Advances in Clinical Medicine
Vol.
13
No.
02
(
2023
), Article ID:
61361
,
8
pages
10.12677/ACM.2023.132264
肉瘤样肾细胞癌1例报告
杨桂楠,李汶轩,苏晓雪,侯四川*
青岛大学附属青岛市立医院泌尿外科,山东 青岛
收稿日期:2023年1月9日;录用日期:2023年2月8日;发布日期:2023年2月15日

摘要
肉瘤样肾细胞癌(sarcomatoid renal cell carcinoma, SRCC)是指肾细胞癌(renal cell carcinoma, RCC)肉瘤样去分化,由于实验室及影像学检查缺乏特异性,确诊依赖病理学检查。目前SRCC的治疗方式存在争议,患者预后极差。现报道1例青岛市市立医院收治的转移性SRCC患者,术后规律行系统治疗至今2年。
关键词
肉瘤样肾细胞癌,肾肿瘤

One Case Report of Sarcomatoid Renal Cell Carcinoma
Guinan Yang, Wenxuan Li, Xiaoxue Su, Sichuan Hou*
Department of Urology, Qingdao Municipal Hospital Affiliated to Qingdao University, Qingdao Shandong
Received: Jan. 9th, 2023; accepted: Feb. 8th, 2023; published: Feb. 15th, 2023

ABSTRACT
Sarcomatoid renal cell carcinoma refers to the sarcomatoid dedifferentiation of renal cell carcinoma. Due to the lack of specificity of laboratory and imaging tests, the diagnosis depends on pathology. The treatment of SRCC is controversial and the prognosis of patients is poor. This paper reports a case of metastatic SRCC patient who has received systematic treatment for 2 years after surgery in Qingdao Municipal Hospital.
Keywords:Sarcomatoid Renal Cell Carcinoma, Carcinoma, Renal Cell

Copyright © 2023 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 背景介绍
肉瘤样肾细胞癌(sarcomatoid renal cell carcinoma, SRCC)是一种临床罕见的肾恶性肿瘤,本病的诊断及治疗有较大争议。本文通过报告一例晚期SRCC患者的临床诊断及治疗过程,并回顾相关文献,旨在总结和探讨转移性SRCC的最佳诊疗方案。
2. 临床资料
患者,女,62岁,不明原因发热1周,体温最高达38.5℃,自服感冒药未见缓解,于外院就诊,行腹部CT平扫发现右肾占位,来我院寻求进一步治疗。患者查体未触及腹部肿物,既往无结核病病史,近期无明显体重减轻。辅助检查:血红蛋白90 g/L,血白细胞计数6.37 × 109/L,肌酐82.09 mmol/L,尿素氮6.88 mmol/L。双肾MR平扫及增强扫描可见腹膜后脊柱旁约4.6 cm × 6.3 cm × 11.8 cm大小,右肾上极约4.6 × 5.0 × 3.9 cm大小2处异常信号灶,呈T2WI稍低信号,DWI高信号(图1、图2)。PET/CT可见右肾上腺区、右肾区高代谢软组织肿块,右颈部及右膈脚后高代谢淋巴结(图3、图4)。患者于2020年8月17日接受腹腔镜下右肾切除术 + 腹腔镜下右侧腹膜后病损切除术,术中见右肾内侧与下腔静脉之间巨大腹膜后肿瘤,大小约12 × 5 cm,无明显包膜,呈糜烂鱼肉样,取部分组织送冰冻病理,结果怀疑肉瘤,遂切除右侧肾脏、同侧肾上腺及腹膜后肿瘤。术后石蜡病理可见癌组织突破肾被膜侵及脂肪囊,未累及肾盂、输尿管断端,瘤组织片状分布,细胞梭形,异型性明显,瘤内可见组织凝固性坏死(图5),免疫组化结果提示Vim、PAX-8、CK、CAIX均(+),ki-67 (约5%+),CD10 (图6)、S-100、Desmin、SMA均(−),诊断为肾肉瘤样癌。患者自2020年11月起,已接受安罗替尼靶向治疗 + 特瑞普利免疫治疗(每日口服安罗替尼8 mg,后调整为12 mg,服药2周,停药1周,每3周行特瑞普利单抗240 mg静滴) 33个疗程,术后1年复查PET/CT提示肿瘤复发及新发转移灶(图7、图8),至今病情未见明显进展。
