Hans Journal of Medicinal Chemistry
Vol.
09
No.
02
(
2021
), Article ID:
42812
,
10
pages
10.12677/HJMCe.2021.92012
萘环类PD-1/PD-L1抑制剂的设计、合成及生物活性评价
赵磊1,余龙波2,欧阳宜强1,郭文洁2,徐 强2,高 健2*,赖宜生1*
1中国药科大学,新药研究中心,天然药物活性组分与药效国家重点实验室,江苏省代谢性疾病药物重点实验室,江苏 南京
2南京大学,生命科学学院,医药生物技术国家重点实验室,江苏 南京
收稿日期:2021年4月30日;录用日期:2021年5月24日;发布日期:2021年5月31日

摘要
以BMS-1018为先导化合物,通过替换联苯片段为萘环以及生物电子等排原理,结合分子对接技术,设计并合成了两个系列共18个新型萘环类PD-1/PD-L1小分子抑制剂,结构经1H-NMR和ESI-MS谱确证。采用均相时间分辨荧光法评价目标化合物对PD-1/PD-L1结合的抑制活性。结果表明,所有目标化合物对PD-1/PD-L1均显示不同程度的抑制活性。其中6个化合物A-8、A-9和B-5、B-6、B-8、B-9的活性较为突出,值得进一步研究。
关键词
肿瘤免疫治疗,免疫检查点,PD-1/PD-L1抑制剂,生物活性

Design, Synthesis and Biological Evaluation of Naphthalene Ring PD-1/PD-L1 Inhibitors
Lei Zhao1, Longbo Yu2, Yiqiang Ouyang1, Wenjie Guo2, Qiang Xu2, Jian Gao2*, Yisheng Lai1*
1China Pharmaceutical University, Jiangsu Key Laboratory of Drug Discovery for Metabolic Diseases, State Key Laboratory of National Medicines, Center of Drug Discovery, Nanjing Jiangsu
2Nanjing University, School of Life Sciences, State Key Laboratory of Pharmaceutical Biotechnology, Nanjing Jiangsu
Received: Apr. 30th, 2021; accepted: May 24th, 2021; published: May 31st, 2021

ABSTRACT
Using BMS-1018 as the lead compound, two series of novel naphthalene-based PD-1/PD-L1 small molecule inhibitors were designed and synthesized by replacing the biphenyl moiety with a naphthalene ring and applying the principle of bioisosterism combined with molecular docking technology. The structures of the target compounds were confirmed by 1H-NMR and ESI-MS. The inhibitory activity of the compounds against the PD-1/PD-L1 interaction was evaluated by homogeneous time-resolved fluorescence. The results showed that all the target compounds displayed different degrees of inhibitory activity. Among them, six compounds A-8, A-9 and B-5, B-6, B-8, B-9 have outstanding activities, which are worthy of further study.
Keywords:Tumor Immunotherapy, Immune Checkpoint, PD-1/PD-L1 Inhibitor, Biological Activity

Copyright © 2021 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
免疫检查点是指通过平衡共刺激和共抑制信号来控制T细胞免疫应答强度的信号通路 [1]。在正常生理状态下,免疫检查点在维持自身耐受和避免自身免疫中发挥重要作用,但在肿瘤患者中,免疫检查点相关蛋白的表达失调,会导致肿瘤免疫逃逸 [2]。自2014年以来,美国FDA已批准6种PD-1/PD-L1单克隆抗体用于癌症治疗 [3],这些单抗具有很好的临床疗效,显著改善了部分此前无法治疗的恶性肿瘤患者的预后。但是高成本、低应答率、免疫原性等问题仍然限制了该类单抗的临床应用 [4]。因此,PD-1/PD-L1小分子抑制剂的研发引起了越来越多学术机构和制药公司的关注 [5]。2015年,百时美施贵宝(BMS)公司首次披露了一系列具有间-(苯氧甲基)联苯骨架的PD-1/PD-L1小分子抑制剂(图1) [6] [7]。随后,Holak课题组发现,BMS-202及其类似物可诱导PD-L1蛋白同源二聚体的形成,从而阻止PD-L1与其受体PD-1的结合,并最终阻断下游信号传导 [8] [9] [10]。受到这一独特作用机制的启发,一系列新型PD-1/PD-L1小分子抑制剂先后被研发,并有望与单抗联用实现协同抗癌作用 [11]。

Figure 1. The representative small-molecule PD-1/PD-L1 inhibitors developed by BMS
图1. BMS研发的代表性PD-1/PD-L1小分子抑制剂的结构
2. 目标化合物的设计与合成
2.1. 目标化合物的设计
在BMS公司公开PD-1/PD-L1小分子抑制剂结构后,Holak等 [8] [9] 揭示了其中的BMS-202、BMS-1001、BMS-1166与靶蛋白结合的晶体结构,发现其联苯片段能够与PD-L1二聚体构成的疏水通道形成关键的作用。具体来讲,联苯中的a环能够与A亚基的Met115形成关键的π-烷基堆积作用,因此增强联苯结构的电子云密度可能有利于提高活性。据此,本文作者尝试将联苯结构替换成萘环,拟在保留联苯结构a环的同时,通过共轭作用提高电子云密度,从而增强其与疏水空腔AMet115的π-烷基作用。再者,萘环与联苯结构的体积相当,有望能够更好地占据疏水空腔。此外,我们还对伸向溶剂区的亲水片段R基团进行优化,以考察其对活性的影响,从而设计了A系列和B系列目标化合物(图2)。
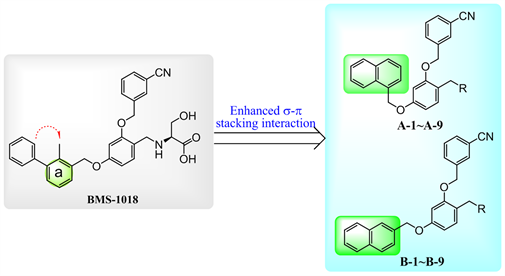
Figure 2. The design strategy of the target compounds A and B
图2. A和B系列目标化合物的设计策略
A系列目标化合物的合成:以1-溴甲基萘为原料,与2,4-二羟基苯甲醛经Williamson醚化反应生成中间体1,1与间氰基苄溴反应生成中间体2,2分别与各种氨基醇或氨基酸经还原胺化反应制得目标化合物A-1~A-9 (图3)。
 试剂和条件:(a) CH3CN, NaHCO3, 90℃, 6 h; (b) 3-(bromomethyl)benzonitrile, DMF, K2CO3, rt, 12 h; (c) DMF, AcOH, NaBH3CN, rt, 24 h.
