Advances in Analytical Chemistry
Vol.
13
No.
02
(
2023
), Article ID:
65827
,
9
pages
10.12677/AAC.2023.132022
非生物胁迫下植物转录组学的研究进展
李晓仪
浙江理工大学生命科学与医药学院,浙江 杭州
收稿日期:2023年4月23日;录用日期:2023年5月13日;发布日期:2023年5月25日

摘要
植物由于其固着性,在其生长过程中面临着多种逆境胁迫,如光、热、重金属等非生物胁迫。为了应对这些压力,植物已经发展出了复杂的机制来避免或抵抗非生物胁迫。由于分子生物学技术的进步,转录组学已成为一门应用广泛的学科,转录组学可以揭示植物在非生物胁迫条件下的整个基因组表达,这有助于了解植物中与胁迫适应和耐受性相关的复杂调控网络。本文综述了植物响应非生物胁迫过程中转录组学研究的最新进展。
关键词
非生物胁迫,转录组,差异表达基因

Advances in Plant Transcriptomics under Abiotic Stress
Xiaoyi Li
College of Life Sciences and Medicine, Zhejiang Sci-Tech University, Hangzhou Zhejiang
Received: Apr. 23rd, 2023; accepted: May 13th, 2023; published: May 25th, 2023

ABSTRACT
Due to its sessile nature, plants face a variety of adversity stresses during their growth, such as light, heat, heavy metals and other abiotic stresses. In response to these stresses, plants have developed complex mechanisms to avoid or resist abiotic stresses. With the development of molecular biology technology, transcriptomics has become a widely used discipline. Under abiotic stress conditions, transcriptomics can reveal the expression of plants at the whole genome level from the whole transcript level, which is helpful for understanding plant Complex regulatory networks associated with stress adaptation and tolerance. This article reviews recent advances in transcriptomic studies in plant responses to abiotic stresses.
Keywords:Abiotic Stress, Transcriptome, Differentially Expressed Genes

Copyright © 2023 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
植物的生长和发育依赖于环境,而环境是多变的,植物在整个生命周期中都面临着生长环境中非生物胁迫挑战 [1] 。非生物胁迫是指任何限制植物生长、发育和生产力的环境因素,如光、热、重金属等 [2] ,植物必须响应和适应非生物胁迫才能在各种环境条件下生存,在这一过程中植物发展出了复杂的胁迫耐受机制,这些机制涉及不同的生理生化变化过程,导致植物发生适应性变化或形态变化 [3] 。由于全球环境的恶化,植物非生物胁迫已成为制约现代农业发展的重要因素,对植物非生物胁迫响应机制的研究也日益增多。
随着高通量测序技术的发展,转录组学成为目前研究和应用广泛的学科,转录组分析正在逐渐提升我们对基于RNA的基因调控网络的理解。转录组是在特定发育阶段和特定生理条件下特定细胞类型或组织的一整套RNA转录本,是从整体水平对细胞内基因转录及调控规律的研究。转录组学中关于差异表达基因(DEGs)的研究是通过比较不同组织和条件下的基因表达谱来确定哪些基因在响应非生物胁迫中起主要作用 [4] ,为植物适应环境胁迫的分子反应的深入研究提供了基础 [5] [6] 。为了提高植物的抗性和生产力,研究的重点已逐渐转向了解植物与环境相互作用的关键分子目标与调控因子 [7] [8] ,因为这将进一步帮助植物家通过基因方式改善或改造植物抗逆境能力,以更好地应对非生物胁迫 [9] 。
