Hans Journal of Agricultural Sciences
Vol.07 No.02(2017), Article ID:20419,12
pages
10.12677/HJAS.2017.72016
Effects of New Variety Selection of Bitter Melon on Modulation of Cell Cycle in 3T3-L1 Preadipocytes
Wei-Tang Chang1, Cheng-Wei Liu2, Tien-Hui Chiu1, Chin-Lin Hsu1,3*
1School of Nutrition, Chung Shan Medical University, Taichung Taiwan
2Department of Post-Modern Agriculture, Ming Dao University, Changhua Taiwan
3Department of Nutrition, Chung Shan Medical University Hospital, Taichung Taiwan

Received: Apr. 10th, 2017; accepted: Apr. 24th, 2017; published: Apr. 30th, 2017

ABSTRACT
The obesity prevalence has been increasing significantly over the past few decades, which is due to change lifestyle and dietary pattern. Bitter melon contains many active ingredients, including triterpenoids, phenolics compounds, and conjugated linolenic acids. It was widely used as herbal medicine for anti-diabetes and anti-inflammatory activities. However, the effects of new variety selection of bitter melon on modulation of cell cycle in 3T3-L1 preadipocytes remain unclear. Therefore, the aim of this study was investigate the effects of different solvents (water, methanol, ethanol, acetone, and ethyl acetate) extracts from the fruit and seed of bitter melon (Momordica charantia, Momordica charantia MDS72, and Momordica charantia ONS33) on cell cycle arrest in 3T3-L1 preadipocytes. Of the 30 bitter melon extracts tested, water extract from the seed of Momordica charantia (WESMC) exhibited the strongest inhibited cell growth in 3T3-L1 preadipocytes. In cell cycle analysis, WESMC caused cell cycle arrest at the G1 phase in 3T3-L1 preadipocytes. In the molecular mechanisms, WESMC significantly increased the protein expressions of p-p53, p27, and p18, and decreased the protein expressions of CDK2, CDK6, cyclin D1, and p-Rb. These results demonstrate that WESMC could inhibit cell growth and cell cycle arrest at G1 phase in 3T3-L1 preadipocytes.
Keywords:3T3-L1 Preadipocytes, Bitter Melon, Cell Cycle
新育种苦瓜调控3T3-L1前脂肪细胞之细胞 周期停滞之影响
张维棠1,刘程炜2,邱天惠1,徐庆琳1,3*
1中山医学大学营养学系,台湾 台中
2明道大学精致农业学系,台湾 彰化
3中山医学大学附设医院营养科,台湾 台中

收稿日期:2017年4月10日;录用日期:2017年4月24日;发布日期:2017年4月30日

摘 要
近年来由于饮食与生活型态改变,使得肥胖盛行率在过去数十年间以倍数方式急遽增加。苦瓜(Momordica charantia)已知含有三萜类、酚类物质与共轭亚麻油酸,过去已被长期使用作为治疗第二型糖尿病与发炎反应上,然而利用新育种苦瓜探讨3T3-L1前脂肪细胞对细胞周期调控之影响,目前仍缺乏相关文献资料。因此,本研究利用三种新育种苦瓜 (苹果苦瓜、苦瓜明道选72与苦瓜冲绳选33) 之果肉与种子,分别以水、甲醇、乙醇、丙酮与乙酸乙酯所制备之萃取物,探讨三种新育种苦瓜对3T3-L1前脂肪细胞之细胞周期调控之影响。结果显示,在30种苦瓜萃取物中,以苹果苦瓜种子水萃取物具有最佳抑制3T3-L1前脂肪细胞生长之作用,在细胞周期分析结果显示,其亦可发现3T3-L1前脂肪细胞之细胞周期停滞于G1期。在分子机制结果显示,苹果苦瓜种子水萃取物可藉由增加p-p53、p27与p18之蛋白表现,进而抑制CDK2、CDK6、cyclin D1与p-Rb之蛋白表现。综合上述结果显示,苹果苦瓜种子水萃取物可藉由抑制3T3-L1前脂肪细胞之增生,并使细胞周期停滞于G1期。
关键词 :3T3-L1前脂肪细胞,新育种苦瓜,细胞周期

Copyright © 2017 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