试剂和条件:(a) CH3CN, NaHCO3, 90℃, 6 h; (b) 3-(bromomethyl)benzonitrile, DMF, K2CO3, rt, 12 h; (c) DMF, AcOH, NaBH3CN, rt, 24 h.
Figure 3. Synthetic route for the target compounds A-1~A-9
图3. 目标化合物A-1~A-9的合成路线
B系列目标化合物的合成:以2-溴甲基萘为原料,与2,4-二羟基苯甲醛经Williamson醚化反应生成中间体3,3与间氰基苄溴反应生成中间体4,4分别与各种氨基醇或氨基酸经还原胺化反应制得目标化合物B-1~B-9 (图4)。
 试剂和条件:(a) CH3CN, NaHCO3, 90℃, 6 h; (b) 3-(bromomethyl)benzonitrile, DMF, K2CO3, rt, 12 h; (c) DMF, AcOH, NaBH3CN, rt, 24 h.
试剂和条件:(a) CH3CN, NaHCO3, 90℃, 6 h; (b) 3-(bromomethyl)benzonitrile, DMF, K2CO3, rt, 12 h; (c) DMF, AcOH, NaBH3CN, rt, 24 h.
Figure 4. Synthetic route for the target compounds B-1~B-9
图4. 目标化合物B-1~B-9的合成路线
2.2. 目标化合物的合成
化合物熔点采用RY-1型熔点仪测定,温度计未经校正;1H-NMR使用ACF-300 MHz核磁共振仪测定,TMS为内标;ESI-MS使用Agilent 1100 Series LC/MSD Trap (SL)质谱仪测定。所有试剂未经特别说明均为市售化学纯或分析纯产品。
2.2.1. 目标化合物A-1~A-9的合成
分别将1-溴甲基萘(2.0 g,9.1 mmol)、2,4-二羟基苯甲醛(1.5 g,10.8 mmol)和碳酸氢钠(1.2 g,14.3 mmol)加入乙腈(30 mL)中,90℃回流反应12 h。加入水(100 mL),乙酸乙酯萃取(50 mL × 3),有机相用无水硫酸镁干燥,抽滤,滤液浓缩,柱层析分离(石油醚/乙酸乙酯,体积比15:1)得白色固体1 (1.5 g,收率59.6%)。1H NMR (300 MHz, DMSO-d6) δ 11.07 (s, 1H), 10.02 (s, 1H), 8.00-7.96 (m, 2H), 7.95 (s, 1H), 7.93 (d, J = 3.2 Hz, 1H), 7.65 (d, J = 8.7 Hz, 1H), 7.58 (dd, J = 8.4, 1.8 Hz, 1H), 7.55-7.52 (m, 2H), 6.70 (dd, J = 8.7, 2.4 Hz, 1H), 6.62 (d, J = 2.3 Hz, 1H), 5.35 (s, 2H)。
分别将1 (1.5 g,5.4 mmol)、碳酸钾(1.1 g,8.1 mmol)和3-氰基苄基溴(1.4 g,7.1 mmol)加入DMF(30 mL)中,室温反应6 h。加入水(150 mL),乙酸乙酯萃取(50 mL × 3),有机相用无水硫酸镁干燥,抽滤,滤液浓缩,柱层析分离(石油醚/乙酸乙酯,体积比8:1)得白色固体2 (1.8 g,收率84.9%)。1H NMR (300 MHz, Chloroform-d) δ 10.38 (d, J = 5.1 Hz, 1H), 7.92 (d, J = 9.5 Hz, 2H), 7.90-7.82 (m, 3H), 7.68 (dd, J = 16.1, 5.9 Hz, 2H), 7.64-7.59 (m, 1H), 7.58-7.51 (m, 2H), 7.48 (dd, J = 14.3, 7.1 Hz, 2H), 6.83-6.68 (m, 1H), 6.59 (d, J = 4.7 Hz, 1H), 5.29 (s, 2H), 5.16 (s, 2H)。
3-(((((1,3-二羟基-2-甲基丙烷-2-基)氨基)甲基)-5-(萘-1-基甲氧基)苯氧基)甲基)苯甲腈(A-1)的制备:将2 (0.2 g,0.5 mmol)溶于DMF(25 mL)中,随后依次加入2-氨基-2-甲基-1,3-丙二醇(0.1 g,1.0 mmol)、冰醋酸(0.06 g,1.0 mmol)和氰基硼氢化钠(0.1 g,1.6 mmol),室温反应24 h。加入水(50 mL),乙酸乙酯萃取(20 mL × 3),有机相用无水硫酸镁干燥,抽滤,滤液浓缩,柱层析分离(二氯甲烷/甲醇,体积比20:1),得0.15 g白色固体A-1,收率61.1%,mp 183℃~185℃。ESI-MS m/z: 481.2 [M-H]-。1H NMR (300 MHz, DMSO-d6) δ 8.10-8.06 (m, 1H), 8.03 (d, J = 2.0 Hz, 1H), 8.00 (dd, J = 7.7, 1.9 Hz, 1H), 7.95 (d, J = 8.2 Hz, 1H), 7.91-7.87 (m, 1H), 7.83 (d, J = 7.7 Hz, 1H), 7.68 (dd, J = 7.0, 1.2 Hz, 1H), 7.62-7.57 (m, 3H), 7.52 (dd, J = 8.2, 7.0 Hz, 1H), 7.44 (d, J = 8.2 Hz, 1H), 6.88 (d, J = 2.3 Hz, 1H), 6.81 (dd, J = 8.4, 2.3 Hz, 1H), 5.60 (s, 2H), 5.40 (s, 2H), 5.22 (s, 2H), 4.12 (s, 2H), 3.55 (s, 2H), 3.54 (s, 2H), 1.12 (s, 3H)。采用类似的方法制备目标化合物A-2~A-9。