2. 非生物胁迫下转录组学的研究
2.1. 光胁迫
影响植物生长发育的关键环境因素之一是光照。光照强度超过植物适应范围会加速反应、细胞损伤并最终导致植物死亡。植物适应强光的一个特征是类黄酮生物合成途径的快速转录激活,导致光保护和抗氧化类黄酮,如花青素,在叶组织中积累 [10] ,花青素通过吸收蓝绿色光和强紫外线来保护细胞免受光抑制和损伤 [11] 。通过分析花青素生物合成基因的表达谱 [12] 发现,花青素生物合成可分为三个阶段:苯丙素途径、类黄酮途径和花青素特异性途径 [13] 。对强光胁迫下的拟南芥转录数据分析发现,有6个DGEs对应转录表达了PAL1、C4H和4CL蛋白,这些蛋白参与了花青素合成途径的起始步骤,另外有三种MYB转录因子基因(MYB11、MYB12和MYB111)在所有强光下均上调 [14] 。MYB、碱性螺旋-环-螺旋(bHLH)和WD40是参与类黄酮生物合成调控的重要转录因子。MYB-bHLH-WD40 (MBW)转录复合物激活类黄酮生物合成的下游基因,介导原花青素和花青素等各种产物的产生 [15] 。MYB-bHLH-WD40复合物在调节花青素中发挥着核心作用,植物对光胁迫的响应主要通过激活或失活该复合物的活性来调节花青素 [16] ,从而保护细胞免受光抑制和光损伤。Ye [17] 等对不同光照条件下茶树的转录组数据分析发现有40个转录因子与类黄酮代谢产物相关,包括23个MYB、14个bHLH和3个WD40。当暴露于较高的光照强度时,在菊花 [18] 、荔枝果皮 [19] 和桃 [20] 的转录组数据分析中也发现bHLH基因显著上调。关于光胁迫下不同植物中关于花青素合成调控转录因子的统计见表1。Zhang等人通过比较在弱光和强光照下的红叶莴苣的转录组数据,鉴定出5个MYB和1个bHLH基因 [21] 。显然植物积极调节着花青素合成途径相关转录因子的转录以响应光胁迫。
Table 1. Statistics of transcription factors regulating anthocyanin biosynthesis in different plants under light stress
表1. 光胁迫下不同植物中关于花青素合成调控转录因子的统计
2.2. 热胁迫
气体排放和人类活动大大增加了温室气体的积累,全球变暖正成为植物学家最关心的问题之一。温室气体将逐渐使全球环境温度升高 [2] ,使植物遭受广泛的热胁迫,严重影响植物的生存、生长和分布。温度是维持细胞代谢的重要因素之一,Wahid等人 [25] 将热胁迫定义为温度上升超过一定水平一段时间,对植物造成不可弥补的损害,热可以扰乱细胞内稳态,最终导致植物生长迟缓甚至死亡。本节综述的是关于利用转录组学揭示热休克蛋白(HSP)功能的分子细节和热休克转录因子(HSF)调控网络的复杂性的最新研究。热休克蛋白在各种生物体中是一个高度保守的家族 [25] ,热激蛋白的类型、亚细胞定位及主要生物学功能见表2。相关研究发现,水稻 [26] 、玉米 [27] 、番茄 [28] 、棉花 [29] 等在热应激条件下均会HSP的合成。植物获得抗热能力的一个重要特点就是在植物体内大量生产HSP。对高温胁迫下马铃薯中的转录水平分析表明,7个HSP90的基因与温度胁迫有关,尤其是StHsp90.2和StHsp90.4,在叶片、茎和根中的表达水平显著上调 [30] 。HSP70家族是蛋白质稳态网络的中心枢纽,其可以通过分子伴侣的形式防止新产生的蛋白质的错误聚集,并利用ATP水解的能量来溶解、转移和调节蛋白质的适当重折叠及去折叠,使植物细胞避免在胁迫条件下受到伤害,对提高植物在逆境下的抵抗能力具有极其重要的作用 [31] 。Ahsan等研究了处于42℃热应激下水稻叶片HSP的基因表达谱,发现在极短的时间内HSP70就会被快速的诱导并大量表达 [32] 。大多数HSP60家族蛋白是热诱导的,也是在热应激条件下防止蛋白质聚集和介导线粒体折叠和重折叠必不可少的 [33] 。Tominaga等的转录组研究证明了温度显著影响孔石菇中UpHSP60基因的表达 [34] 。植物体内的sHSPs在常温下不表达,但在热激条件下会迅速合成,对维持植物细胞蛋白质的稳定性和生物膜结构具有重要作用 [35] 。羊毛铁线莲和厚叶铁线莲热应激反应的比较转录组分析发现DEGs主要集中在sHSPs中,包括HSP17、HSP17.8、HSP18.1、HSP20和HSP26.5 [36] 。目前的研究已知有四种途径,包括热休克转录因子–热休克蛋白(Hsf-Hsp)途径、钙离子–钙调蛋白(Ca2+-CaM)途径、活性氧途径和激素途径来调节热应激 [37] [38] 。在本节主要综述植物(Hsf-Hsp)途径对热胁迫的响应,如图1所示,热信号被质膜感知,然后将热信号迅速传递给HSFA1,作为调节下游热应激响应基因的主体,提高热胁迫下植物的耐热性 [39] 。在热休克转录因子(HSFs)的控制下,热休克蛋白(HSPs)的积累被认为在植物的热应激反应(HSR)和获得性耐热性中发挥着核心作用。例如在拟南芥 [40] 、百合 [41] 、
