Material Sciences
Vol.
12
No.
06
(
2022
), Article ID:
53009
,
11
pages
10.12677/MS.2022.126069
不同孔隙率钽涂层钛合金材料对细胞成骨的 影响
李学谦,赵仁良,傅绍菱,王诚,陈城,施忠民,邹剑*
上海交通大学附属第六人民医院骨科,上海
收稿日期:2022年5月8日;录用日期:2022年6月22日;发布日期:2022年6月29日

摘要
目的:研究不同孔隙率钽涂层钛合金材料对SD大鼠原代骨髓间充质干细胞(rBMSC)黏附、增殖及成骨分化的影响并进行对比。方法:3D打印60%、70%和80%三种不同孔隙率的钛合金支架5 × 2 mm,并完成钽涂层的覆盖。在体外提取rBMSC进行原代培养至第三代,各组分别进行细胞黏附、CCK8技术、碱性磷酸酶(ALP)定量检测、茜素红S染色、活死细胞染色及实时定量聚合酶链反应(qRT-PCR)检测成骨基因实验包括Runx-2、Osterix和ALP基因,并以纯钽(孔隙率为0)作为阴性对照,各组间进行对比。结果:细胞黏附实验中60%、70%和80%三种不同孔隙率的钛合金支架黏附性能优于纯钽;CCK8法检测细胞活性在第1、3天各组间无明显差异,第5天80%孔隙率钽涂层钛合金支架的增殖活性最高;第7天检测细胞ALP活性可见80%孔隙率钽涂层钛合金支架的明显高于其他三组;细胞培养第14、21天茜素红S染色80%孔隙率钽涂层钛合金支架均明显高于其他三组;细胞培养第1、3、5天活死细胞染色80%孔隙率钽涂层钛合金支架活细胞数明显增多,死细胞数减少,而其他三组活细胞、死细胞数均增多;qRT-PCR检测Runx-2、Osterix和ALP基因均在80%孔隙率钽涂层钛合金支架组表达最高。结论:相对于纯钽、60%和70%孔隙率钽涂层钛合金支架,80%孔隙率钽涂层钛合金支架能更好地促进rBMSC增殖、黏附和成骨基因诱导。
关键词
孔隙率,钽,涂层

Effect of Tantalum-Coated Titanium Alloy with Different Porosities on Cell Osteogenesis
Xueqian Li, Renliang Zhao, Shaoling Fu, Cheng Wang, Cheng Chen, Zhongmin Shi, Jian Zou*
Department of Orthopedics, The Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University, Shanghai
Received: May 8th, 2022; accepted: Jun. 22nd, 2022; published: Jun. 29th, 2022

ABSTRACT
Objective: To study and compare the effects of different porosities tantalum-coated titanium alloy materials on the adhesion, proliferation and osteogenesis differentiation of SD rats’ primary bone marrow mesenchymal stem cells (rBMSCs) in vitro. Methods: 3D printing 5 × 2 mm pure tantalum scaffolds and 60%, 70% and 80% porosities titanium alloy scaffolds coating tantalum coverage by chemical vapor deposition technology (CVD). In vitro, rBMSCs were extracted for primary culture to the third generation, and each group was subjected to cell adhesion, CCK8 technology, alkaline phosphatase (ALP) quantitative detection, alizarin red S staining, live and dead cell staining, and real-time quantitative polymerase chain reaction (qRT-PCR) detection of osteogenetic genes including Runx-2, Osterix and ALP genes. Pure tantalum (the porosity was zero) was regarded as a negative control, and comparisons were made between groups. Results: In cell adhesion experiments, the adhesion performance of tantalum-coated titanium alloy scaffolds with different porosities of 60%, 70% and 80% was better than that of pure tantalum. The CCK8 method detected no significant differences between the groups on days 1 and 3, and the proliferative activity of 80% porosity tantalum-coated titanium alloy scaffolds on day 5 was the highest, and the detection of cell ALP activity on day 7 showed that the 80% porosity tantalum-coated titanium alloy scaffolds were significantly higher than those of the other three groups. On days 14 and 21 of cell culture, alizarin red S stained revealed that 80% porosity tantalum-coated titanium alloy scaffolds were significantly higher than those of the other three groups. On the 1st, 3rd and 5th days of cell culture, the number of live cells stained with 80% porosity tantalum-coated titanium alloy scaffold increased significantly, and the number of dead cells decreased, while the other three groups of living cells and dead cells both increased. qRT-PCR showed that Runx-2, Osterix and ALP gene expression were all highest in 80% porosity tantalum-coated titanium alloy scaffolds. Conclusion: Compared with pure tantalum, 60% and 70% porosities tantalum-coated titanium alloy scaffolds, 80% porosity tantalum-coated titanium alloy scaffolds can better promote rBMSC proliferation, adhesion and osteogenetic gene induction.