3-((2-((呋喃-2-基甲基)氨基)甲基)-5-(萘-1-基甲氧基)苯氧基)甲基)苯甲腈(A-2),白色固体,收率62.2%,mp 172℃~174℃。ESI-MS m/z: 473.2 [M-H]-。1H NMR (300 MHz, Chloroform-d) δ 7.92 (td, J = 6.2, 3.0 Hz, 2H), 7.87 (d, J = 8.0 Hz, 1H), 7.72 (dd, J = 8.9, 1.7 Hz, 2H), 7.62 (dt, J = 7.7, 1.4 Hz, 1H), 7.54 (dd, J = 6.4, 3.3 Hz, 2H), 7.51 (d, J = 1.1 Hz, 1H), 7.50-7.48 (m, 2H), 7.47 (s, 1H), 7.33 (d, J = 1.8 Hz, 1H), 6.68 (dd, J = 8.4, 2.3 Hz, 1H), 6.59 (d, J = 3.4 Hz, 1H), 6.55 (d, J = 2.3 Hz, 1H), 6.30 (dd, J = 3.3, 1.9 Hz, 1H), 5.34 (s, 2H), 5.10 (s, 2H), 3.97 (s, 2H), 3.94 (s, 2H)。
3-((2-((1,3-二羟基丙烷-2-基)氨基)甲基)-5-(萘-1-基甲氧基)苯氧基)甲基)苯甲腈(A-3),白色固体,收率42.0%,mp 175℃~177℃。ESI-MS m/z: 467.2 [M-H]-。1H NMR (300 MHz, Chloroform-d) δ 7.99 (t, J = 8.0 Hz, 1H), 7.86 (dt, J = 15.1, 7.4 Hz, 2H), 7.73 (d, J = 5.6 Hz, 1H), 7.60 (d, J = 6.5 Hz, 1H), 7.53 (dq, J = 7.7, 4.1 Hz, 4H), 7.43 (d, J = 7.3 Hz, 2H), 7.33 (s, 1H), 6.68 (d, J = 7.6 Hz, 1H), 6.57 (d, J = 5.7 Hz, 1H), 5.41 (s, 2H), 5.08 (s, 2H), 4.07 (s, 2H), 3.74 (d, J = 7.1 Hz, 4H), 3.00-2.94 (m, 1H), 2.90 (d, J = 7.4 Hz, 2H)。
N-(2-((2-((3-氰基苯)氧基)-4-(萘-1-甲氧基)苄基)氨基)乙基)乙酰胺(A-4),白色固体,收率41.0%,mp 165℃~167℃。ESI-MS m/z: 478.2 [M-H]-。1H NMR (300 MHz, Chloroform-d) δ 8.06-7.96 (m, 1H), 7.92-7.87 (m, 1H), 7.85 (s, 1H), 7.73 (d, J = 6.7 Hz, 1H), 7.66 (d, J = 8.1 Hz, 1H), 7.56 (t, J = 5.6 Hz, 2H), 7.53 (s, 1H), 7.51-7.44 (m, 2H), 7.34 (d, J = 8.5 Hz, 1H), 7.10 (s, 1H), 6.71 (d, J = 8.2 Hz, 1H), 6.60 (d, J = 14.7 Hz, 1H), 5.44 (s, 2H), 5.15 (s, 2H), 4.11 (s, 2H), 3.44 (t, J = 10.4 Hz, 2H), 3.12 (t, J = 6.4 Hz, 2H), 1.93 (s, 3H)。
N-(2-((3-氰基苯)氧基)-4-(萘-1-基甲氧基)苄基)-N-甲基甘氨酸(A-5),白色固体,收率56.2%,mp 171℃~173℃。ESI-MS m/z: 465.2 [M-H]-。1H NMR (300 MHz, DMSO-d6) δ 8.09-8.05 (m, 1H), 7.98-7.93 (m, 2H), 7.91 (d, J = 5.1 Hz, 2H), 7.79 (t, J = 8.4 Hz, 2H), 7.65 (d, J = 7.1 Hz, 1H), 7.57 (dd, J = 8.1, 4.3 Hz, 3H), 7.51 (d, J = 7.6 Hz, 1H), 6.78 (d, J = 2.3 Hz, 1H), 6.70 (d, J = 7.9 Hz, 1H), 5.51 (s, 2H), 5.19 (s, 2H), 3.56 (s, 2H), 3.55 (s, 2H), 2.14 (s, 3H)。
(S)-1-(2-((3-氰基苯甲酰基)氧基)-4-(萘-1-基甲氧基)苄基)哌啶-2-羧酸(A-6),白色固体,收率46.6%,mp 172℃~174℃。ESI-MS m/z: 505.2 [M-H]-。1H NMR (300 MHz, DMSO-d6) δ 8.08 (d, J = 7.8 Hz, 1H), 8.01-7.92 (m, 3H), 7.82 (dd, J = 12.0, 7.7 Hz, 2H), 7.67 (d, J = 7.0 Hz, 1H), 7.59 (q, J = 7.5, 6.5 Hz, 3H), 7.52 (t, J = 7.6 Hz, 1H), 7.36 (d, J = 8.4 Hz, 1H), 6.83 (s, 1H), 6.79 (d, J = 8.2 Hz, 1H), 5.54 (s, 2H), 5.22 (s, 2H), 4.04-3.97 (m, 2H), 3.86 (s, 1H), 3.19 (dd, J = 8.1, 4.1 Hz, 1H), 3.00 (dd, J = 11.5, 5.9 Hz, 1H), 1.86 (t, J = 9.3 Hz, 1H), 1.73 (q, J = 10.7, 9.3 Hz, 1H), 1.53 (m, 3H), 1.37 (q, J = 7.5, 7.1 Hz, 1H)。
