, 3112-3135. https://doi.org/10.1039/c3ee41272e
- 6. Pang, H., Masuda, T. and Ye, J. (2018) Semiconductor-Based Photoe-lectrochemical Conversion of Carbon Dioxide: Stepping towards Artificial Photosynthesis. Chemistry Asian Journal, 13, 127-142. https://doi.org/10.1002/asia.201701596
- 7. Chang, X., Wang, T. and Gong, J. (2016) CO2 Photo-Reduction: In-sights into CO2 Activation and Reaction on Surfaces of Photocatalysts. Energy Environment Science, 9, 2177-2196. https://doi.org/10.1039/C6EE00383D
- 8. Qin, J., Wang, S. and Wang, X. (2017) Visible-Light Reduction CO2 with Dodecahedral Zeolitic Imidazolate Framework ZIF-67 as an Efficient Co-Catalyst. Applied Catalysis B: Environment, 209, 476-482. https://doi.org/10.1016/j.apcatb.2017.03.018
- 9. Su, Y., Zhang, Z., Liu, H., Wang, Y. (2017) Cd0.2Zn0.8S@UiO-66-NH2 Nanocomposites as Efficient and Stable Visible-Light-Driven Photocatalyst for H2 Evolution and CO2 Reduction. Applied Catalysis B: Environment, 200, 448-457. https://doi.org/10.1016/j.apcatb.2016.07.032
- 10. Xie, S., Zhang, Q., Liu, G. and Wang, Y. (2016) Photocatalytic and Photoelectrocatalytic Reduction of CO2 Using Heterogeneous Catalysts with Controlled Nanostructures. Chemistry Communication, 52, 35-59. https://doi.org/10.1039/C5CC07613G
- 11. Ji, Y. and Luo, Y. (2016) New Mechanism for Photocatalytic Reduc-tion of CO2 on the Anatase TiO2(101) Surface: The Essential Role of Oxygen Vacancy. Journal of the American Chemical Society, 138, 15896-15902. https://doi.org/10.1021/jacs.6b05695
- 12. Gao, S., Gu, B., Jiao, X., Sun, Y., Zu, X., Yang, F., Zhu, W., Wang, C., Feng, Z., Ye, B. and Xie, Y. (2017) Highly Efficient and Exceptionally Durable CO2 Photoreduction to Methanol over Freestanding Defective Single-Unit-Cell Bismuth Vanadate Layers. Journal of the American Chemistry Society, 139, 3438-3445. https://doi.org/10.1021/jacs.6b11263
- 13. 黄亚辉. CdS复合半导体光催化剂的制备及其光解水制氢性能研究[D]: [硕士学位论文]. 南昌: 南昌大学化学系, 2008.
NOTES
*通讯作者。