肥胖为已开发和开发中国家之严重公共健康议题之一,亦是造成许多慢性疾病发生之危害因子,例如:第二型糖尿病、心血管疾病、高血压、血脂异常、肝脏疾病、骨关节炎与癌症。世界卫生组织(world health organization, WHO)在2008年的统计数据显示,目前世界上约有15亿的成年人口有体重过重的情形,亦有超过十分之一的人口具有肥胖的问题,预计2023年将有超过23亿人会有体重过重的问题 [1] 。在2005~2008年的台湾「国民营养健康状况变迁调查」报告显示,在近十年间,成年男性与女性体重过重与肥胖的比例,分别增加17.4%和5.2% [2] 。此外,根据卫生福利部统计,2012年国人十大死因中就有八项与肥胖有密切相关,此显示台湾民众因生活型态的改变,使得肥胖问题逐年增加。营养流行病学研究指出,用药物和手术等方式治疗肥胖,可能带给身体极大的副作用 [3] [4] [5] [6] 。因此,藉由摄食天然蔬果而获取机能性成分,作为改善肥胖之作用,已知是一种对身体负担较小,且能达到预防或推迟疾病的方法,亦是目前医学相关研究着重的目标和趋势之一。
苦瓜学名为Momordica charantia,别名为锦荔枝、癞瓜或凉瓜,为开花之葫芦科藤本植物。研究指出,苦瓜含有多种生理活性之成份,例如:生物碱(alkaloids)、固醇类葡萄醣苷(steroidal glucosides)、酚类物质(phenolics compounds)、溶血磷脂酰胆碱(lysophosphatidylcholines, LPC)、共轭亚麻油酸异构体(conjugated linolenic acid isomers)与葫芦烷型三萜类化合物(cucurbitane-type triterpenoids) [7] [8] [9] [10] 。许多文献指出,苦瓜能有效的增加胰岛素敏感性 [11] 、降低发炎反应 [12] [13] 、降血脂 [14] ,以及抑制癌细胞增生 [15] [16] 。研究亦指出,苦瓜能藉由降低PPARg、SREBP与perilipin之基因表现,并增加脂解作用,而达到抑制人类初代脂肪细胞的分化 [17] 。在本研究室先前研究显示,利用分离、纯化与鉴定等方法,从新育种苦瓜中分离出四种已知的三萜类化合物,且此分离之三萜类化合物能显著抑制LPS所诱导RAW 264.7巨噬细胞之一氧化氮与发炎相关激素之分泌 [18] 。
肥胖是由于前脂肪细胞的增生与分化,进而导致脂肪油滴之累积。因此,透过抑制前脂肪细胞的有丝分裂与脂肪细胞分化机制,将有助于抑制肥胖与相关疾病之发生 [19] 。由于肥胖的问题日渐严重,相对的抗肥胖的健康食品市场是具有发展潜力,但目前在台湾提供消费者可选择之不易形成体脂肪健康食品主要以纤维成份、茶类产品与结构性油脂为主,可选择之产品相当有限,且缺乏新颖性之材料作为抗肥胖之素材。因此,本研究将以新育种苦瓜作为材料并利用3T3-L1前脂肪细胞试验模式,探讨三种新育种苦瓜之果肉和种子利用不同溶剂(水、甲醇、乙醇、丙酮和乙酸乙酯)所萃取制得之萃取物,对3T3-L1前脂肪细胞生长和细胞周期停滞之影响,并厘清其分子作用机转。
2. 方法
2.1. 试验样品
本研究使用之三种新育种苦瓜材料来源由台湾明道大学精致农业学系刘程炜老师提供,其为利用纯化选种与田间试验培育出三种新育种苦瓜品系,分别为苹果苦瓜(Momordica charantia)、苦瓜明道选72 (Momordica charantia MDS72)与苦瓜冲绳选33 (Momordica charantia ONS33)。苹果苦瓜为早生种,果色白,果面珍珠粒,果型短肥型,果重约500公克,为白玉苦瓜突变种,为台湾中部地方品种;苦瓜明道选72亲本为台湾山苦瓜与印度苦瓜,人工杂交后经5年选拔后之新品种,果色深绿,果面刺排粒,果型细长型,果重约450公克;苦瓜冲绳选33为收集自日本琉球冲绳地区之苦瓜品种,经5年栽培驯化后选拔之地方品种,果色绿,果面珍珠粒,果型细长型,果重约600公克,分别收集此三种苦瓜之种子与果肉并将其冷冻干燥,再制成苦瓜种子与果肉粉末。
2.2. 不同品系、部位与溶剂之苦瓜萃取物制备
分别秤取4克苹果苦瓜、苦瓜明道选72与苦瓜冲绳选33之苦瓜之果肉与种子粉末于离心管中,再加入40 mL之不同极性溶剂(水、甲醇、乙醇、丙酮与乙酸乙酯),以parafilm将管壁封好,利用面震荡器(rotator shaker)于室温下震荡(100 rpm、24小时)后,经过滤后以减压浓缩或冷冻干燥(针对水萃物)方式进行浓缩与定量,每一种溶剂进行三重复试验,并计算其萃取率。不同品系、部位与溶剂之萃取物缩写名称为:苹果苦瓜(Momordica charantia, MC)、苦瓜明道选72 (Momordica charantia MDS72, MCMDS72)、苦瓜冲绳选33 (Momordica charantia ONS33, MCONS33)、种子(seed, S)、果肉(fruit, F)、水萃取物(water extract, WE)、甲醇萃取物(methanol extract, ME)、乙醇萃取物(ethanol extract, EE)、丙酮萃取物(acetone extract, AE)与乙酸乙酯萃取物(ethyl acetate extract, EAE)。
2.3. 3T3-L1前脂肪细胞之培养
3T3-L1前脂肪细胞株(BCRC No.60159)购自财团法人食品工业发展研究所,生物资源保存及研究中心(台湾,新竹)。以培养基[90% Dulbecco’s Modified Eagle’s Medium (DMEM 含有4 mM L-glutamine与4.5 g/L glucose), 1.5 g/L sodium bicarbonate, 10% bovine calf serum与1% Penicillin-Streptomycin],于37˚C、5%二氧化碳培养箱培养,平均每两天更换一次培养液。
2.4. 3T3-L1前脂肪细胞之细胞生长测定
将3T3-L1前脂肪细胞依1 ´ 104 cells/well种入96 well plate中,以200 mL high-glucose DMEM培养,并分别加入不同品系、部位与溶剂之苦瓜萃取物(0, 50, 100与250 mg/mL),进行48小时培养。先从每个well中取出110 mL之培养液,再分别加入10 mL之MTT [3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide] solution (5 mg/mL)于培养箱中培养3小时后,将所有培养液移除,再加入80 mL之DMSO后,置于暗室2小时,以溶出formazan,并利用全波长酵素免疫分析仪(UVM340, Biochrome, USA),以570 nm测定吸光值,计算数值变化。
2.5. 3T3-L1前脂肪细胞之细胞周期分析
将培养于10 cm dish之3T3-L1前脂肪细胞(1 ´ 106 cell/mL)以不同品系、部位与溶剂之苦瓜萃取物处理后(0, 25, 50与100 mg/mL、24小时),以trypsin-EDTA切下后,以1000 rpm、4℃离心5分钟收集细胞,加入1 mL之70%乙醇固定细胞,室温下反应20分钟后,以4℃、900 rpm离心5分钟除去乙醇,并以1 mL之PBS清洗两次后,加入0.5 mL之PI solution (40 mg/mL propidium iodide),在37℃下暗室反应15分钟,最后以FACScan流式细胞仪(Becton-Dickinson Immunocytometery Systems, San Jose, CA, USA)进行分析。