Keywords:Porosity, Tantalum, Coating

Copyright © 2022 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
目前,由于创伤、炎症、骨肿瘤手术等原因造成的骨缺损仍然是治疗难题,尤其是负重部位的骨缺损。自体骨移植或同种异体骨移植是处理骨缺损的金标准。与此同时,金属合金材料也越来越多地应用于临床。其中,金属钛及其合金由于较好的生物相容性、高强度等成为近年来研究热点 [1] [2]。随着钛合金3D打印技术的逐步成熟及在医学上的研究与应用,3D打印的多孔钛合金骨填充材料逐渐取代了传统致密的钛合金制品。但是钛及其合金的耐腐蚀性较差,这会延迟组织的愈合,此外由于与人骨的弹性模量和质量难以匹配等问题,限制了其临床效能 [3] [4] [5]。
钽金属因其生物相容性和物理特性上的优势逐渐被发觉,其硬度大、抗磨损性能强、理化性质稳定,并且在一定程度上可以降低手术感染的风险,刺激细胞增殖,提高成骨细胞的成骨能力,植入人体后有利于新生骨组织的长入,增强与骨组织的连结能力,促进骨组织的再生和重建,增加植入器械的长期生物稳定性 [6] [7] [8]。但纯钽支架作为骨缺损填充物成本高,价格昂贵,且其生物理化性能尚值得研究,因此是否可以在钛合金表面涂钽,将两者的优点结合,成为了目前研究的一个热点。
本研究拟通过体外实验观察不同孔隙率钽涂层钛合金材料对于成骨诱导的差异,通过细胞实验发现对于骨生成最佳的孔隙率的钽涂层钛合金支架,为临床骨缺损的修复重建提供一种有效的材料。
2. 材料与方法
2.1. 钽涂层钛合金材料的制备
通过选择性激光烧结法(SLM技术) [9] 制备孔隙率分别为60%、70%和80%的多孔钛合金支架,直径5 mm,厚2 mm (上海昕健医疗技术有限公司,上海)。再通过化学气相沉积技术完成钽涂层的覆盖(东方钽业,宁夏) [10]。
2.2. 分组
本实验分为四组,一组为纯钽组,孔隙率为0,另三组分别为孔隙率60%、70%和80%的多孔钽涂层钛合金支架。
2.3. 细胞培养
按照文献汇报的方法 [11] 提取SD大鼠原代骨髓间充质干细胞(rat Bone Marrow Mesenchymal Stem cells, rBMSC),P3代用于细胞实验。常规脱水、干燥后行扫描电子显微镜(SEM,S-3400N,HITACHI,日本)成像。本研究已获得上海市第六人民医院动物实验室伦理批准,编号DWSY2020-233。
2.4. CCK8法检测细胞增殖
采用CCK-8试剂盒(Cell Counting Kit-8,北京索莱宝科技有限公司)对不同孔隙率支架进行细胞增殖活性检测。
2.5. 碱性磷酸酶活性测量
在细胞培养第7天使用P0321试剂盒(上海碧云天生物技术有限公司)检测rBMSC的ALP活性。
2.6. rBMSCs细胞的茜素红S染色
细胞培养第14天和21天,进行茜素红S染色。
2.7. 活死细胞染色
细胞培养第1、3、7天用活细胞/死细胞双染试剂盒(Calcein AM/PI Double Stain Kit,上海懋康生物科技有限公司)进行染色。
2.8. 统计学处理
采用SPSS 26.0统计学软件以及Graphpad prism 9软件,应用Shapiro-Wilk法进行正态性检验,符合正态分布的计量资料以χ ± sd描述,不符合正态分布的计量资料以M (Q1, Q3)表示,组间比较通过单因素方差分析(One Way ANOVA)分析,以P < 0.05表示差异有统计学意义。
3. 结果
3.1. 电子显微镜扫描图像
四组材料扫描电镜图像见图1。可见孔隙率越高,材料内部支架越细,正方形孔径越大。
 (a)
(a)
 (b)
(b)
 (c)
(c)
 (d)
(d)
Figure 1. SEM of four sets of different porosity scaffolds materials: (a) Pure tantalum; (b) 60% porosity; (c) 70% porosity; (d) 80% porosity
图1. 四组不同孔隙率支架材料SEM图:(a) 纯钽;(b) 孔隙率60%;(c) 孔隙率70%;(d) 孔隙率80%
3.2. 细胞形态和分布
细胞形态及在多孔钽涂层钛合金支架的分布如图2所示,可见细胞分布在支架表面,在80%、70%、60%孔隙率的支架表面细胞伸展更加充分,细胞伸出伪足,分泌的细胞外基质包绕于细胞周围,黏附性能较好,纯钽表面细胞分布欠佳。
 (a)
(a)
 (b)
(b)
 (c)
(c)
 (d)
(d)
 (e)
(e)
 (f)
(f)
 (g)
(g)
 (h)
(h)