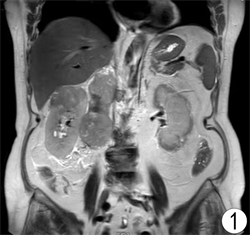
Figure 1. MRI image of bilateral kidneys before surgical operation (T2W2)
图1. 术前双肾MRI图像(T2WI)
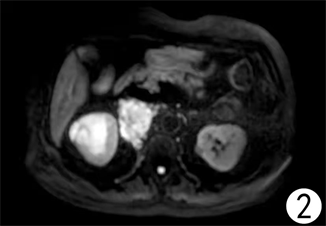
Figure 2. MRI image of bilateral kidneys before surgical operation (DWI)
图2. 术前双肾MRI图像(DWI)
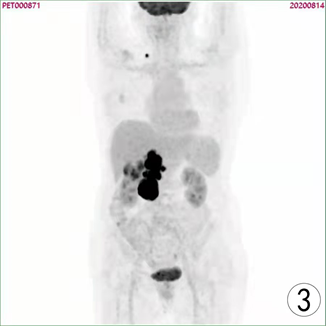
Figure 3. PET/CT images before surgical operation
图3. 术前PET/CT图像
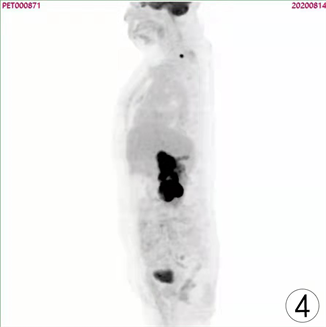
Figure 4. PET/CT images before surgical operation
图4. 术前PET/CT图像
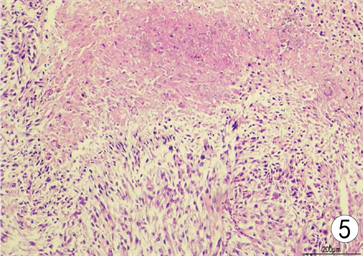
Figure 5. Paraffin section (hematoxylin-eosin staining × 100)
图5. 石蜡病理(苏木精–伊红染色 × 100)
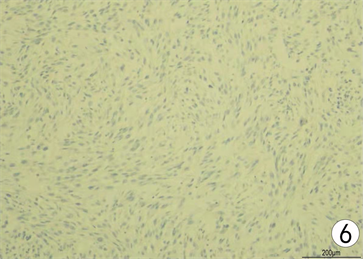
Figure 6. Immunohistochemistry of CD10 (SP staining × 100)
图6. CD10免疫组织化学染色(SP法染色 × 100)
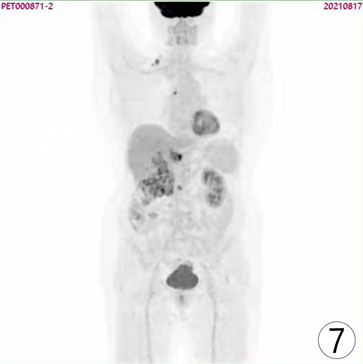
Figure 7. PET/CT images of patient one year after surgical operation
图7. 患者术后1年PET/CT图像
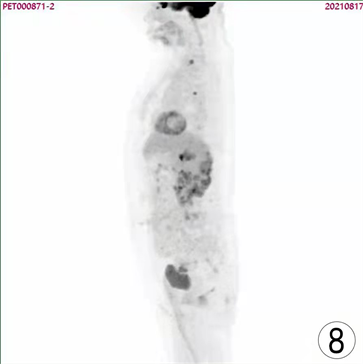
Figure 8. PET/CT images of patient one year after surgical operation
图8. 患者术后1年PET/CT图像
3. 讨论
SRCC是1968年由Farrow [1] 等首次提出,是以RCC肉瘤样去分化,高侵袭性为特征性病理表现的一种较少见的恶性肿瘤,它可以出现在所有组织学亚型的肾恶性肿瘤中,因此不被单独列为肾恶性肿瘤的亚型 [2] [3] [4] 。SRCC患者约86%~89%有临床表现,常见包括疼痛(51%~52%)、血尿(22%~34%)、体重减轻(18%~22%)、乏力(15%)、低热或盗汗(6%~12%)、咳嗽或呼吸困难(6%~12%) [4] [5] 。
目前还不存在一种可靠的术前诊断SRCC的影像学方法,Schieda [6] 等通过SRCC和肾透明细胞癌(clear cell renal cell carcinoma, CCRCC)患者的CT表现的对比研究发现,SRCC患者肿瘤有体积巨大、密度不均、边缘浸润的假包膜破裂、瘤内易坏死,常见瘤旁较粗大新生血管形成等特征,但这些特征是非特异性的。SRCC和CCRCC的MRI表现同CT结论类似,两者有非特异性的微妙差异,如SRCC形态更不规则,更容易观察到周围浸润的表现 [7] 。回顾性分析表明,SRCC患者MRI在T2像表现为信号不均一,所有患者肿瘤内均有不同程度的组织坏死,半数患者坏死比例超过50% [6] [8] 。国内学者收集29例肾肉瘤样癌与82例肾透明细胞癌患者的PET/CT图像对比,发现SRCC可以摄取更多的氟代脱氧葡萄糖,因此标准摄取值较CCRCC更高,由于瘤组织坏死及瘤周新生血管较RCC更为常见,“环状标志”有助于SRCC的诊断 [9] 。
SRCC肿瘤的平均直径多在9.2~11 cm之间,肉瘤样分化区一般为灰白色,边缘浸润,切面呈鱼肉样或纤维状,质地坚韧,90%以上的肉瘤样癌可以观察到肿瘤组织凝固性坏死 [4] [10] [11] 。显微镜SRCC一般可见间充质细胞(即肉瘤样癌细胞)及上皮样癌细胞 [5] ,而后者在肾肉瘤中缺失。有报道显示嫌色细胞RCC肉样转化率高于其他组织学亚型的RCC [11] ,但CCRCC较高的发病率使这一发现难以验证 [12] 。部分SRCC肿瘤中分化良好的上皮成分和去分化的肉瘤样成分可能只是局部存在,在缺乏低分化上皮细胞的情况下,通过肿瘤外观及显微镜下细胞形态诊断SRCC仍有局限性,因为如肉瘤样尿路上皮癌、血管平滑肌脂肪瘤、肉瘤样肾上腺皮质癌、孤立性纤维瘤和滑膜肉瘤均可见梭形肿瘤细胞,而免疫组化可以有效弥补这一不足。PAX-2和PAX-8是建立肾系细胞和肾脏形成所需的转录因子 [13] [14] ,是肾肿瘤的有效标记,CAIX是CCRCC的特异性标记物,为SRCC的来源提供了有力证据,此外,上皮分化的标记物包括细胞角蛋白、上皮膜抗原通常在SRCC中局部表达,而在肾肉瘤中不表达 [15] 。