2.6. 蛋白质表现分析
3T3-L1前脂肪细胞以不同时间点(1, 3, 6, 9与12小时)加入25 mg/mL之苹果苦瓜种子水萃取物处理,经PBS冲洗二次后,加入300 mL之lysis buffer solution,将细胞以刮刀刮下后,置于冰浴中,利用超音波破碎机进行细胞破碎,取得细胞破碎液后,以bio-rad protein assay (Bio-rad Laboratories, Hercules, CA, USA)进行蛋白质浓度测试,并使用bovine serum albumin作标准物之定量分析。细胞破碎液加入60 mL (5×) protein loading dye,于100℃沸水中加热10分钟后,立即置于冰上快速冷却,并存放于−20℃冰箱备用。将制备好之细胞液和标示标准分子量的multimarker (1~2 mL/lane)于10%聚丙烯酰胺胶体电泳的胶体孔槽中,进行垂直式电泳分离,通以电压70伏特约15分钟,待样品通过stacking gel后,将电压调整为140伏特,约电泳60分钟。电泳结束后,利用bio-rad mini protein湿式转渍设备,以400 mA进行转渍,1小时后取出PVDF membrane,置于5%之脱脂牛奶中[脱脂奶粉溶于PBST buffer (PBS加入0.1% Tween-20)],作为空白处抑制(blocking),于室温下摇晃1小时,接着以PBST buffer,清洗5分钟2次,加入一级抗体(primary antibody),于4℃下作用隔夜,再以PBST清洗8分钟3次后,加入二级抗体(secondary antibody)于室温下作用1小时,经PBST清洗8分钟3次后,以ECL kit进行呈色,并利用冷光影像分析系统(Luminescent image analyze, LAS-1000, FUJIFLM, Japan)进行分析。
2.7. 统计分析
实验数据使用SPSS计算机统计软件进行分析。变异数分析则以Student’s t-test进行分析,p < 0.05为具有显著性差异。
3. 结果与讨论
3.1. 不同品系、部位及溶剂之苦瓜萃取物对3T3-L1前脂肪细胞之细胞生长影响
脂肪细胞形成之过程,包括有前脂肪细胞的增生与脂肪细胞的分化,而前脂肪细胞的增生已被证实是脂肪细胞分化的先决条件 [20] [21] 。因此,本实验利用不同品系、部位及溶剂之苦瓜萃取物,探讨其对3T3-L1前脂肪细胞之细胞生长和细胞周期调控之影响。表1~3为不同品系、部位与溶剂之苦瓜萃取物对
Table 1. Effects of different solvent extracts from the seed and fruit of Momordica charantia on cell growth in 3T3-L1 preadipocytes
表1. 苹果苦瓜种子和果肉不同溶剂萃取物对3T3-L1前脂肪细胞之细胞生长影响
Cell growth (%) is expressed as control at 100%. Data are expressed as the mean ±SD (n = 3).*p < 0.05 indicates significant difference from the control. 3T3-L1 preadipocytes were incubated with 0 - 250 mg/mL of bitter melon extracts for 48 h at 37˚C in 5% CO2 incubator. WE, water extract; ME, methanol extract; EE, ethanol extract; AE, acetone extract; EAE, ethyl acetate extract; S, seed; F, fruit; MC, Momordica charantia
Table 2. Effects of different solvent extracts from the seed and fruit of Momordica charantia MDS72 on cell growth in 3T3-L1 preadipocytes
表2. 苦瓜明道选72种子和果肉不同溶剂萃取物对3T3-L1前脂肪细胞之细胞生长影响
Cell growth (%) is expressed as control at 100%. Data are expressed as the mean ± SD (n = 3). *p < 0.05 indicates significant difference from the control. 3T3-L1 preadipocytes were incubated with 0 - 250 mg/mL of bitter melon extracts for 48 h at 37˚C in 5% CO2 incubator. MCMDS72, Momordica charantia MDS72
3T3-L1前脂肪细胞之细胞生长影响。表1为苹果苦瓜种子与果肉不同溶剂萃取物对3T3-L1前脂肪细胞之细胞生长影响,结果得知,苹果苦瓜种子水萃取物(WESMC)与苹果苦瓜种子甲醇萃取物(MESMC)在浓度50至250 mg/mL时,皆可显著抑制3T3-L1前脂肪细胞之细胞生长(p < 0.05),且其最佳抑制作用分别为52.7% (250 mg/mL WESMC)与86.4% (100 mg/mL MESMC)。苹果苦瓜种子丙酮萃取物(AESMC)于浓度
Table 3. Effects of different solvent extracts from the seed and fruit of Momordicacharantia ONS33 on cell growth in 3T3-L1 preadipocytes
表3. 苦瓜冲绳选33种子和果肉不同溶剂萃取物对3T3-L1前脂肪细胞之细胞生长影响
Cell growth (%) is expressed as control at 100%. Data are expressed as the mean ± SD (n = 3). *p < 0.05 indicates significant difference from the control. 3T3-L1 preadipocytes were incubated with 0 - 250 mg/mL of bitter melon extracts for 48 h at 37 ˚C in 5% CO2 incubator. MCONS33, Momordica charantia ONS33.