Figure 2. Observation of cell adhesion of pure tantalum and 60%, 70% and 80% porous tantalum-coated titanium scaffolds by SLM: (a) Pure tantalum imaging; (b) 60% porosity imaging; (c) 70% porosity imaging; (d) 80% porosity imaging; (e) Gold-jet manipulation SLM imaging PS software conduction after cell coloration pure tantalum group cell morphology; (f) 60% porosity scaffolds cell color rendering; (g) 70% porosity scaffolds cell color rendering; (h) 80% porosity scaffolds cell color rendering. It can be seen that the cells stretch well
图2. 激光共聚焦电子显微镜观察纯钽和60%、70%和80%的多孔钽涂层钛合金支架细胞黏附:(a) 纯钽细胞黏附SLM成像;(b) 60%支架细胞黏附SLM成像;(c) 70%支架细胞黏附SLM成像;(d) 80%支架细胞黏附SLM成像;(e) 喷金操作SLM成像PS软件处理后细胞显色纯钽组细胞形态;(f) 60%孔隙率组细胞显色;(g) 70%支架细胞显色;(h) 80%支架细胞显色,可见细胞伸展较好
3.3. 活死细胞染色
细胞培养第1、3、5天分别通过活死细胞染色,结果如图3。第1、3、5天各组活细胞均明显增多,其中纯钽组和60%支架组死细胞第3天与第1天相比均明显增多,第5天比第3天增多不明显;70%组第3天比第1天死细胞明显增多,第5天比第3天明显增多;而80%组第1、3天死细胞略增多,第5天则明显减少。在第1天,纯钽死细胞数与60%组接近,而70%和80%组略增加;第3天纯钽、60%、70%、80%孔隙率组死细胞数依次明显减少;第5天亦然。
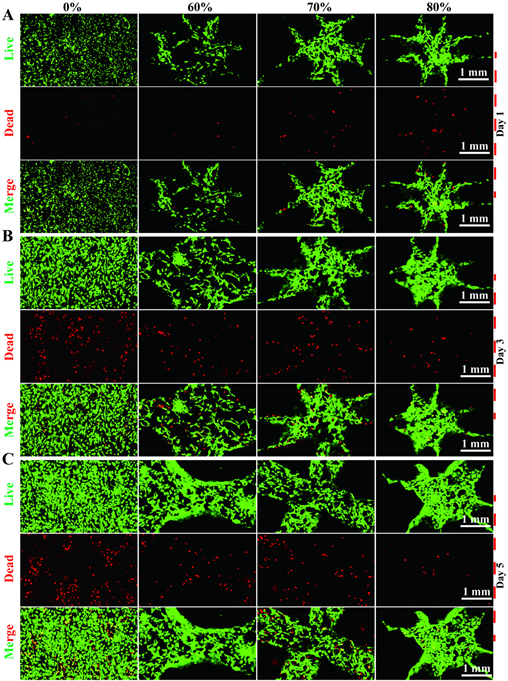
Figure 3. On the 1st, 3rd, and 5th days of cell culture, the number of living cells in each group increased, while on the 5th day, the number of dead cells in the 80% porosity group decreased, but the number of dead cells in other groups increased significantly
图3. 细胞培养第1、3、5天各组活细胞数增多,而第5天可见80%孔隙率组死细胞数减少,其他组死细胞数明显增多
3.4. CCK8法检测细胞增殖
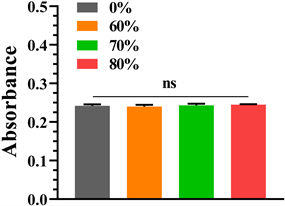
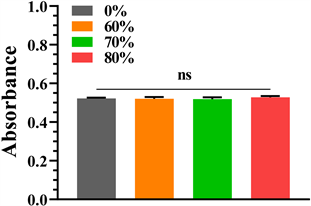
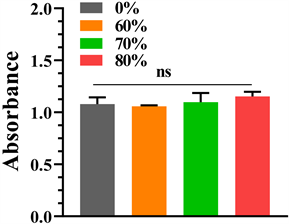
细胞培养第1、3、5天分别通过CCK8法检测细胞增殖活性,检测结果如图4所示,可见各组第1、3、5天细胞增殖活性均增加,第1天各组增殖活性相近,各组间无统计学意义(P < 0.05,表1);第3天各组细胞增殖活性相近,各组间无统计学意义(P < 0.05);第5天可见80%孔隙率钽涂层钛合金支架增殖活性最高,其次为70%、纯钽,60%孔隙率组略低于纯钽,但各组间无统计学意义(P < 0.05)。
3.5. ALP活性
细胞培养第7天检测各组ALP活性,结果如图5,可见纯钽ALP活性最低,60%孔隙率的支架ALP活性略高于纯钽,70%孔隙率的支架略高于60%,三组之间差异无统计学意义(P > 0.05,表2);而80%孔隙率的支架ALP活性明显高于其他三组,差异有统计学意义(P < 0.05)。
 (a)
(a)
 (b)
(b)
 (c)
(c)
Figure 4. Cell proliferative activity was detected by CCK8 method on days 1, 3, and 5 of cell culture, respectively. (a) The 1st day; (b) The 3rd day; (c) The 5th day
图4. 细胞培养第1、3、5天分别通过CCK8法检测细胞增殖活性:(a) 细胞培养第1天;(b) 细胞培养第3天;(c) 细胞培养第5天
Table 1. The CCK8 method detected the P values between each group of the absorbance at a wavelength of 450 nm on days 1, 3, and 5 of cell culture
表1. CCK8法检测细胞增殖细胞培养第1、3、5天在450nm波长处的吸光度各组间P值
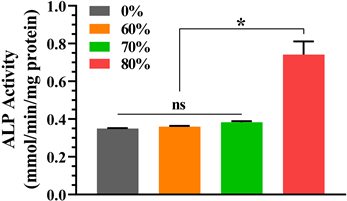
Figure 5. The comparison of ALP activity of pure tantalum and 60%, 70% and 80% porous tantalum-coated titanium alloy scaffolds
图5. 纯钽和60%、70%和80%的多孔钽涂层钛合金支架ALP活性比较