(2-((3-氰基苯甲酰基)氧基)-4-(萘-1-基甲氧基)苄基)-L-丝氨酸(A-7),白色固体,收率44.8%,mp 167℃~169℃。ESI-MS m/z: 481.2 [M-H]-。1H NMR (300 MHz, DMSO-d6) δ 8.07 (d, J = 8.0 Hz, 1H), 8.00 (s, 2H), 7.94 (d, J = 8.4 Hz, 1H), 7.89 (d, J = 8.1 Hz, 1H), 7.80 (d, J = 7.7 Hz, 1H), 7.66 (d, J = 7.0 Hz, 1H), 7.63-7.53 (m, 4H), 7.52 (d, J = 7.7 Hz, 1H), 7.42 (d, J = 8.4 Hz, 1H), 6.86 (s, 1H), 6.79 (d, J = 8.3 Hz, 1H), 5.55 (s, 2H), 5.24 (s, 2H), 4.17 (d, J = 11.7 Hz, 2H), 3.84 (s, 2H), 3.43 (s, 1H)。
2-((2-((3-氰基苯甲酰基)氧基)-4-(萘-1-基甲氧基)苄基)氨基)-3-羟基丁酸(A-8),白色固体,收率41.5%,mp 161℃~163℃。ESI-MS m/z: 495.2 [M-H]-。1H NMR (300 MHz, DMSO-d6) δ 8.08-8.05 (m, 1H), 7.98 (d, J = 9.3 Hz, 2H), 7.94 (d, J = 8.3 Hz, 1H), 7.88 (d, J = 7.9 Hz, 1H), 7.80 (d, J = 7.7 Hz, 1H), 7.66 (d, J = 6.8 Hz, 1H), 7.61-7.56 (m, 3H), 7.53-7.49 (m, 1H), 7.32 (d, J = 8.3 Hz, 1H), 6.81 (d, J = 2.2 Hz, 1H), 6.74 (d, J = 8.3 Hz, 1H), 5.53 (s, 2H), 5.22 (s, 2H), 3.91 (d, J = 6.3 Hz, 2H), 3.84 (d, J = 4.5 Hz, 1H), 3.81 (d, J = 6.1 Hz, 1H), 1.11 (d, J = 6.2 Hz, 3H)。
(2-((3-氰基苯甲酰基)氧基)-4-(萘-1-基甲氧基)苄基)-L-脯氨酸(A-9),白色固体,收率43.9%,mp 174℃~176℃。ESI-MS m/z: 491.2 [M-H]-。1H NMR (300 MHz, Chloroform-d) δ 8.02-7.98 (m, 1H), 7.94-7.88 (m, 2H), 7.72 (d, J = 7.8 Hz, 1H), 7.68 (s, 1H), 7.60 (s, 1H), 7.55 (dd, J = 4.0, 2.4 Hz, 2H), 7.51-7.45 (m, 2H), 7.39 (d, J = 8.3 Hz, 1H), 7.28 (s, 1H), 6.70 (dd, J = 8.3, 2.3 Hz, 1H), 6.57 (d, J = 2.3 Hz, 1H), 5.43 (s, 2H), 5.14 (s, 2H), 4.28 (s, 2H), 3.91 (d, J = 4.1 Hz, 1H), 3.58 (q, J = 3.8, 3.3 Hz, 1H), 2.94 (d, J = 9.5 Hz, 1H), 2.38-2.27 (m, 2H), 1.97 (dd, J = 10.1, 5.9 Hz, 2H)。
2.2.2. 目标化合物B-1~B-9的合成
分别将2-溴甲基萘(2.0 g,9.1 mmol)、2,4-二羟基苯甲醛(1.5 g,10.8 mmol)和碳酸氢钠(1.2 g,14.3 mmol)加入乙腈(30 mL)中,90℃回流反应12 h。加入水(100 mL),乙酸乙酯萃取(50 mL × 3),有机相用无水硫酸镁干燥,抽滤,滤液浓缩,柱层析分离(石油醚/乙酸乙酯,体积比15:1)得白色固体3 (1.4 g,收率55.6%)。1H NMR (300 MHz, DMSO-d6) δ 10.97 (s, 1H), 9.92 (s, 1H), 7.95-7.92 (m, 2H), 7.90 (s, 1H), 7.87 (s, 1H), 7.62 (d, J = 6.7 Hz, 1H), 7.55 (dd, J = 7.5, 1.7 Hz, 1H), 7.45 (d, J = 7.5 Hz, 2H), 6.65 (dd, J = 7.7, 2.1 Hz, 1H), 6.53 (d, J = 1.3 Hz, 1H), 5.35 (s, 2H)。
分别将3 (1.5 g,5.4 mmol)、碳酸钾(1.1 g,8.1 mmol)和3-氰基苄基溴(1.4 g,7.1 mmol)加入DMF (30 mL)中,室温反应6 h。加入水(150 mL),乙酸乙酯萃取(50 mL × 3),有机相用无水硫酸镁干燥,抽滤,滤液浓缩,柱层析分离(石油醚/乙酸乙酯,体积比 8:1)得白色固体4 (1.7 g,收率80.2%)。1H NMR (300 MHz, Chloroform-d) δ 10.39 (s, 1H), 7.93 (s, 1H), 7.90 (d, J = 2.2 Hz, 2H), 7.88 (s, 2H), 7.71 (s, 1H), 7.65 (d, J = 5.7 Hz, 1H), 7.62 (s, 1H), 7.56 (d, J = 3.7 Hz, 1H), 7.53 (dd, J = 3.8, 2.1 Hz, 2H), 7.51 (d, J = 2.6 Hz, 1H), 6.76 (dd, J = 8.8, 2.2 Hz, 1H), 6.59 (d, J = 2.2 Hz, 1H), 5.31 (s, 2H), 5.17 (s, 2H)。