Alevizakos [16] 的回顾性分析表明SRCC I期、II期、III期、IV期疾病患者的5年疾病特异性生存率分别为77.7%、67.8%、35.4%和3.5%。Main [4] 的研究发现,所有患者中位生存期仅9个月,这与Blum等 [3] [16] 的研究结果一致。对于非转移性SRCC,根治性肾切除术辅以全身治疗仍是最重要的治疗手段,但术后26个月复发率仍高达80% [5] [17] 。对于有淋巴结侵犯或远处转移的SRCC,患者是否从减瘤手术中获益仍有争议,Alevizakos [16] 的研究中有472例转移性SRCC患者,接受手术的患者1年、3年和5年的疾病特异性生存率分别为33.7%、10.8%和6.2%,未接受手术的患者的1年、3年和5年疾病特异性生存率分别为11.5%、1.9%和0%。而Main等 [4] [5] [18] 学者认为SRCC具有高侵袭性,与单独接受系统治疗相比,接受肾切除术并系统治疗的转移性疾病患者无明显生存优势,手术也会延迟系统治疗,因此建议转移性SRCC患者应首先接受系统治疗,对证实系统治疗有效的患者进行手术,对系统治疗无反应的,或有周围器官侵犯或多发转移如肝脏、颅内或骨转移的患者,应避免手术。目前想要明确转移性SRCC是否必要接受减瘤手术,其难点在于术前影像学或组织穿刺活检检出率低,在一项对199名SRCC患者的研究中,在肾切除术或转移瘤切除术前对原发肿瘤或转移部位进行活检,仅有7.5%的患者存在肉瘤样特征,多数患者依赖术后的病理学诊断明确肿瘤性质 [3] [19] [20] [21] 。通常认为,RCC是对放射治疗不敏感的肿瘤,同样的,在一项对408例非转移性SRCC患者的研究中,未能证明放疗对SRCC有效,接受辅助放疗的患者在1年、3年和5年的总体或疾病特异性生存率与仅接受手术治疗的患者无显著差异,目前放疗主要用于转移性疾病或复发性局部肿瘤生长的患者的姑息性治疗 [22] 。Kunene [23] 曾报道23例接受舒尼替尼靶向治疗的SRCC患者,无进展生存期及中位总生存期分别为5.7和15.7个月,体能状态(performance status, PS)良好的患者接受舒尼替尼治疗尤其有效,PS 0~1、2~3分患者的中位总生存期分别为20.9个月、5.0个月。免疫检查点抑制剂与其他治疗SRCC的全身疗法相比取得了最大的进展,Joseph [24] 的研究中显示与非肉瘤样RCC相比,SRCC在肿瘤细胞上PD-1/PD-L1的表达水平更高,SRCC表达PD-1/PD-L1的比例分别是96%和54%,提示SRCC存在免疫抵抗的模式,应用抗PDL1和PD1药物治疗SRCC有较好的前景,2018年1篇国外病例报告 [25] ,报道1名SRCC患者在接受舒尼替尼 + 吉西他尼治疗4个月发现多发转移灶,更换纳武单抗治疗1个月后1处转移灶消退,6个月复查PET/CT已知转移灶全部消退,术后2年复查无复发迹象。另一篇文献报道了2名有PDL1在9p24.1处扩增的SRCC患者对免疫治疗有显著反应,两例治疗前均提示多器官转移,其中1例接受阿替利珠单抗治疗14个月病情稳定,另一例接受帕博利珠单抗治疗16个月后病情稳定 [26] 。
4. 结论
综上所述,关于SRCC的治疗,泌尿或肿瘤指南中没有标准化的建议,因为它的发病率低,术前诊断困难,缺乏疾病特异性试验,在临床工作中应以患者为中心,制定个性化的治疗方案。本例转移性SRCC患者在减瘤手术的基础上接受长期、规律的靶向治疗和免疫治疗,术后2年病情进展缓慢,对晚期SRCC诊疗方案的制定有较高的参考价值。
文章引用
杨桂楠,李汶轩,苏晓雪,侯四川. 肉瘤样肾细胞癌1例报告
One Case Report of Sarcomatoid Renal Cell Carcinoma[J]. 临床医学进展, 2023, 13(02): 1906-1913. https://doi.org/10.12677/ACM.2023.132264
参考文献
- 1. Farrow, G.M., Harrison Jr., E.G., Utz, D.C. and Remine, W.H. (1968) Sarcomas and Sarcomatoid and Mixed Malignant Tumors of the Kidney in Adults—Part I. Cancer, 22, 545-550. https://doi.org/10.1002/1097-0142(196809)22:3<545::AID-CNCR2820220308>3.0.CO;2-4
- 2. Shuch, B., Bratslavsky, G., Linehan, W.M. and Srinivasan, R. (2012) Sarcomatoid Renal Cell Carcinoma: A Comprehensive Re-view of the Biology and Current Treatment Strategies. The Oncologist, 17, 46-54. https://doi.org/10.1634/theoncologist.2011-0227