Table 2. The ALP activity on day 7 of cell culture was quantitatively detected between the groups
表2. 细胞培养第7天碱性磷酸酶活性定量检测各组间P值
3.6. ARS活性
分别于第14天、21天检测各种金属支架ARS活性,结果如图6。第14天纯钽的ARS活性最低,80%组明显高于其他三组,差异有统计学意义(P < 0.05,表3),其次为70%孔隙率组,差异有统计学意义(P < 0.05),而60%孔隙率组虽然高于纯钽,但差异无统计学意义(P > 0.05);第21天时纯钽和60%、70%和80%的多孔钽涂层钛合金支架ARS活性依次增高,且第21天时各组ARS活性均明显高于第14天,各组差异均有统计学意义(P均 > 0.05)。

Figure 6. The comparison of ARS activity of pure tantalum and 60%, 70% and 80% porous tantalum-coated titanium alloy scaffolds
图6. 纯钽和60%、70%和80%的多孔钽涂层钛合金支架ARS活性比较
Table 3. On days 14 and 21 of cell culture, the P value of each group of ARS staining was semi-quantitatively detected
表3. 细胞培养第14、21天茜素红S染色半定量检测各组间P值
3.7. qRT-PCR
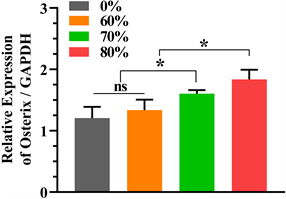
细胞培养第7天检测纯钽和60%、70%和80%的多孔钽涂层钛合金支架qRT-PCR,结果如图7所示。可见Osterix和ALP与GAPDH的比值均在80%孔隙率组中表达最高,差异有统计学意义(P < 0.05,表4),70%、60%和纯钽依次减低,而在Runx-2/GAPDH中80%略高于70%孔隙率组,两组之间差异无统计学意义(P > 0.05),上述两组表达高于60%孔隙率组和纯钽组,差异有统计学意义(P < 0.05)。
4. 讨论
由于各类原因造成的骨缺损仍然是一个棘手的问题。小面积的缺损可以采用自体骨或是异体骨移植,而大面积的骨缺损则较难处理。尽管目前3D打印的各类金属支架材料已经在临床上得到普遍运用,但在金属材料的选择上仍存在不少争议。在各类金属支架中,以钛、钽及其合金目前应用较多,他们具有高抗压性、高力学强度、高延展性和抗疲劳性,可用于负重部位的骨缺损 [12]。然而,钛金属及合金虽然能加速细胞的黏附、增殖与分化,但其耐腐蚀性能较差,释放到体液中的金属离子会对周围组织产生一定的毒副作用,会延迟组织的愈合,并且难以与人骨的弹性模量和质量匹配,这些问题限制了其应用 [3] [4] [5]。而钽金属的理化性质比较稳定,抗腐蚀性较强,其表面会形成一层氧化膜保护基体免受侵蚀,并防止钽离子发生电化学反应 [13];此外,钽金属表面容易发生细胞黏附与增殖,生物亲和性比较好 [14];此外还有研究发现钽的抗菌性能相比于钛更好 [15] 等,这些观点提示对于钽金属能更好地应用于骨缺损。但是钽金属价格比较昂贵,会增加患者的经济负担,且熔点高,难以加工等,这些方面限制了其应用。
 (a)
(a)
 (b)
(b)
 (c)
(c)
Figure 7. qRT-PCR gene expression data: (a) Osterix/GAPDH; (b) Runx-2/GAPDH; (c) ALP/GAPDH
图7. qRT-PCR基因表达数据:(a) Osterix/GAPDH;(b) Runx-2/GAPDH;(c) ALP/GAPDH
Table 4. QRT-PCR on the 7th day of cell culture to detect osteogenetic gene Osterix, Runx2, ALP expression by Gapdh correction of the P values between each group
表4. 细胞培养第7天qRT-PCR法检测成骨基因Osterix、Runx2、ALP表达通过Gapdh校正各组间P值
金属支架作为骨缺损的材料应在足够抗压强度与良好的生物相容性的同时允许细胞黏附与增殖,因此金属支架具有一定的孔隙率和孔径对于细胞张入是有必要的,有研究表明较高孔隙率(75%~85%)的金属支架率显著降低了多孔钽材料的密度,同时也进一步降低了弹性模量,多孔钽的弹性模量为1.3~10 GPa,与人骨的弹性模量(0.4~20.0 GPa)相似 [16]。此外有研究表明孔隙率为75%的钽金属支架在体外和体内实验中都表现出更好的生物力学性能 [17],因此本研究拟设计孔隙率分别为60%、70%和80%的钽金属支架,同时以孔隙率为0的纯钽块作为阴性对照,分别研究其生物学特性。
大鼠骨髓间充质干细胞(rBMSCs)是一种多能干细胞,具有分化为成骨细胞、软骨细胞、脂肪细胞及其他组织细胞的潜力,且相对容易被分离出来用于其它研究,为骨组织工程提供了良好的骨祖细胞来源 [18]。Sagomonyants等 [13] 研究发现成骨细胞在黏附在钽上的细胞数量明显多于钛网和塑料培养基(多25%~30%);对年轻和老年患者的细胞增殖测定显示钽组分别比钛网组增长3.7和5.9倍,比塑料组分别提高11.6和16.4倍。本研究将细胞培养第3代rBMSCs与各组钽金属支架共培养,通过喷金造作图片处理,结果显示除纯钽支架组,其他各组细胞明显增多且分泌细胞外基质,广泛分布于支架内外表面,形态变扁呈梭形,细胞伸出丝状,伸展性比较好。