50 mg/mL时可显著抑制细胞生长至93.8% (p < 0.05),苹果苦瓜种子乙酸乙酯萃取物(EAESMC)在100 mg/mL可降低细胞生长至94.7% (p < 0.05),而苹果苦瓜种子乙醇萃取物(EESMC)则无显著抑制3T3-L1前脂肪细胞生长之作用。苹果苦瓜果肉水萃取物 (WEFMC) 于浓度50至250 mg/mL时,皆具有显著降低3T3-L1前脂肪细胞生长之能力(p < 0.05),且于浓度250 mg/mL时可降低细胞生长至54.8%。苹果苦瓜果肉甲醇萃取物(MEFMC)于浓度100 mg/mL时,能显著降低细胞生长至89.1%,苹果苦瓜果肉乙醇萃取物(EEFMC)、苹果苦瓜果肉丙酮萃取物(AEFMC)与苹果苦瓜果肉乙酸乙酯萃取物(EAEFMC)则无法抑制3T3-L1前脂肪细胞之生长。研究指出,以苦瓜萃取物(1 mg/mL)处理3T3-L1前脂肪细胞48和72小时后,其可显著抑制3T3-L1前脂肪细胞之细胞增生 [22] 。
苦瓜明道选72种子与果肉不同溶剂萃取物对3T3-L1前脂肪细胞生长之影响,由表2得知,苦瓜明道选72种子水萃取物(WESMCMDS72)于浓度50、100与250 mg/mL时,皆具显著抑制3T3-L1前脂肪细胞生长之作用(p < 0.05),其可分别降低至47.1%、45.2%与34.3%。苦瓜明道选72种子乙酸乙酯萃取物(EAESMCMDS72)于浓度250 mg/mL时,可降低3T3-L1前脂肪细胞生长至70.9% (p < 0.05)。但是苦瓜明道选72种子甲醇萃取物(MESMCMDS72)、苦瓜明道选72种子乙醇萃取物(EESMCMDS72)与苦瓜明道选72种子丙酮萃取物(AESMCMDS72)则对3T3-L1前脂肪细胞之生长不具有抑制作用。苦瓜明道选72果肉水萃取物(WEFMCMDS72)于浓度250 mg/mL时,能显著降低3T3-L1前脂肪细胞生长至62.4% (p < 0.05),苦瓜明道选72果肉乙酸乙酯萃取物(EAEFMCMDS72)于浓度50与100 mg/mL时皆具显著抑制作用(p < 0.05),其分别降低至93.8%与88.6%。苦瓜明道选72果肉甲醇萃取物(MEFMCMDS72),苦瓜明道选72果肉乙醇萃取物(EEFMCMDS72)与苦瓜明道选72果肉丙酮萃取物(AEFMCMDS72)则不具抑制3T3-L1前脂肪细胞生长之能力。研究显示,以2%、5%和10%苦瓜汁处理人类初代脂肪细胞后,均可显著抑制其细胞增生之作用 [17] 。
表3为苦瓜冲绳选33种子与果肉不同溶剂萃取物对3T3-L1前脂肪细胞生长之影响,结果显示,苦瓜冲绳选33种子水萃取物(WESMCONS33)于浓度250 mg/mL时,可显著降低3T3-L1前脂肪细胞生长至71.1% (p < 0.05),苦瓜冲绳选33种子甲醇萃取物(MESMCONS33)于浓度100 mg/mL时,可降低细胞生长至88.9% (p < 0.05)。苦瓜冲绳选33种子乙醇萃取物(EESMCONS33)、苦瓜冲绳选33种子丙酮萃取物(AESMCONS33)与苦瓜冲绳选33种子乙酸乙酯萃取物(EAESMCONS33)无法显著抑制3T3-L1前脂肪细胞生长之能力。苦瓜冲绳选33果肉丙酮萃取物(AEFMCONS33)于浓度50、100与250 mg/mL时皆具显著抑制作用(p < 0.05),其最佳降低生长之作用为87.5% (100 mg/mL)。但是,苦瓜冲绳选33果肉水萃取物(WEFMCONS33)、苦瓜冲绳选33果肉甲醇萃取物(MEFMCONS33)、苦瓜冲绳选33果肉乙醇萃取物(EEFMCONS33)与苦瓜冲绳选33果肉乙酸乙酯萃取物(EAEFMCONS33)则无法显著降低3T3-L1前脂肪细胞之生长。苦瓜萃取物中富含三萜类(葫芦烷型三萜类与三萜皂苷类)、酚类物质(没食子酸、儿茶素和表儿茶素)与共轭亚麻油酸等成分物质,过去报导指出,其可显著降低3T3-L1前脂肪细胞之增生 [23] [24] [25] [26] [27] 。利用富含三萜类之苦瓜与苦瓜种子萃取物处理3T3-L1前脂肪细胞,其可显著降低3T3-L1前脂肪细胞之细胞存活率 [28] [29] 。研究指出,酚类物质可显著降低3T3-L1前脂肪细胞增生,进而降低3T3-L1脂肪细胞之成脂作用 [30] [31] 。此外,Chou等(2012)研究亦指出,苦瓜所含之9c, 11t, 13t共轭亚麻油酸可显著降低3T3-L1前脂肪细胞之存活率,并透过活化ERK,促使PPARg磷酸化,进而抑制3T3-L1前脂肪细胞的分化 [32] 。综合上述结果得知,在30种苦瓜萃取物中,以苹果苦瓜种子水萃取物(WESMC)、苹果苦瓜果肉水萃取物(WEFMC)与苦瓜明道选72果肉水萃取物(WEFMCMDS72)具有较佳抑制3T3-L1前脂肪细胞生长之能力,故后续研究将针对此三种苦瓜萃取物,探讨其对3T3-L1前脂肪细胞之细胞周期调控与诱发细胞凋亡之影响。
3.2. 苹果苦瓜种子水萃取物、苹果苦瓜果肉水萃取物与苦瓜明道选72果肉水萃取物对 3T3-L1前脂肪细胞之细胞周期调控影响