Table 2. Types, subcellular localization and main biological functions of heat shock protein
表2. 热激蛋白的类型、亚细胞定位及主要生物学功能
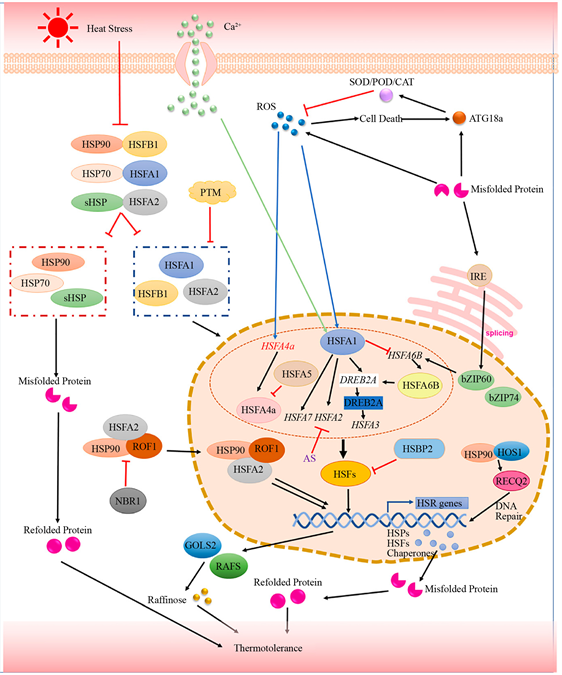
Figure 1. Schematic representation of HSFs-and HSPs-mediated heat stress response (HSR) in plants [39]
图1. 植物中HSF和HSP介导的热应激反应(HSR)的示意图 [39]
番茄 [42] 的热调节机制研究中发现,植物主要通过HSF-HSP途径来响应热胁迫。 [40] 使用RNA测序系统分析了拟南芥植物暴露于高温后的转录调控网络,确定了热休克因子A1家族蛋白(HSFA1s)是介导HS诱导的第一波基因表达的主要转录因子,帮助植物适应白天的高温。Li等人对百合幼苗 [41] 进行不同时间长度的热处理,RNA-seq测序发现在0.5 h~1 h和1 h~3 h的热处理期间,HSF-HSP通路DEGs被显著且高度诱导。Giorno等人对番茄 [42] 的花药和花粉发育阶段进行转录分析,结果表明HsfA2可能直接参与热胁迫期间番茄花药中保护机制的激活,从而可能有助于番茄在不利温度下的坐果。
2.3. 重金属胁迫
由于施肥、工业废物处理不当、污水处理不规范 [43] 等,导致Mn、Cu、Ni、Co、Cd、Fe、Zn和Hg等重金属在土壤中积累。有研究认为,重金属胁迫干扰了必需金属离子与生物分子的取代反应,破坏膜的完整性,导致光合能力、呼吸等的变化 [44] ,常见的症状有植物生长减缓、光合作用受阻、水分平衡被扰乱、生物量积累减少,引发衰老和褪绿 [45] 。重金属胁迫还通过刺激过氧化氢、超氧化自由基和羟基自由基的产生来诱导氧化应激 [46] 。为了避免或耐受重金属的毒性,植物已经发展出复杂的防御机制,包括重金属在细胞器中的吸收和积累,通过与有机螯合物形成复合物进行固定,通过使用大量转运蛋白、离子通道、信号级联和转录元件进行提取等 [47] 。本节综述了植物对这些应激源的反应和适应策略中关于转运蛋白的最新进展。转运蛋白主要有助于金属的摄取、转运和再分配。转录组学技术被广泛应用于研究拟南芥属 [48] [49] [50] 和景天属植物 [51] 的基因表达谱,这些物种的特征是高度可变的金属超耐受性和超积累性。转录组学研究表明,在金属超积累植物根的高度表达的基因中,有一些属于ZRT/IRT样蛋白(ZIP)转运蛋白基因家族 [52] [53] [54] ,这些转运蛋白在超积累植物的根中吸收和转运重金属。另一个被提及较多的是铁调节转运蛋白基因(IRT1),在拟南芥属根的镉吸收中发挥作用 [55] 。另一个在镉/锌金属转运、动员和分配中发挥作用的基因是寡聚肽转运子3 (OPT3) [56] ,转录组数据显示,与非超积累植物相比,其在金属超积累植物如欧洲拟南芥的根中的表达高得多。在重金属胁迫条件下分析不同的植物转录组时,已经观察到保守的趋势,转运蛋白的参与受到了研究者的广泛关注,关于不同植物中转运蛋白的统计见表3。利用转录组学研究金属转运蛋白有助于筛选和生产耐金属胁迫的作物,而不会影响其生产力,可以减缓农业逐渐增加的压力。
Table 3. Statistics of transporters in different plants under heavy metal stress
表3. 重金属胁迫下不同植物中关于转运蛋白的统计
3. 展望
如上所述,过去几十年来,转录组分析技术被广泛应用于了解植物基因水平上的胁迫耐受机制。对植物应对非生物胁迫基因功能的重点研究所产生的信息将成为一波新的战略跳板,以改善和提升重要植物抵抗非生物胁迫的能力。转录组技术可以快速预测相关的胁迫防御因子,揭示植物的代谢途径、信号转导与防御反应之间的关系,对研究和了解植物的抗逆性机制具有重要意义。但是,植物对非生物胁迫的反应是通过一系列复杂的细胞、分子和生理过程,它们的代谢网络是复杂的。虽然已经逐渐阐明了其中一些分子机制,但对植物抗胁迫的认识仍然有限,这些通路的协同作用或其他关联需要进一步研究。