3-((2-((1,3-二羟基-2-甲基丙烷-2-基)氨基)甲基)-5-(萘-2-基甲氧基)苯氧基)甲基)苯甲腈(B-1)的制备:将4 (0.2 g,0.5 mmol)溶于DMF(25 mL)中,随后依次加入2-氨基-2-甲基-1,3-丙二醇(0.1 g,1.0 mmol)、冰醋酸(0.06 g,1.0 mmol)和氰基硼氢化钠(0.1 g,1.6 mmol),室温反应24 h,加入水(50 mL),乙酸乙酯萃取(30 mL × 3),有机相用无水硫酸镁干燥,抽滤,滤液浓缩,柱层析分离(二氯甲烷/甲醇,体积比20:1),得0.15 g白色固体B-1,收率40.7%,mp 182℃~184℃。ESI-MS m/z: 481.2 [M-H]-。1H NMR (300 MHz, DMSO-d6) δ 8.24 (s, 1H), 8.02 (s, 1H), 7.98 (s, 1H), 7.97-7.93 (m, 2H), 7.92 (d, J = 4.9 Hz, 1H), 7.88 (d, J = 7.9 Hz, 1H), 7.82 (d, J = 7.7 Hz, 1H), 7.63-7.57 (m, 2H), 7.56 (q, J = 1.8 Hz, 1H), 7.54-7.53 (m, 1H), 7.38 (d, J = 8.4 Hz, 1H), 6.86 (d, J = 2.3 Hz, 1H), 6.74 (dd, J = 8.4, 2.4 Hz, 1H), 5.37 (s, 2H), 5.34 (s, 2H), 5.22 (s, 2H), 4.10 (s, 2H), 3.52 (s, 4H), 1.10 (s, 3H)。用类似的方法制备目标化合物B-2~B-9。
3-((2-((1,3-二羟基丙烷-2-基)氨基)甲基)-5-(萘-2-基甲氧基)苯氧基)甲基)苯甲腈(B-2),白色固体,收率56.0%,mp 163℃~165℃。ESI-MS m/z: 467.2 [M-H]-。1H NMR (300 MHz, Chloroform-d) δ 7.86 (d, J = 2.5 Hz, 1H), 7.84 (s, 1H), 7.82 (t, J = 2.8 Hz, 2H), 7.75 (s, 1H), 7.63 (d, J = 7.8 Hz, 1H), 7.56-7.53 (m, 1H), 7.49 (dd, J = 6.4, 2.9 Hz, 3H), 7.47-7.40 (m, 2H), 7.28 (s, 1H), 6.62 (dd, J = 8.3, 2.2 Hz, 1H), 6.56 (d, J = 2.3 Hz, 1H), 5.14 (s, 2H), 5.11 (s, 2H), 4.08 (s, 2H), 3.74 (d, J = 7.0 Hz, 2H), 3.69-3.58 (m, 2H), 2.96-2.90 (m, 1H)。
N-(2-((2-((3-氰基苯甲酰基)氧基)-4-(萘-2-基甲氧基)苄基)氨基)乙基)乙酰胺(B-3),白色固体,收率61.5%,mp 170℃~172℃。ESI-MS m/z: 478.2 [M-H]-。1H NMR (300 MHz, Chloroform-d) δ 7.87 (d, J = 3.5 Hz, 1H), 7.85 (d, J = 4.3 Hz, 2H), 7.83 (d, J = 2.8 Hz, 1H), 7.75 (d, J = 1.7 Hz, 1H), 7.68-7.64 (m, 1H), 7.60-7.56 (m, 1H), 7.53-7.50 (m, 2H), 7.50 (d, J = 2.2 Hz, 1H), 7.48-7.46 (m, 1H), 7.33 (d, J = 8.4 Hz, 1H), 7.28 (s, 1H), 6.63 (dd, J = 8.4, 2.3 Hz, 1H), 6.57 (d, J = 2.3 Hz, 1H), 5.16 (s, 4H), 4.07 (s, 2H), 3.49 (s, 1H), 3.45 (d, J = 4.9 Hz, 2H), 3.06 (t, J = 5.0 Hz, 2H), 1.93 (s, 3H)。
3-((2-((2-羟乙基)氨基)甲基)-5-(萘-2-基甲氧基)苯氧基)甲基)苯甲腈(B-4),白色固体,收率49.3%,mp 167℃~169℃。ESI-MS m/z: 437.2 [M-H]-。1H NMR (300 MHz, Chloroform-d) δ 7.86 (d, J = 2.8 Hz, 1H), 7.84 (d, J = 3.3 Hz, 1H), 7.82 (d, J = 2.3 Hz, 1H), 7.73 (d, J = 1.7 Hz, 1H), 7.67-7.64 (m, 1H), 7.54 (s, 1H), 7.51 (s, 1H), 7.50 (d, J = 3.0 Hz, 1H), 7.48 (d, J = 2.5 Hz, 1H), 7.46 (d, J = 1.8 Hz, 1H), 7.32 (d, J = 8.4 Hz, 1H), 7.28 (s, 1H), 6.62 (dd, J = 8.4, 2.3 Hz, 1H), 6.57 (d, J = 2.3 Hz, 1H), 5.16 (s, 2H), 5.15 (s, 2H), 4.10 (s, 2H), 3.71 (t, J = 4.8 Hz, 2H), 2.96 (t, J = 4.9 Hz, 2H)。