- 3. Blum, K.A., Gupta, S., Tickoo, S.K., et al. (2020) Sarcoma-toid Renal Cell Carcinoma: Biology, Natural History and Management. Nature Reviews Urology, 17, 659-678. https://doi.org/10.1038/s41585-020-00382-9
- 4. Mian, B.M., Bhadkamkar, N., Slaton, J.W., et al. (2002) Prog-nostic Factors and Survival of Patients with Sarcomatoid Renal Cell Carcinoma. The Journal of Urology, 167, 65-70. https://doi.org/10.1016/S0022-5347(05)65384-0
- 5. Lebacle, C., Pooli, A., Bessede, T., et al. (2019) Epidemiol-ogy, Biology and Treatment Of Sarcomatoid RCC: Current State of the Art. World Journal of Urology, 37, 115-123. https://doi.org/10.1007/s00345-018-2355-y
- 6. Schieda, N., Thornhill, R.E., Al-Subhi, M., et al. (2015) Diagno-sis of Sarcomatoid Renal Cell Carcinoma with CT: Evaluation by Qualitative Imaging Features and Texture Analysis. AJR American Journal of Roentgenology, 204, 1013-1023. https://doi.org/10.2214/AJR.14.13279
- 7. Takeuchi, M., Kawai, T., Suzuki, T., et al. (2015) MRI for Differentiation of Renal Cell Carcinoma with Sarcomatoid Component from Other Renal Tumor Types. Abdominal Imaging, 40, 112-119. https://doi.org/10.1007/s00261-014-0185-y
- 8. Rosenkrantz, A.B., Chandarana, H. and Melamed, J. (2011) MRI Findings of Sarcomatoid Renal Cell Carcinoma in Nine Cases. Clinical Imaging, 35, 459-464. https://doi.org/10.1016/j.clinimag.2010.11.002
- 9. Gao, J., Xu, Q., Fu, Y., et al. (2021) Comprehensive Evalua-tion of 68Ga-PSMA-11 PET/CT Parameters for Discriminating Pathological Characteristics in Primary Clear-Cell Renal Cell Carcinoma. European Journal of Nuclear Medicine and Molecular Imaging, 48, 561-569. https://doi.org/10.1007/s00259-020-04916-6
- 10. de Peralta-Venturina, M., Moch, H., Amin, M., et al. (2001) Sarcomatoid Differentiation in Renal Cell Carcinoma: A Study of 101 Cases. The American Journal of Surgical Pathol-ogy, 25, 275-284. https://doi.org/10.1097/00000478-200103000-00001