文章引用
李晓仪. 非生物胁迫下植物转录组学的研究进展
Advances in Plant Transcriptomics under Abiotic Stress[J]. 分析化学进展, 2023, 13(02): 181-189. https://doi.org/10.12677/AAC.2023.132022
参考文献
- 1. Dolferus, R. (2014) To Grow or Not to Grow: A Stressful Decision for Plants. Plant Science, 229, 247-261.
https://doi.org/10.1016/j.plantsci.2014.10.002 - 2. Imran, Q.M., Falak, N., Hussain, A.,Mun, B.G. and Yun, B.W. (2021) Abiotic Stress in Plants; Stress Perception to Molecular Response and Role of Biotechnological Tools in Stress Resistance. Agronomy, 11, Article No. 1579.
https://doi.org/10.3390/agronomy11081579 - 3. Urano, K., Kurihara, Y., Seki, M. and Shinozaki, K. (2010) ‘Omics’ Analyses of Regulatory Networks in Plant Abiotic Stress Responses. Current Opinion in Plant Biology, 13, 132-138.
https://doi.org/10.1016/j.pbi.2009.12.006 - 4. Finotello, F. and Di Camillo, B. (2015) Measuring Differential Gene Expression with RNA-Seq: Challenges and Strategies for Data Analysis. Briefings in Functional Genomics, 14, 130-142.
https://doi.org/10.1093/bfgp/elu035 - 5. Matsui, A., Ishida, J., Morosawa, T., Mochizuki, Y., Kaminuma, E., Endo, T.A., Okamoto, M., Nambara, E., Nakajima, M., Kawashima, M., Satou, M., Kim, J.-M., Kobayashi, N., Toyoda, T., Shinozaki, K. and Seki, M. (2008) Arabidopsis Transcriptome Analysis under Drought, Cold, High-Salinity and ABA Treatment Conditions Using a Tiling Array. Plant and Cell Physiology, 49, 1135-1149.
https://doi.org/10.1093/pcp/pcn101 - 6. Fowler, S. and Thomashow, M.F. (2002) Arabidopsis Transcriptome Profiling Indicates That Multiple Regulatory Pathways Are Activated during Cold Acclimation in Addition to the CBF Cold Response Pathway. The Plant Cell, 14, 1675-1690.
https://doi.org/10.1105/tpc.003483 - 7. Mosa, K.A., Ismail, A. and Helmy, M. (2017) Omics and System Biology Approaches in Plant Stress Research. In: Plant Stress Tolerance. SpringerBriefs in Systems Biology, Springer, Cham, 21-34.