细胞周期停滞与细胞凋亡被视为是细胞增生受阻之主要反应,细胞停滞可提供受损DNA之修补机会,而细胞凋亡则会造成严重之DNA受损而促使细胞走向死亡 [33] 。利用流式细胞分析技术评估具潜力之苦瓜萃取物,由本研究得知,30种苦瓜萃取物并无法从诱发3T3-L1前脂肪细胞凋亡之方式达到减低前脂肪细胞增生之作用,因此本研究将针对3T3-L1前脂肪细胞之细胞周期调控进行探讨。图1为苹果苦瓜种子水萃取物(WESMC)、苹果苦瓜果肉水萃取物(WEFMC)与苦瓜明道选72果肉水萃取物(WEFMCMDS72)对3T3-L1前脂肪细胞之细胞周期调控之影响。由图1得知,苹果苦瓜种子水萃取物(WESMC)于浓度0 mg/mL时,其G0/G1期之分布为62.7%,而处理苹果苦瓜种子水萃取物25至100 mg/mL时,G0/G1期分布百分比分别为81.3%、80.6%与80.0% (p < 0.05)。苹果苦瓜果肉水萃取物(WEFMC)于浓度0 mg/mL时,其G0/G1期细胞周期分布为68.8%,而处理苹果苦瓜果肉水萃取物(50 mg/mL WEFMC)后,其G0/G1期分布提升至72.3% (p < 0.05)。由图1得知,苦瓜明道选72果肉水萃取物(WEFMCMDS72)于浓度0 mg/mL时,其细胞周期分布于G0/G1期之比例为64.0%,而处理苦瓜明道选72果肉水萃取物(WEFMCMDS72) 25、50与100 mg/mL后,G0/G1期分布分别显著提升至80.8%、76.6%与67.5% (p < 0.05)。综合上述结果得知,苹果苦瓜种子水萃取物(WESMC)对3T3-L1前脂肪细胞具有最佳调控细胞周期停滞于G0/G1期之结果。Popovich等(2010)研究结果指出,富含三萜类化合物之苦瓜萃取物,可使3T3-L1前脂肪细胞之细胞周期停滞于G2/M期,进而抑制3T3-L1前脂肪细胞之增生与油脂堆积 [28] 。综合以上结果得知,苹果苦瓜种子水萃取物(WESMC)对3T3-L1前脂肪细胞周期停滞于G0/G1期之作用最为显著,故后续研究将针对苹果苦瓜种子水萃取物(WESMC)进行细胞周期停滞相关之分子机制探讨。

Figure 1. Effects of WESMC, WEFMC, and WESMCMDS72 on modulation of cell cycle in 3T3-L1 preadipocytes. Cell cycle distribution (%) is expressed as control at 100%. Data are expressed as the mean ± SD (n = 3). *p < 0.05 indicates significant difference from the control. WESMC, water extract from the seed of Momordica charantia; WEFMC, water extract from the fruit of Momordica charantia; WESMCMDS72, water extract from the seed of Momordica charantia MDS72
图1. 苹果苦瓜种子水萃取物、苹果苦瓜果肉水萃取物与苦瓜明道选72果肉水萃取物对3T3-L1前脂肪细胞之细胞周期影响。细胞周期分布(%)表示是以控制组设定为100%。数据的呈现是以平均值 ± 标准偏差(重复数 = 3)。*p < 0.05表示与控制组有显著差异。WESMC为苹果苦瓜种子水萃取物,WEFMC苹果苦瓜果肉水萃取物,WESMCMDS72苦瓜明道选72果肉水萃取物
3.3. 苹果苦瓜种子水萃取物对3T3-L1前脂肪细胞之细胞周期调控相关蛋白表现之影响
细胞周期调控主要由两个蛋白家族所调控,包括细胞周期蛋白依赖型激酶(CDKs)与细胞周期蛋白 (cyclins)。研究指出,cyclins与CDKs结合后才具有调控细胞周期之进程,能使细胞通过细胞周期的不同阶段。CDK2会与D型或E型cyclin结合,CDK4和CDK6则与D型cyclin结合,进而调控G1期的进程。CKI (CDK inhibitor) 为cyclin/CDK复合体之抑制子,包含p21家族(p21、p27与p57)与INK4家族(p15、p16、p18与p19),主要作为调节CDKs,进而抑制受损细胞从G1期进入S期 [34] 。肿瘤抑制子p53与视网膜母细胞瘤蛋白(retinoblastoma protein, Rb)之功能相似,为细胞与肿瘤生长之抑制子,能调控细胞从G1期进入S期 [35] 。研究指出,Rb蛋白的磷酸化(失活态),会使cyclin D1与CDK4和CDK6形成复合体,导致细胞周期进入S期。此外,Rb蛋白失活时易导致细胞不当增生与肿瘤形成,进而导致癌症的发生 [36] [37] 。图2为苹果苦瓜种子水萃取物(WESMC)对3T3-L1前脂肪细胞之细胞周期相关蛋白表现之影响。已知p18、CDK6、cyclin D1与pRB为调控G1期进程之相关蛋白,由图2结果显示,以25 mg/mL之苹果苦瓜种子水萃取物(WESMC)处理3T3-L1前脂肪细胞后,p18蛋白质表现于1至12小时均具有显