细胞的增殖活性与毒性是判断生物材料的一个重要方面。本研究在细胞培养第1,3,5天分别通过CCK8法检测细胞增殖活性发现细胞增殖活性随时间的延长而升高,虽然在第5天80%孔隙率组钽金属支架的增殖活性高于其他组,但各组间无统计学意义。这与Luo [17] 的研究结果不同,Luo发现在培养第7天时,以75%和85%孔隙率支架细胞增殖活性显著高于25%和55%孔隙率组,其中以75%孔隙率支架组在450 nm波长处OD值最强。究其原因,可能是本研究细胞培养时间较短,虽然80%孔隙率组在第5天开始增殖活性开始高于其他三组,但由于细胞培养时间不够所以各组尚无统计学意义,随培养时间的延长可能细胞增殖的差异更加明显。此外,本研究通过活死细胞染色发现细胞培养第5天80%孔隙率钽金属支架组相比于其他组死细胞术更少,提示生物相容性更好。
不同孔隙率钽金属支架诱导细胞成骨分化能力主要通过碱性磷酸酶(ALP)活性检测及茜素红S (ARS)半定量分析来评价 [19]。Luo等 [17] 在成骨诱导第5天半定量检测25%、55%、75%和85%四组孔隙率钽金属支架的ALP活性发现各种ALP活性水平都比较高,而各组间无统计学意义;第21天ARS半定量检测中发现75%孔隙率活性最高,其次85%,两组间无统计学差异,与其他两组有统计学差异。本研究在成骨诱导第7天检测ALP活性发现80%孔隙率组明显高于60%、70%孔隙率组和纯钽组,差异有统计学意义;第14天和21天ARS半定量检测80%孔隙率组高于其他三组,差异均有统计学意义。由此推断,孔隙率为80%的钽金属支架在早期成骨诱导的早期作用更强,能水解磷酸酯,为羟基磷灰石的沉积提供必要的磷酸,同时水解焦磷酸盐,解除其对骨盐形成的抑制作用,更有利于成骨作用。
在成骨相关基因表达方面,本研究通过qRT-PCR测定ALP、Runx-2和Osterix三种因子,同时以GAPDH mRNA作为对照组,获得Ct值进行数据分析。Runx-2是细胞成骨分化和成熟的关键转录因子之一,主要调控细胞分裂周期并协同其它转录因子发挥作用 [20]。Osterix (Osx)是一种成骨细胞特异性转录因子,在骨骼的生长和动态平衡中发挥重要的调控作用 [21] [22]。Lu等 [23] 在骨质疏松模型下研究钽与钛成骨基因发现与钛组相比,钽组的RUNX2和ALP表达水平均占有明显的优势,约为钛组的1.2~1.8倍。在Luo等 [17] 的研究中发现在成骨诱导第7天ALP在75%和85%孔隙率支架组表达高于25%和55%孔隙率组,差异有统计学意义,而75%和85%两组之间无统计学意义,而本研究中ALP在80%孔隙率支架组表达最高,差异有统计学意义,与本研究结论相似;RUNX2在成骨诱导第7天在75%孔隙率支架组最高,但差异无统计学意义,而本研究中Runx-2在80%和70%孔隙率支架组表达最高,差异有统计学意义,虽然80%孔隙率支架组更高,但两组间差异无统计学意义。此外,本研究发现Osterix在80%孔隙率支架组表达最高,差异有统计学意义。综上,我们认为高孔隙率(80%)钽金属支架在成骨相关基因表达方面作用更有优势。
本研究尚有不足之处,首先,本研究为体外细胞实验,缺乏动物实验进一步验证材料的安全性及生物活性;其次,本研究未对成骨的细胞机制做出研究,需要进一步研究探索;最后,本研究初步得出80%孔隙率金属支架在细胞成骨方面活性最强的结论,可进一步细化支架的孔隙率探讨最优的孔隙率以达到实际应用于临床的目的。
5. 结论
80%孔隙率的钽金属支架相比于70%、60%孔隙率和纯钽支架在诱导细胞成骨和成骨相关基因的表达方面表现出更强的优势;各组之间细胞增殖活性未发现明显差异,但是80%孔隙率钽金属支架死细胞数相比于其他三组更少,而80%、70%、60%孔隙率的钽金属支架相比于纯钽细胞黏附性能更好。综合上述观点,我们认为80%孔隙率支架组作为骨缺损材料对细胞成骨最有优势。
基金项目
2020年度宁夏回族自治区重点研发计划,基金号:2020BCH01001;
2020年度上海“科技创新行动计划”国内科技合作项目编号:20025800200;
2019年度“上海交通大学医工(理)交叉基金资助”基金号:YG 2019 QNB 16。
文章引用
李学谦,赵仁良,傅绍菱,王 诚,陈 城,施忠民,邹 剑. 不同孔隙率钽涂层钛合金材料对细胞成骨的影响
Effect of Tantalum-Coated Titanium Alloy with Different Porosities on Cell Osteogenesis[J]. 材料科学, 2022, 12(06): 631-641. https://doi.org/10.12677/MS.2022.126069
参考文献