https://doi.org/10.1007/978-3-319-59379-1_2 - 8. Parida, A.K., Panda, A. and Rangani, J. (2018) Metabolomics-Guided Elucidation of Abiotic Stress Tolerance Mechanisms in Plants. In: Ahmad, P., Ahanger, M.A., Singh, V.P., Tripathi, D.K., Alam, P. and Alyemeni, M.N., Eds., Plant Metabolites and Regulation under Environmental Stress, Academic Press, Cambridge, 89-131.
https://doi.org/10.1016/B978-0-12-812689-9.00005-4 - 9. Soda, N., Wallace, S. and Karan, R. (2015) Omics Study for Abiotic Stress Responses in Plants. Advances in Plants & Agriculture Research, 2, 28-34.
https://doi.org/10.15406/apar.2015.02.00037 - 10. Araguirang, G.E. and Richter, A.S. (2022) Activation of Anthocyanin Biosynthesis in High Light—What Is the Initial Signal? New Phytologist, 236, 2037-2043.
https://doi.org/10.1111/nph.18488 - 11. Li, J., Ou-Lee, T.-M., Raba, R., Amundson, R.G. and Last, R.L. (1993) Arabidopsis Flavonoid Mutants Are Hypersensitive to UV-B Irradiation. The Plant Cell, 5, 171-179.
https://doi.org/10.2307/3869583 - 12. Solfanelli, C., Poggi, A., Loreti, E., Alpi, A. and Perata, P. (2006) Sucrose-Specific Induction of the Anthocyanin Biosynthetic Pathway in Arabidopsis. Plant Physiology, 140, 637-646.
https://doi.org/10.1104/pp.105.072579 - 13. Shi, M.-Z. and Xie, D.-Y. (2014) Biosynthesis and Metabolic Engineering of Anthocyanins in Arabidopsis thaliana. Recent Patents on Biotechnology, 8, 47-60.
https://doi.org/10.2174/1872208307666131218123538 - 14. Huang, J., Zhao, X. and Chory, J. (2019) The Arabidopsis Transcriptome Responds Specifically and Dynamically to High Light Stress. Cell Reports, 29, 4186-4199.
https://doi.org/10.1016/j.celrep.2019.11.051 - 15. Li, S. and Zachgo, S. (2013) TCP 3 Interacts with R2R3-MYB Proteins, Promotes Flavonoid Biosynthesis and Nega-tively Regulates the Auxin Response in Arabidopsis thaliana. The Plant Journal, 76, 901-913.
https://doi.Org/10.1111/Tpj.12348 - 16. Zoratti, L., Karppinen, K., Luengo Escobar, A., Häggman, H. and Jaakola, L. (2014) Light-Controlled Flavonoid Biosynthesis in Fruits. Frontiers in Plant Science, 5, Article 534.
https://doi.org/10.3389/fpls.2014.00534 - 17. Ye, J.-H., Lv, Y.-Q., Liu, S.-R., Jin, J., Wang, Y.-F., Wei, C.-L. and Zhao, S.-Q. (2021) Effects of Light Intensity and Spectral Composition on the Transcriptome Profiles of Leaves in Shade Grown Tea Plants (Camellia sinensis L.) and Regulatory Network of Flavonoid Biosynthesis. Molecules, 26, Article No. 5836.
https://doi.org/10.3390/molecules26195836 - 18. Hong, Y., Tang, X., Huang, H., Zhang, Y. and Dai, S. (2015) Transcriptomic Analyses Reveal Species-Specific Light- Induced Anthocyanin Biosynthesis in Chrysanthemum. BMC Genomics, 16, Article No. 202.
https://doi.org/10.1186/s12864-015-1428-1 - 19. Zhang, H.-N., Li, W.-C., Wang, H.-C., Shi, S.-Y., Shu, B., Liu, L.-Q., Wei, Y.-Z. and Xie, J.-H. (2016) Transcriptome Profiling of Light-Regulated Anthocyanin Biosynthesis in the Pericarp of Litchi. Frontiers in Plant Science, 7, Article 963.
https://doi.org/10.3389/fpls.2016.00963 - 20. Liu, T., Song, S., Yuan, Y., Wu, D., Chen, M., Sun, Q., et al. (2015) Improved Peach Peel Color Development by Fruit Bagging. Enhanced Expression of Anthocyanin Biosynthetic and Regulatory Genes Using White Non-Woven Polypropylene as Replacement for Yellow Paper. Scientia Horticulturae, 184, 142-148.