- 11. Cheville, J.C., Lohse, C.M., Zincke, H., et al. (2004) Sarcomatoid Renal Cell Carcinoma: An Examination of Underlying Histologic Subtype and an Analysis of Associations with Patient Outcome. The American Journal of Surgical Pathology, 28, 435-441. https://doi.org/10.1097/00000478-200404000-00002
- 12. Kuroda, N., Tamura, M., Hamaguchi, N., et al. (2011) Acquired Cystic Disease-Associated Renal Cell Carcinoma with Sarcomatoid Change and Rhabdoid Features. Annals of Diagnostic Pathology, 15, 462-466. https://doi.org/10.1016/j.anndiagpath.2010.07.008
- 13. Tong, G.-X., Yu, W.M., Beaubier, N.T., et al. (2009) Ex-pression of PAX8 in Normal and Neoplastic Renal Tissues: An Immunohistochemical Study. Modern Pathology, 22, 1218-1227. https://doi.org/10.1038/modpathol.2009.88
- 14. Ozcan, A., de la Roza, G., Ro, J.Y., Shen, S.S. and Truong, L.D. (2012) PAX2 and PAX8 Expression in Primary and Metastatic Renal Tumors: A Comprehensive Com-parison. Archives of Pathology & Laboratory Medicine, 136, 1541-1551. https://doi.org/10.5858/arpa.2012-0072-OA
- 15. Tickoo, S.K., Alden, D., Olgac, S., et al. (2007) Immunohisto-chemical Expression of Hypoxia Inducible Factor-1α and Its Downstream Molecules in Sarcomatoid Renal Cell Carci-noma. The Journal of Urology, 177, 1258-1263. https://doi.org/10.1016/j.juro.2006.11.100
- 16. Alevizakos, M., Gaitanidis, A., Nasioudis, D., Msaouel, P. and Appleman, L.J. (2019) Sarcomatoid Renal Cell Carcinoma: Population-Based Study of 879 Patients. Clinical Genitouri-nary Cancer, 17, e447-e453. https://doi.org/10.1016/j.clgc.2019.01.005
- 17. Merrill, M.M., Wood, C.G, Tannir, N.M., et al. (2015) Clinically Nonmetastatic Renal Cell Carcinoma with Sarcomatoid Dedifferentiation: Natural History and Outcomes after Surgical Resection with Curative Intent. Urologic Oncology: Seminars and Original Investigations, 33, 166.e21-166.e29. https://doi.org/10.1016/j.urolonc.2014.11.021
- 18. Kutikov, A., Uzzo, R.G., Caraway, A., et al. (2010) Use of Systemic Therapy and factors Affecting Survival for Patients Undergoing Cytoreductive Nephrectomy. BJU Internation-al, 106, 218-223. https://doi.org/10.1111/j.1464-410X.2009.09079.x