- 1. Witte, F., Kaese, V., Haferkamp, H., Switzer, E., Meyer-Lindenberg, A., Wirth, C.J. and Windhagen, H. (2005) In Vivo Corrosion of Four Magnesium Alloys and the Associated Bone Response. Biomaterials, 26, 3557-3563. https://doi.org/10.1016/j.biomaterials.2004.09.049
- 2. Xu, L., Pan, F., Yu, G., Yang, L., Zhang, E. and Yang, K. (2009) In Vitro and in Vivo Evaluation of the Surface Bioactivity of a Calcium Phosphate Coated Magnesium Alloy. Biomaterials, 30, 1512-1523. https://doi.org/10.1016/j.biomaterials.2008.12.001
- 3. Lee, J.W., Han, H.S., Han, K.J., Park, J., Jeon, H., Ok, M.R., Seok, H.K., Ahn, J.P., Lee, K.E., Lee, D.H., Yang, S.J., Cho, S.Y., Cha, P.R., Kwon, H., Nam, T.H., Han, J.H., Rho, H.J., Lee, K.S., Kim, Y.C. and Mantovani, D. (2016) Long-Term Clinical Study and Multiscale Analysis of in Vivo Biodegradation Mechanism of Mg Alloy. Proceedings of the National Academy of Sciences of the United States of America, 113, 716-721. https://doi.org/10.1073/pnas.1518238113
- 4. Kim, D.G., Huja, S.S., Tee, B.C., Larsen, P.E., Kennedy, K.S., Chien, H.H., Lee, J.W. and Wen, H.B. (2013) Bone Ingrowth and Initial Stability of Titanium and Porous Tantalum Dental Implants: A Pilot Canine Study. Implant Dentistry, 22, 399-405. https://doi.org/10.1097/ID.0b013e31829b17b5
- 5. Bencharit, S., Byrd, W.C., Altarawneh, S., Hosseini, B., Leong, A., Reside, G., Morelli, T. and Offenbacher, S. (2014) Development and Applications of Porous Tantalum Trabecular Metal-Enhanced Titanium Dental Implants. Clinical Implant Dentistry and Related Research, 16, 817-826. https://doi.org/10.1111/cid.12059
- 6. Liu, Z.H., Guo, W.S., Li, Z.R., Cheng, L.M., Zhang, Q.D., Yue, D.B., Shi, Z.C., Wang, B.L., Sun, W. and Zhang, N.F. (2014) Porous Tantalum Rods for Treating Osteonecrosis of the Femoral Head. Genetics and Molecular Research, 13, 8342-8352. https://doi.org/10.4238/2014.October.20.10
- 7. Pakos, E.E., Megas, P., Paschos, N.K., Syggelos, S.A., Kouzelis, A., Georgiadis, G. and Xenakis, T.A. (2015) Modified Porous Tantalum Rod Technique for the Treatment of Femoral Head Osteonecrosis. World Journal of Orthopedics, 6, 829-837. https://doi.org/10.5312/wjo.v6.i10.829
- 8. Tsao, A.K., Roberson, J.R., Christie, M.J., Dore, D.D., Heck, D.A., Robertson, D.D. and Poggie, R.A. (2005) Biomechanical and Clinical Evaluations of a Porous Tantalum Implant for the Treatment of Early-Stage Osteonecrosis. The Journal of Bone & Joint Surgery, 87, 22-27. https://doi.org/10.2106/00004623-200511002-00003