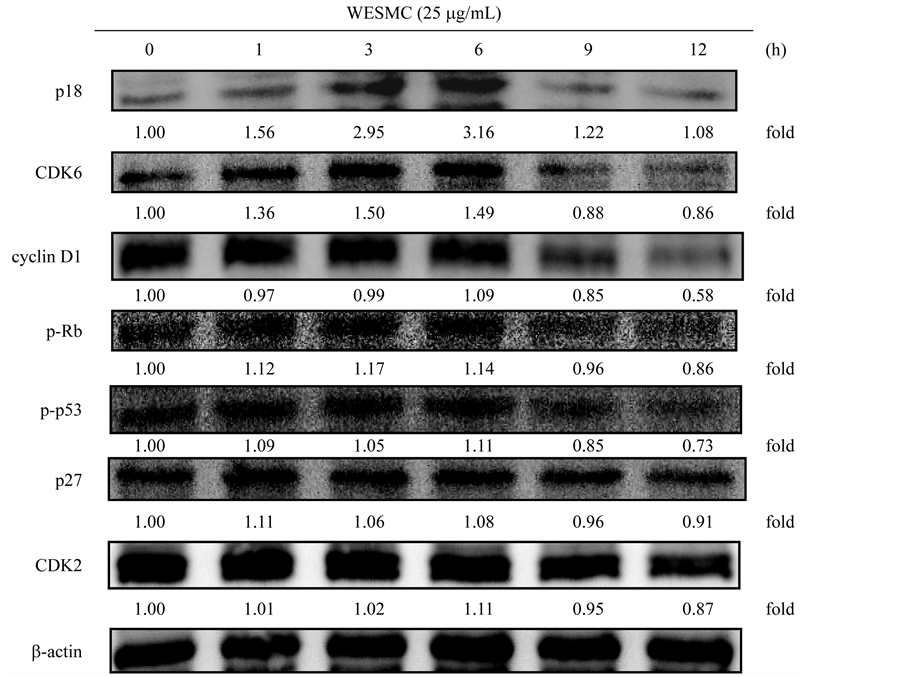
Figure 2. Effect of WESMC on protein expressions of p18, CDK6, cyclin D1, p-RB, p-p53, p27, and CDK2 in 3T3-L1 preadipocytes. 3T3-L1 preadipocytes were incubated with 25 mg/mL of WESMC for 0 - 12 h at 37˚C in 5% CO2 incubator. The relative protein expression was quantified using densitometry and Image J software, and calculated in reference to the b-actin reference bands (n = 3). WESMC, water extract from the seed of Momordica charantia
图2. 苹果苦瓜种子水萃取物对3T3-L1前脂肪细胞之p18、CDK6、cyclin D1、p-RB、p-p53、p27与CDK2蛋白质表现之影响。3T3-L1前脂肪细胞培养于37˚C、5%二氧化碳培养箱中,以25 mg/mL苹果苦瓜种子水萃取物处理0 - 12 h。相对蛋白质表现之定量是以Image J软件进行分析,并以b-actin蛋白质表现量为参考进行计算(重复数 = 3)。WESMC为苹果苦瓜种子水萃取物
著提升作用(p < 0.05),且最大作用为6小时(3.16);CDK6在12小时具有最佳抑制作用,其抑制能力为0.86;在细胞周期蛋白cyclin D1之表现上以苹果苦瓜种子水萃取物(WESMC)处理9与12小时皆具抑制作用,其抑制蛋白表现分别为0.85与0.58。在p-Rb蛋白表现上,于9与12小时皆具显著抑制作用(p < 0.05),其最大抑制蛋白表现为0.86 (12小时)。p-p53、p27和CDK2为G1期进入S期之重要调控蛋白。此外,p-p53与p27蛋白质表现于1至6小时皆具显著提升作用(p < 0.05),且最大表现作用分别为1.11 (6小时)与1.11 (1小时);CDK2则在12小时具有最佳抑制作用,其抑制表现为0.87。研究指出,富含三萜类化合物之苦瓜叶乙醇萃取物,能藉由提升p21与p27,并抑制CDK2、CDK4、cyclin D1与cyclin E之表现,进而诱导前列腺癌细胞之细胞周期停滞在G1期 [38] 。
4. 结论
综合上述结果得知,本研究利用不同品系、部位与溶剂萃取所得之苦瓜萃取物,由结果发现30种苦瓜萃取物中以苹果苦瓜种子水萃取物(WESMC)最具抑制3T3-L1前脂肪细胞之生长和调控细胞周期停滞,其可透过提升p-p53与CDK抑制子p18与p27之蛋白质表现,并降低CDK2、CDK6、cyclin D1与pRb之蛋白质表现,进而达到减低3T3-L1前脂肪细胞增生数目。故本研究成果证实苹果苦瓜种子水萃取物,确实可透过细胞周期之调控,而抑制3T3-L1前脂肪细胞之细胞生长。
致谢
本研究承蒙台湾行政院国家科学委员会计划(NSC 102-2313-B-040-002)经费支持,特此感谢。
文章引用
张维棠,刘程炜,邱天惠,徐庆琳. 新育种苦瓜调控3T3-L1前脂肪细胞之细胞周期停滞之影响
Effects of New Variety Selection of Bitter Melon on Modulation of Cell Cycle in 3T3-L1 Preadipocytes[J]. 农业科学, 2017, 07(02): 128-139. http://dx.doi.org/10.12677/HJAS.2017.72016
参考文献 (References)
- 1. Mariela, G. and Itamar, R. (2011) Present and Future: Pharmacologic Treatment of Obesity. Journal of Obesity, 2011, 636181.
- 2. Yeh, C.J., Chang, H.Y. and Pan, W.H. (2011) Time Trend of Obesity, the Metabolic Syndrome and Related Dietary Pattern in Taiwan: From NAHSIT 1993-1996 to NAHSIT 2005-2008. Asia Pacific Journal of Clinical Nutrition, 20, 292-300.