N-(2-((3-氰基苯)氧基)-4-(萘-2-基甲氧基)苄基)-N-甲基甘氨酸(B-5),白色固体,收率50.6%,mp 182℃~184℃。ESI-MS m/z: 465.2 [M-H]-。1H NMR (300 MHz, DMSO-d6) δ 7.96 (d, J = 4.4 Hz, 2H), 7.93-7.90 (m, 3H), 7.79 (dd, J = 12.1, 7.5 Hz, 2H), 7.58 (d, J = 2.9 Hz, 1H), 7.55 (s, 1H), 7.53-7.50 (m, 2H), 7.26 (d, J = 8.1 Hz, 1H), 6.77 (d, J = 6.4 Hz, 1H), 6.65 (d, J = 7.8 Hz, 1H), 5.25 (s, 2H), 5.21 (s, 2H), 3.57 (s, 2H), 3.40 (s, 2H), 2.17 (s, 3H)。
(S)-1-(2-((3-氰基苯)氧基)-4-(萘-2-甲氧基)苄基)哌啶-2-羧酸(B-6),白色固体,收率46.6%,mp 161℃~163℃。ESI-MS m/z: 505.2 [M-H]-。1H NMR (300 MHz, DMSO-d6) δ 7.98 (s, 1H), 7.94 (q, J = 6.8, 5.9 Hz, 4H), 7.81 (dd, J = 12.9, 7.8 Hz, 2H), 7.59 (s, 1H), 7.56 (s, 1H), 7.56-7.52 (m, 2H), 7.33 (d, J = 8.3 Hz, 1H), 6.79 (d, J = 2.4 Hz, 1H), 6.70 (dd, J = 8.5, 2.3 Hz, 1H), 5.26 (s, 2H), 5.22 (s, 2H), 3.90 (s, 2H), 3.75 (s, 1H), 3.11 (dd, J = 8.3, 4.2 Hz, 1H), 2.99-2.93 (m, 1H), 2.39-2.31 (m, 1H), 1.80 (s, 1H), 1.73 (t, J = 8.1 Hz, 1H), 1.34 (s, 1H), 1.24 (d, J = 6.1 Hz, 1H), 1.16 (dt, J = 13.9, 6.9 Hz, 1H)。
(2-((3-氰基苯甲酰基)氧基)-4-(萘-1-基甲氧基)苄基)-L-丝氨酸(B-7),白色固体,收率53.0%,mp 163℃~165℃。ESI-MS m/z: 481.2 [M-H]-。1H NMR (300 MHz, DMSO-d6) δ 8.01-7.95 (m, 3H), 7.91 (d, J = 8.4 Hz, 3H), 7.79 (d, J = 7.5 Hz, 1H), 7.58 (s, 1H), 7.57-7.50 (m, 3H), 7.36 (d, J = 8.3 Hz, 1H), 6.83 (d, J = 2.2 Hz, 1H), 6.75-6.68 (m, 1H), 5.29 (s, 2H), 5.24 (s, 2H), 4.09 (s, 2H), 3.77 (d, J = 3.9 Hz, 2H), 3.70 (t, J = 6.1 Hz, 1H)。
(2S)-2-((2-((3-氰基苯甲酰基)氧基)-4-(萘-2-基甲氧基)苄基)氨基)-3-羟基丁酸(B-8),白色固体,收率58.1%,mp 173℃~175℃。ESI-MS m/z: 495.2 [M-H]-。1H NMR (300 MHz, DMSO-d6) δ 8.00 (s, 1H), 7.97 (s, 1H), 7.95 (d, J = 4.5 Hz, 1H), 7.93 (s, 2H), 7.88 (d, J = 8.2 Hz, 1H), 7.80 (d, J = 7.6 Hz, 1H), 7.61-7.57 (m, 2H), 7.54 (q, J = 3.5, 3.0 Hz, 3H), 7.35 (d, J = 8.4 Hz, 1H), 6.82 (d, J = 2.3 Hz, 1H), 6.71 (dd, J = 8.3, 2.3 Hz, 1H), 5.28 (s, 2H), 5.24 (s, 2H), 5.20 (s, 1H), 4.03 (d, J = 14.7 Hz, 2H), 3.91 (d, J = 6.2 Hz, 1H), 3.01 (d, J = 5.7 Hz, 1H), 1.15 (d, J = 6.2 Hz, 3H)。
(2-((3-氰基苯甲酰基)氧基)-4-(萘-1-基甲氧基)苄基)-L-脯氨酸(B-9),白色固体,收率47.9%,mp 165℃~167℃。ESI-MS m/z: 491.2 [M-H]-。1H NMR (300 MHz, Chloroform-d) δ 7.87 (d, J = 3.1 Hz, 1H), 7.84 (q, J = 3.5, 2.9 Hz, 2H), 7.82 (d, J = 2.4 Hz, 1H), 7.72-7.67 (m, 2H), 7.60-7.56 (m, 1H), 7.50 (dt, J = 6.1, 3.0 Hz, 3H), 7.48-7.46 (m, 1H), 7.32 (d, J = 8.4 Hz, 1H), 6.62 (dd, J = 8.3, 2.3 Hz, 1H), 6.57 (d, J = 2.3 Hz, 1H), 5.16 (s, 4H), 4.26 (d, J = 5.9 Hz, 2H), 3.92 (dd, J = 8.7, 5.3 Hz, 1H), 3.57 (dt, J = 11.2, 5.5 Hz, 1H), 2.97-2.92 (m, 1H), 2.35-2.26 (m, 2H), 1.98-1.90 (m, 2H)。
3. 生物活性评价