- 9. Trevisan, F., Calignano, F., Aversa, A., Marchese, G., Lombardi, M., Biamino, S., Ugues, D. and Manfredi, D. (2018) Additive Manufacturing of Titanium Alloys in the Biomedical Field: Processes, Properties and Applications. Journal of Applied Biomaterials & Functional Materials, 16, 57-67. https://doi.org/10.5301/jabfm.5000371
- 10. 蔡宏中, 易健宏, 吴霏, 魏燕, 张诩翔, 胡昌义. 多孔泡沫炭上钽涂层的化学气相沉积制备与表征[J]. 稀有金属, 2020, 44(10): 1108-1112. https://doi.org/10.13373/j.cnki.cjrm.XY19040006
- 11. Rubin, C.T., Capilla, E., Luu, Y.K., Busa, B., Crawford, H., Nolan, D.J., Mittal, V., Rosen, C.J., Pessin, J.E. and Judex, S. (2007) Adipogenesis Is Inhibited by Brief, Daily Exposure to High-Frequency, Extremely Low-Magnitude Mechanical Signals. Proceedings of the National Academy of Sciences of the United States of America, 104, 17879-17884. https://doi.org/10.1073/pnas.0708467104
- 12. Schmitz, J.P. and Hollinger, J.O. (1986) The Critical Size Defect as an Experimental Model for Craniomandibulofacialnonunions. Clinical Orthopaedics and Related Research, 205, 299-308. https://doi.org/10.1097/00003086-198604000-00036
- 13. Sagomonyants, K.B., Hakim-Zargar, M., Jhaveri, A., Aronow, M.S. and Gronowicz, G. (2011) Porous Tantalum Stimulates the Proliferation and Osteogenesis of Osteoblasts from Elderly Female Patients. Journal of Orthopaedic Research, 29, 609-616. https://doi.org/10.1002/jor.21251
- 14. Balla, V.K., Banerjee, S., Bose, S. and Bandyopadhyay, A. (2010) Direct Laser Processing of a Tantalum Coating on Titanium for Bone Replacement Structures. Acta Biomaterialia, 6, 2329-2334. https://doi.org/10.1016/j.actbio.2009.11.021
- 15. Schildhauer, T.A., Robie, B., Muhr, G. and Köller, M. (2006) Bacterial Adherence to Tantalum versus Commonly Used Orthopedic Metallic Implant Materials. Journal of Orthopaedic Trauma, 20, 476-484. https://doi.org/10.1097/00005131-200608000-00005
- 16. Liu, Y., Bao, C., Wismeijer, D. and Wu, G. (2015) The Physicochemical/Biological Properties of Porous Tantalum and the Potential Surface Modification Techniques to Improve Its Clinical Application in Dental Implantology. Materials Science and Engineering: C, 49, 323-329. https://doi.org/10.1016/j.msec.2015.01.007