- 3. Lawson, M.L., Kirk, S., Mitchell, T., Chen, M.K., Loux, T.J., Daniels, S.R., Harmon, C.M., Clements, R.H., Garcia, V.F. and Inge, T.H. (2006) Pediatric Bariatric Study Group. One-Year Outcomes of Roux-en-Y Gastric Bypass for Morbidly Obese Adolescents: A Multicenter Study from the Pediatric Bariatric Study Group. Journal of Pediatric Surgery, 41, 137-143. https://doi.org/10.1016/j.jpedsurg.2005.10.017
- 4. Nadler, E.P., Youn, H.A., Ren, C.J. and Fielding, G.A. (2008) An Update on 73 US Obese Pediatric Patients Treated with Laparoscopic Adjustable Gastric Banding: Comorbidity Resolution and Compliance Data. Journal of Pediatric Surgery, 43, 141-146. https://doi.org/10.1016/j.jpedsurg.2007.09.035
- 5. Bobowicz, M., Lehmann, A., Orlowski, M., Lech, P. and Michalik, M. (2011) Preliminary Outcomes 1 Year after Laparoscopic Sleeve Gastrectomy Based on Bariatric Analysis and Reporting Outcome System (BAROS). Obesity Surgery, 21, 1843-1848. https://doi.org/10.1007/s11695-011-0403-4
- 6. Dietrich, M.O. and Horvath, T.L. (2012) Limitations in Anti-Obesity Drug Development: The Critical Role of Hunger-Promoting Neurons. Nature Reviews. Drug Discovery, 11, 675-691. https://doi.org/10.1038/nrd3739
- 7. Chuang, C.Y., Hsu, C., Chao, C.Y., Wein, Y.S., Kuo, Y.H. and Huang, C.J. (2006) Fractionation and Identification of 9c, 11t, 13t-Conjugated Linolenic Acid as an Activator of PPAR Alpha in Bitter Gourd (Momordica charantia L.). Journal of Biomedical Science, 13, 763-772. https://doi.org/10.1007/s11373-006-9109-3
- 8. Krawinkel, M.B. and Keding, G.B. (2006) Bitter Gourd (Momordica charantia): A Dietary Approach to Hyperglycemia. Nutrition Reviews, 64, 331-337. https://doi.org/10.1111/j.1753-4887.2006.tb00217.x
- 9. Chang, C.I., Tseng, H.I., Liao, Y.W., Yen, C.H., Chen, T.M., Lin, C.C. and Cheng, H.L. (2011) In Vivo and In Vitro Studies to Identify the Hypoglycemic Constituents of Momordica charantia Wild Variant WB24. Food Chemistry, 125, 521-528. https://doi.org/10.1016/j.foodchem.2010.09.043
- 10. Hsu, C., Hsieh, C.L., Kuo, Y.H. and Huang, C.J. (2011) Isolation and Identification of Cucurbitane-Type Triterpenoids with Partial Agonist/Antagonist Potential for Estrogen Receptors from Momordica charantia. Journal of Agricultural and Food Chemistry, 59, 4553-4561. https://doi.org/10.1021/jf200418g
- 11. Klomann, S.D., Mueller, A.S., Pallauf, J. and Krawinkel, M.B. (2010) Antidiabetic Effects of Bitter Gourd Extracts in Insulin-Resistant db/db Mice. The British Journal of Nutrition, 104, 1613-1620. https://doi.org/10.1017/S0007114510002680
- 12. Lii, C.K., Chen, H.W., Yun, W.T. and Liu, K.L. (2009) Suppressive Effects of Wild Bitter Gourd (Momordica charantia Linn. var. abbreviata ser.) Fruit Extracts on Inflammatory Responses in RAW 264.7 Macrophages. Journal of Ethnopharmacology, 122, 227-233. https://doi.org/10.1016/j.jep.2009.01.028
- 13. Cheng, H.L., Kuo, C.Y., Liao, Y.W. and Lin, C.C. (2012) EMCD, a Hypoglycemic Triterpene Isolated from Momordica charantia Wild Variant, Attenuates TNF-α-Induced Inflammation in FL83B Cells in an AMP-Activated Protein Kinase-Independent Manner. European Journal of Pharmacology, 689, 241-248. https://doi.org/10.1016/j.ejphar.2012.05.033
- 14. Matsui, S., Yamane, T., Takita, T., Oishi, Y. and Kobayashi-Hattori, K. (2013) The Hypocholesterolemic Activity of Momordica charantia Fruit Is Mediated by the Altered Cholesterol- and Bile Acid-Regulating Gene Expression in Rat Liver. Nutrition Research, 33, 580-585. https://doi.org/10.1016/j.nutres.2013.05.002
- 15. Li, C.J., Tsang, S.F., Tsai, C.H., Tsai, H.Y., Chyuan, J.H. and Hsu, H.Y. (2012) Momordica charantia Extract Induces Apoptosis in Human Cancer Cells through Caspase- and Mitochondria-Dependent Pathways. Evidence-Based Complementary and Alternative Medicine, 2012, Article ID: 261971. https://doi.org/10.1155/2012/261971
- 16. Tabata, K., Hamano, A., Akihisa, T. and Suzuki, T. (2012) Kuguaglycoside C, a Constituent of Momordica charantia, Induces Caspase-Independent Cell Death of Neuroblastoma Cells. Cancer Science, 103, 2153-2158. https://doi.org/10.1111/cas.12021
- 17. Nerurkar, P.V., Lee, Y.K. and Nerurkar, V.R. (2010) Momordica charantia (Bitter Melon) Inhibits Primary Human Adipocyte Differentiation by Modulating Adipogenic Genes. BMC Complementary and Alternative Medicine, 10, 34. https://doi.org/10.1186/1472-6882-10-34
- 18. Hsu, C.L., Fang, S.C., Liu, C.W. and Chen, Y.F. (2013) Inhibitory Effects of New Varieties of Bitter Melon on Lipopolysaccharide-Stimulated Inflammatory Response in RAW 264.7 Cells. Journal of Functional Foods, 5, 1829-1837. https://doi.org/10.1016/j.jff.2013.09.002
- 19. Wang, Y.W. and Jones, P.J. (2004) Conjugated Linoleic Acid and Obesity Control: Efficacy and Mechanisms. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity, 28, 941-955. https://doi.org/10.1038/sj.ijo.0802641
- 20. Zhang, J.W., Tang, Q.Q., Vinson, C. and Lane, M.D. (2004) Dominant-Negative C/EBP Disrupts Mitotic Clonal Expansion and Differentiation of 3T3-L1 Preadipocytes. Proceedings of the National Academy of Sciences of the United States of America, 101, 43-47. https://doi.org/10.1073/pnas.0307229101
- 21. Hung, P.F., Wu, B.T., Chen, H.C., Chen, Y.H., Chen, C.L., Wu, M.H., Liu, H.C., Lee, M.J. and Kao, Y.H. (2005) Antimitogenic Effect of Green Tea (-)-Epigallocatechin Gallate on 3T3-L1 Preadipocytes Depends on the ERK and Cdk2 Pathways. American Journal of Physiology Cell Physiology, 288, C1094-C1108. https://doi.org/10.1152/ajpcell.00569.2004
- 22. Sahib, N.G., Hamid, A.A., Kitts, D., Purnama, M., Saari, N. and Abas, F. (2011) The Effects of Morinda citrifolia, Momordica charantia and Centella asiatica Extracts on Lipoprotein Lipase and 3T3-L1 Preadipocytes. Journal of Food Biochemistry, 35, 1186-1205. https://doi.org/10.1111/j.1745-4514.2010.00444.x
- 23. Murakami, T., Emoto, A., Matsuda, H. and Yoshikawa, M. (2001) Medicinal Foodstuffs. XXI. Structures of New Cucurbitane-Type Triterpene Glycosides, Goyaglycosidesa, -b, -c, -d, -e, -f, -g, and -h, and New Oleanane-Type Triterpene Saponins, Goyasaponins I, II, and III, from the Fresh Fruit of Japanese Momordica charantia. Chemical & Pharmaceutical Bulletin, 49, 54-63. https://doi.org/10.1248/cpb.49.54
- 24. Chanchai, M. (2003) Analysis of Charantin from Momordica charantia L. MS Thesis, Faculty of Graduate Studies, Mahidol University, Bangok.