- 19. Abel, E.J., Carrasco, A., Culp, S.H., et al. (2012) Limita-tions of Preoperative Biopsy in Patients with Metastatic Renal Cell Carcinoma: Comparison to Surgical Pathology in 405 Cases. BJU International, 110, 1742-1746. https://doi.org/10.1111/j.1464-410X.2012.11124.x
- 20. Keskin, S.K., Msaouel, P., Hess, K.R., et al. (2017) Out-comes of Patients with Renal Cell Carcinoma and Sarcomatoid Dedifferentiation Treated with Nephrectomy and Systemic Therapies: Comparison between the Cytokine and Targeted Therapy Eras. The Journal of Urology, 198, 530-537. https://doi.org/10.1016/j.juro.2017.04.067
- 21. Shuch, B., Said, J., La Rochelle, J.C., et al. (2009) Cytoreductive Nephrectomy for Kidney Cancer with Sarcomatoid Histology—Is up-Front Resection Indicated and, If Not, Is It Avoida-ble? The Journal of Urology, 182, 2164-2171. https://doi.org/10.1016/j.juro.2009.07.049
- 22. Siva, S., Kothari, G., Muacevic, A., et al. (2017) Radiotherapy for Renal Cell Carcinoma: Renaissance of an Overlooked Approach. Nature Reviews Urology, 14, 549-563. https://doi.org/10.1038/nrurol.2017.87
- 23. Kunene, V., Miscoria, M., Pirrie, S., et al. (2014) Sarcomatoid Renal Cell Carcinoma: Clinical Outcome and Survival after Treatment with Sunitinib. Clinical Genitourinary Cancer, 12, 251-255. https://doi.org/10.1016/j.clgc.2013.12.001
- 24. Joseph, R.W., Millis, S.Z., Carballido, E.M., et al. (2015) PD-1 and PD-L1 Expression in Renal Cell Carcinoma with Sarcomatoid Differentiation. Cancer Immunology Research, 3, 1303-1307. https://doi.org/10.1158/2326-6066.CIR-15-0150
- 25. El Mouallem, N., Smith, S.C. and Paul, A.K. (2018) Com-plete Response of a Patient with Metastatic Sarcomatoid Renal Cell Carcinoma to a Programmed Death-1 Checkpoint In-hibitor. Journal of Oncology Practice, 14, 511-513. https://doi.org/10.1200/JOP.18.00213
- 26. Gupta, S., Cheville, J.C., Jungbluth, A.A., et al. (2019) JAK2/PD-L1/PD-L2 (9p24.1) Amplifications in Renal Cell Carcinomas with Sarcomatoid Transformation: Implications for Clinical Management. Modern Pathology, 32, 1344-1358. https://doi.org/10.1038/s41379-019-0269-x
NOTES
*通讯作者Email: housichuandoctor@126.com