- 17. Luo, C., Wang, C., Wu, X., Xie, X., Wang, C., Zhao, C., Zou, C., Lyu, F., Huang, W. and Liao, J. (2021) Influence of Porous Tantalum Scaffold Pore Size on Osteogenesis and Osteointegration: A Comprehensive Study Based on 3D-Printing Technology. Materials Science and Engineering: C, 129, Article ID: 112382. https://doi.org/10.1016/j.msec.2021.112382
- 18. Kosinski, M., Figiel-Dabrowska, A., Lech, W., Wieprzowski, L., Strzalkowski, R., Strzemecki, D., et al. (2020) Bone Defect Repair Using a Bone Substitute Supported by Mesenchymal Stem Cells Derived from the Umbilical Cord. Stem Cells International, 2020, Article ID: 1321283. https://doi.org/10.1155/2020/1321283
- 19. Dou, X.J., Wei, X.W., Liu, G., Wang, S., Lyu, Y., Li, J., et al. (2019) Effect of Porous Tantalum on Promoting the Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells In Vitro through the MAPK/ERK Signal Pathway. Journal of Orthopaedic Translation, 19, 81-93. https://doi.org/10.1016/j.jot.2019.03.006
- 20. Tseng, P.C., Hou, S.M., Chen, R.J., Peng, H.W., Hsieh, C.F., Kuo, M.L. and Yen, M.L. (2011) Resveratrol Promotes Osteogenesis of Human Mesenchymal Stem Cells by Upregulating RUNX2 Gene Expression Via the SIRT1/FOXO3A Axis. Journal of Bone and Mineral Research, 26, 2552-2563. https://doi.org/10.1002/jbmr.460
- 21. Neuss, S., Schneider, R.K., Tietze, L., Knüchel, R. and Jahnen-Dechent, W. (2010) Secretion of Fibrinolytic Enzymes Facilitates Human Mesenchymal Stem Cell Invasion into Fibrin Clots. Cells Tissues Organs, 191, 36-46. https://doi.org/10.1159/000215579
- 22. Lee, S.H., Lee, Y.J., Song, C.H., Ahn, Y.K. and Han, H.J. (2010) Role of FAK Phosphorylation in Hypoxia-Induced HMSCS Migration: Involvement of VEGF as Well as MAPKS and ENOS Pathways. American Journal of Physiology-Cell Physiology, 298, C847-C856. https://doi.org/10.1152/ajpcell.00418.2009
- 23. Lu, M.M., Wu, P.S., Guo, X.J., Yin, L.L., Cao, H.L. and Zou, D. (2018) Osteoinductive Effects of Tantalum and Titanium on Bone Mesenchymal Stromal Cells and Bone Formation in Ovariectomizedrats. European Review for Medical and Pharmacological Sciences, 22, 7087-7104. https://doi.org/10.26355/eurrev_201811_16241
NOTES
*通讯作者。