https://doi.org/10.1016/j.scienta.2015.01.003 - 21. Zhang, Y., Xu, S., Cheng, Y., Peng, Z. and Han, J. (2018) Transcriptome Profiling of Anthocyanin-Related Genes Reveals Effects of Light Intensity on Anthocyanin Biosynthesis in Red Leaf Lettuce. PeerJ, 6, e4607.
https://doi.org/10.7717/peerj.4607 - 22. Xiang, L.-L., Liu, X.-F., Li, X., Yin, X.-R., Grierson, D., Li, F. and Chen, K.-S. (2015) A Novel bHLH Transcription Factor Involved in Regulating Anthocyanin Biosynthesis in Chrysanthemums (Chrysanthemum morifolium Ramat.). PLOS ONE, 10, e0143892.
https://doi.org/10.1371/journal.pone.0143892 - 23. Lin-Wang, K., McGhie, T.K., Wang, M., Liu, Y., Warren, B., Storey, R., Espley, R.V. and Allan, A.C. (2014) Engineering the Anthocyanin Regulatory Complex of Strawberry (Fragaria vesca). Frontiers in Plant Science, 5, Article 651.
https://doi.org/10.3389/fpls.2014.00651 - 24. Tao, R., Yu, W., Gao, Y., Ni, J., Yin, L., Zhang, X., et al. (2020) Light-Induced Basic/Helix-Loop-Helix64 Enhances Anthocyanin Biosynthesis and Undergoes CONSTITUTIVELY PHOTOMORPHOGENIC1-Mediated Degradation in Pear. Plant Physiology, 184, 1684-1701.
https://doi.org/10.1104/pp.20.01188 - 25. Efeoğlu, B. (2009) Heat Shock Proteins and Heat Shock Response in Plants. Gazi University Journal of Science, 22, 67-75.
- 26. Da Costa, M.V.J., Ramegowda, V., Ramakrishnan, P., Nataraja, K.N. and Sheshshayee, M.S. (2022) Comparative Metabolite Profiling of Rice Contrasts Reveal Combined Drought and Heat Stress Signatures in Flag Leaf and Spikelets. Plant Science, 320, Article ID: 111262.
https://doi.org/10.1016/j.plantsci.2022.111262 - 27. El-Sappah, A.H., Rather, S.A., Wani, S.H., Elrys, A.S., Bilal, M., Huang, Q., et al. (2022) Heat Stress-Mediated Constraints in Maize (Zea mays) Production: Challenges and Solutions. Frontiers in Plant Science, 13, Article 879366.
https://doi.org/10.3389/fpls.2022.879366 - 28. Xu, X., Wang, Q., Li, W., Hu, T., Wang, Q., Yin, Y., et al. (2022) Overexpression of SlBBX17 Affects Plant Growth and Enhances Heat Tolerance in Tomato. International Journal of Biological Macromolecules, 206, 799-811.
https://doi.org/10.1016/j.ijbiomac.2022.03.080 - 29. Masoomi-Aladizgeh, F., Kamath, K.S., Haynes, P.A. and Atwell, B.J. (2022) Genome Survey Sequencing of Wild Cotton (Gossypium robinsonii) Reveals Insights into Proteomic Responses of Pollen to Extreme Heat. Plant, Cell & Environment, 45, 1242-1256.
https://doi.org/10.1111/pce.14268 - 30. Li, W., Chen, Y., Ye, M.H., Wang, D.D. and Chen, Q. (2020) Evolutionary History of the Heat Shock Protein 90 (Hsp90) Family of 43 Plants and Characterization of Hsp90s in Solanum tuberosum. Molecular Biology Reports, 47, 6679-6691.
https://doi.org/10.1007/s11033-020-05722-x - 31. Imamoglu, R., Balchin, D., Hayer-Hartl, M. and Hartl, F.U. (2020) Bacterial Hsp70 Misfolded States and Accelerates Productive Folding of a Multi-Domain Protein. Nature Communication, 11, Article No. 365.
https://doi.org/10.1038/s41467-019-14245-4 - 32. Lee, D.G., Ahsan, N., Kim, Y.G., Kim, K.H., Lee, S.H., Lee, K.W., et al. (2013) Expression of Heat Shock Protein and Antioxidant Genes in Rice Leaf under Heat Stress. Journal of the Korean Society of Grassland Science, 33, 159-166.