采用均相时间分辨荧光法 [12] 评价目标化合物对PD-1/PD-L1的抑制活性。PD-1/PD-L1结合分析试剂盒(641CP01PEG)购自Cisbio。将化合物用DMSO配置成所设置的浓度,加入稀释缓冲液,混合均匀后取2 μL加到384孔白色酶标板中。将PD-1和PD-L1用缓冲液稀释,分别取4 μL加到上述384孔板中,孵育15 min。将10 μL Anti-tag1-Eu和Anti-tag2-XL665混合液加入检测缓冲溶液中,混合均匀后加入上述384孔板中,然后在室温条件下孵育2 h。用Infinite® M1000多功能酶标仪检测665 nm和620 nm处荧光信号,根据荧光比值计算化合物对蛋白结合的抑制率。实验结果如表1所示。
Table 1. Inhibitory activity of the target compounds A-1~A-9 and B-1~B-9 against the PD-1/PD-L1 interaction
表1. 目标化合物A-1~A-9和B-1~B-9对PD-1/PD-L1相互作用的抑制活性
BMS-1018: Positive control compound.
4. 结果与讨论
本文合成了18个未见文献报道的萘环类目标化合物,结构均经1H-NMR和ESI-MS谱确证。从表1的活性数据可以看出,所有的目标化合物在10 μmol/L浓度下对PD-1/PD-L1相互作用均显示出不同程度的抑制活性,其中6个化合物(A-8、A-9和B-5、B-6、B-8、B-9)的活性相对突出。
从表1的数据可以获得以下构效关系规律:1) 从整体来看,B系列化合物的整体活性优于A系列(A-1 vs B-1、A-5 vs B-5和A-6 vs B-6);2) 在A系列化合物中,伸入溶剂区的末端取代基为氨基酸时(A-6、A-7、A-8和A-9)活性优于氨基醇类化合物(A-1和A-3);3) 末端取代基含有较多极性基团如羟基(A-3)时活性优于含有非极性基团如呋喃环(A-2);4) 相似地,B系列化合物末端取代基为氨基酸时(B-5、B-6、B-8和B-9)活性也普遍优于氨基醇类化合物(B-1、B-2和B-4);5) 在两个系列目标化合物中,乙酰基乙二胺衍生物的活性均较弱(A-4和B-3),但肌氨酸衍生物则显示A-5弱而B-5强,此外,苏氨酸和脯氨酸的衍生物均显示出最强的活性(A-8、A-9和B-8、B-9)。
为了进一步说明目标化合物的构效关系,本文作者通过Discovery Studio 4.0中的CDOCKER模块,选取了活性最强的A-8和B-8进行分子对接实验,分析了小分子与PD-L1蛋白的结合模式。从图5可以看出,化合物A-8和B-8均处于PD-L1蛋白二聚体构成的夹缝中。从图5(A)可知,A-8和B-8与靶蛋白的结合模式与BMS-1018相似。具体来说,两者的萘环与BMS-1018的联苯片段朝向一致,而且中心苯环的3位间氰基苄氧基的朝向也一致。此外,两者的a环都能与BMet115形成σ-π相互作用,而中心苯环则与BTyr56形成π-π堆积作用。进一步地,A-8的氰基氮原子可以与AArg125形成氢键作用,其末端苏氨酸可以与AAla18、AAsp122、ALys124形成三个氢键。而B-8末端苏氨酸残基则可以与AAla18、APhe19、AAsp122和BGln66形成四个氢键相互作用,这可能是B系列化合物的活性普遍优于A系列的原因。上述构效关系信息为后续开展结构优化提供了理论指导。
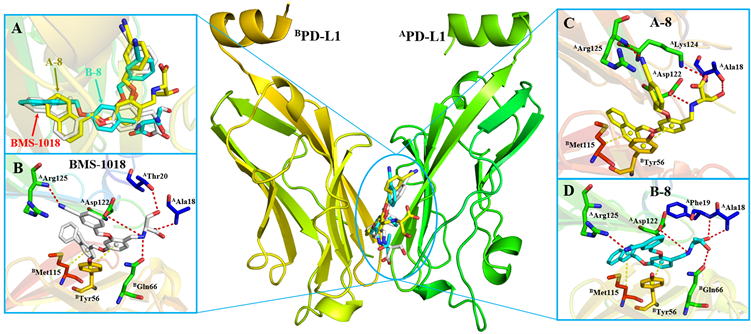
Figure 5. Proposed binding mode of BMS-1018, A-8 and B-8 (PDB ID: 5J89)
图5. BMS-1018、A-8、B-8与靶蛋白的结合模式
基金项目
国家自然科学基金项目(21977117)。
文章引用
赵 磊,余龙波,欧阳宜强,郭文洁,徐 强,高 健,赖宜生. 萘环类PD-1/PD-L1抑制剂的设计、合成及生物活性评价
Design, Synthesis and Biological Evaluation of Naphthalene Ring PD-1/PD-L1 Inhibitors[J]. 药物化学, 2021, 09(02): 94-103. https://doi.org/10.12677/HJMCe.2021.92012
参考文献
- 1. Chen, L. and Flies, D.B. (2013) Molecular Mechanisms of T Cell Co-Stimulation and Co-Inhibition. Nature Reviews Immunology, 13, 227-242. https://doi.org/10.1038/nri3405
- 2. Mohme, M., Riethdorf, S. and Pantel, K. (2017) Circulating and Disseminated Tumour Cells-Mechanisms of Immune Surveillance and Escape. Nature Reviews Clinical Oncology, 14, 155-167. https://doi.org/10.1038/nrclinonc.2016.144
- 3. Lin, X., Lu, X., Luo, G. and Xiang, H. (2020) Progress in PD-1/PD-L1 Pathway Inhibitors: From Biomacromolecules to Small Molecules. European Journal of Medicinal Chemistry, 186, 111876-111905. https://doi.org/10.1016/j.ejmech.2019.111876