- 25. Jesada, P., Sutawadee, C., Motonobu, G., Weena, J., Mitsuru, S. and Artiwan, S. (2007) New Approach for Extraction of Charantin from Momordica charantia with Pressurized Liquid Extraction. Separation and Purification Technology, 52, 416-422. https://doi.org/10.1016/j.seppur.2005.11.037
- 26. Horax, R., Hettiarachchy, N. and Chen, P. (2010) Extraction, Quantification, and Antioxidant Activities of Phenolics from Pericarp and Seeds of Bitter Melons (Momordica charantia) Harvested at Three Maturity Stages (Immature, Mature, and Ripe). Journal of Agricultural and Food Chemistry, 58, 4428-4433. https://doi.org/10.1021/jf9029578
- 27. Pawar, R.S., Tamta, H., Ma, J., Krynitsky, A.J., Grundel, E., Wamer, W.G. and Rader, J.I. (2013) Updates on Chemical and Biological Research on Botanical Ingredients in Dietary Supplements. Analytical and Bioanalytical Chemistry, 405, 4373-4384. https://doi.org/10.1007/s00216-012-6691-2
- 28. Popovich, D.G., Li, L. and Zhang, W. (2010) Bitter Melon (Momordica charantia) Triterpenoid Extract Reduces Preadipocyte Viability, Lipid Accumulation and Adiponectin Expression in 3T3-L1 Cells. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 48, 1619-1626. https://doi.org/10.1016/j.fct.2010.03.035
- 29. Popovich, D.G., Li, L. and Zhang, W. (2011) Momordica charantia Seed Extract Reduces Preadipocyte Viability, Affects Lactate Dehydrogenase Release, and Lipid Accumulation in 3T3-L1 Cells. Journal of Medicinal Food, 14, 201- 208. https://doi.org/10.1089/jmf.2010.1150
- 30. Hsu, C.L., Lo, W.H. and Yen, G.C. (2007) Gallic Acid Induces Apoptosis in 3T3-L1 Preadipocytes via a Fas- and Mitochondria-Mediated Pathway. Journal of Agricultural and Food Chemistry, 55, 7359-7365. https://doi.org/10.1021/jf071223c
- 31. Hsu, C.L., Lin, Y.J., Ho, C.T. and Yen, G.C. (2012) Inhibitory Effects of Garcinol and Pterostilbene on Cell Proliferation and Adipogenesis in 3T3-L1 Cells. Food & Function, 3, 49-57. https://doi.org/10.1039/C1FO10209E
- 32. Chou, Y.C., Su, H.M., Lai, T.W., Chyuan, J.H. and Chao, P.M. (2012) cis-9, trans-11, trans-13-Conjugated Linolenic Acid Induces Apoptosis and Sustained ERK Phosphorylation in 3T3-L1 Preadipocytes. Nutrition, 28, 803-811. https://doi.org/10.1016/j.nut.2011.11.019
- 33. Alenzi, F.Q. (2004) Links between Apoptosis, Proliferation and the Cell Cycle. British Journal of Biomedical Science, 61, 99-102. https://doi.org/10.1080/09674845.2004.11732652
- 34. Donjerkovic, D. and Scott, D.W. (2000) Regulation of the G1 Phase of the Mammalian Cell Cycle. Cell Research, 10, 1-16. https://doi.org/10.1038/sj.cr.7290031
- 35. Kuerbitz, S.J., Plunkett, B.S., Walsh, W.V. and Kastan, M.B. (1992) Wild-Type p53 Is a Cell Cycle Checkpoint Determinant Following Irradiation. Proceedings of the National Academy of Sciences of the United States of America, 89, 7491-7495https://doi.org/10.1073/pnas.89.16.7491
- 36. Gregorc, V., Ludovini, V., Pistola, L.,Darwish, S., Floriani, I., Bellezza, G., Sidoni, A., Cavaliere, A., Scheibel, M., De Angelis, V., Bucciarelli, E. and Tonato, M. (2003) Relevance of p53, bcl-2 and Rb Expression on Resistance to Cisplatin-Based Chemotherapy in Advanced Non-Small Cell Lung Cancer. Lung Cancer, 39, 41-48. https://doi.org/10.1016/S0169-5002(02)00391-4
- 37. Raghvan, D. (2003) Molecular Targeting and Pharmacogenomics in the Management of Advanced Bladder Cancer. Cancer, 97, 2083-2089. https://doi.org/10.1002/cncr.11281
- 38. Pitchakarn, P., Suzuki, S., Ogawa, K., Pompimon, W., Takahashi, S., Asamoto, M., Limtrakul, P. and Shirai, T. (2011) Induction of G1 Arrest and Apoptosis in Androgen-Dependent Human Prostate Cancer by Kuguacin J, A Triterpenoid from Momordica charantia Leaf. Cancer Letters, 306, 142-150. https://doi.org/10.1016/j.canlet.2011.02.041
NOTES
*通讯作者。