- 4. Li, X., Shao, C., Shi, Y. and Han, W. (2018) Lessons Learned from the Blockade of Immune Checkpoints in Cancer Immunotherapy. Journal of Hematology & Oncology, 11, 31-56. https://doi.org/10.1186/s13045-018-0578-4
- 5. van der Zanden, S.Y., Luimstra, J.J., Neefjes, J., Borst, J. and Ovaa, H. (2020) Opportunities for Small Molecules in Cancer Immunotherapy. Trends in Immunology, 41, 493-511. https://doi.org/10.1016/j.it.2020.04.004
- 6. Chupak, L.S. and Zheng, X. (2015) Compounds Useful as Immuno-modulators. WO2015034820 A1.
- 7. Chupak, L.S., Ding, M., Martin, S.W., Zheng, X., Hewawasam, P., Connolly, T.P., Xu, N., Yeung, K., Zhu, J. and Langley, D.R. (2015) Compounds Useful as Immunomodulators. WO2015160641 A1.
- 8. Skalniak, L., Zak, K.M., Guzik, K., Magiera, K., Musielak, B., Pachota, M., Szelazek, B., Kocik, J., Grudnik, P., Tomala, M., Krzanik, S., Pyrc, K., Dömling, A., Dubin, G. and Holak, T.A. (2017) Small-Molecule Inhibitors of PD-1/PD-L1 Immune Checkpoint Alleviate the PD-L1-Induced Exhaustion of T-Cells. Oncotarget, 8, 72167-72181. https://doi.org/10.18632/oncotarget.20050
- 9. Zak, K.M., Grudnik, P., Guzik, K., Zieba, B.J., Musielak, B., Döm-ling, A., Dubin, G. and Holak, T.A. (2016) Structural Basis for Small Molecule Targeting of the Programmed Death Ligand 1 (PD-L1). Oncotarget, 7, 30323-30335. https://doi.org/10.18632/oncotarget.8730
- 10. Guzik, K., Zak, K.M., Grudnik, P., Magiera, K., Musielak, B., Törner, R., Skalniak, L., Dömling, A., Dubin, G. and Holak, T.A. (2017) Small-Molecule Inhibitors of the Programmed Cell Death-1/Programmed Death-Ligand 1 (PD-1/PD-L1) Interaction via Transiently Induced Protein States and Dimeri-zation of PD-L1. Journal of Medicinal Chemistry, 60, 5857-5867. https://doi.org/10.1021/acs.jmedchem.7b00293
- 11. Qin, M., Cao, Q., Wu, X., Liu, C., Zheng, S., Xie, H., Tian, Y., Xie, J., Zhao, Y., Hou, Y., Zhang, X., Xu, B., Zhang, H. and Wang, X. (2020) Discovery of the Programmed Cell Death-1/Programmed Cell Death-Ligand 1 Interaction Inhibitors Bearing an Indoline Scaffold. European Journal of Me-dicinal Chemistry, 186, 111856-111868. https://doi.org/10.1016/j.ejmech.2019.111856
- 12. Cheng, B., Ren, Y., Niu, X., Wang, W., Wang, S., Tu, Y., Liu, S., Wang, J., Yang, D., Liao, G. and Chen, J. (2020) Discovery of Novel Resorcinol Dibenzyl Ethers Targeting the Pro-grammed Cell Death-1/Programmed Cell Death-Ligand 1 Interaction as Potential Anticancer Agents. Journal of Medici-nal Chemistry, 63, 8338-8358. https://doi.org/10.1021/acs.jmedchem.0c00574
NOTES
*通讯作者。