https://doi.org/10.5333/KGFS.2013.33.3.159 - 33. Sharma, S., Reddy, P., Rohilla, M.S. and Tiwari, P.K. (2006) Expression of HSP60 Homologue in Sheep Blowfly Lucilia cuprina during Development and Heat Stress. Journal of Thermal Biology, 31, 546-555.
https://doi.org/10.1016/j.jtherbio.2006.05.010 - 34. Tominaga, H., Coury, D.A., Amano, H., Miki, W. and Kakinuma, M. (2012) CDNA Cloning and Expression Analysis of Two Heat Shock Protein Genes, Hsp90 and Hsp60, from a Sterile Ulva pertusa (Ulvales, Chlorophyta). Fisheries Science, 78, 415-429.
https://doi.org/10.1007/s12562-011-0451-7 - 35. Jackson, S.E. (2013) Small Heat-Shock Proteins: Paramedics of the Cell. In: Jackson, S., Ed., Molecular Chaperones. Topics in Current Chemistry, Vol. 328, Springer, Berlin, 69-98.
https://doi.org/10.1007/128_2012_324 - 36. Qian, R., Hu, Q., Ma, X., Zhang, X., Ye, Y., Liu, H., Gao, H. and Zheng, J. (2022) Comparative Transcriptome Analysis of Heat Stress Responses of Clematis lanuginosa and Clematis crassifolia. BMC Plant Biology, 22, Article No. 138.
https://doi.org/10.1186/s12870-022-03497-w - 37. Reddy, A.S., Ali, G.S., Celesnik, H. and Day, I.S. (2011) Coping with Stresses: Roles of Calcium- and Calcium/Cal- modulin-Regulated Gene Expression. The Plant Cell, 23, 2010-2032.
https://doi.org/10.1105/tpc.111.084988 - 38. Mittler, R., Finka, A. and Goloubinoff, P. (2012) How Do Plants Feel the Heat? Trends in Biochemical Sciences, 37, 118-125.
https://doi.org/10.1016/j.tibs.2011.11.007 - 39. Zhou, Y., Xu, F., Shao, Y. and He, J. (2022) Regulatory Mechanisms of Heat Stress Response and Thermomorphogenesis in Plants. Plants, 11, Article No. 3410.
https://doi.org/10.3390/plants11243410 - 40. Li, B., Gao, Z., Liu, X., Sun, D. and Tang, W. (2019) Transcriptional Profiling Reveals a Time-of-Day-Specific Role of REVEILLE 4/8 in Regulating the First Wave of Heat Shock-Induced Gene Expression in Arabidopsis. The Plant Cell, 31, 2353-2369.
https://doi.org/10.1105/tpc.19.00519 - 41. Zhou, Y., Wang, Y., Xu, F., Song, C., Yang, X., Zhang, Z., et al. (2022) Small HSPs Play an Important Role in Crosstalk between HSF-HSP and ROS Pathways in Heat Stress Response through Transcriptomic Analysis in Lilies (Lilium longiflorum). BMC Plant Biology, 22, Article No. 202.
https://doi.org/10.1186/s12870-022-03587-9 - 42. Giorno, F., Wolters-Arts, M., Grillo, S., Scharf, K. D., Vriezen, W.H. and Mariani, C. (2010) Developmental and Heat Stress-Regulated Expression of HsfA2 and Small Heat Shock Proteins in Tomato Anthers. Journal of Experimental Botany, 61, 453-462.
https://doi.org/10.1093/jxb/erp316 - 43. Ghori, N.-H., Ghori, T., Hayat, M.Q., Imadi, S.R., Gul, A., Altay, V. and Ozturk, M. (2019) Heavy Metal Stress and Responses in Plants. International Journal of Environmental Science and Technology, 16, 1807-1828.
https://doi.org/10.1007/s13762-019-02215-8 - 44. Hossain, M.A., Piyatida, P., da Silva, J.A.T. and Fujita, M. (2012) Molecular Mechanism of Heavy Metal Toxicity and Tolerance in Plants: Central Role of Glutathione in Detoxification of Reactive Oxygen Species and Methylglyoxal and in Heavy Metal Chelation. Journal of Botany, 2012, Article ID: 872875.
https://doi.org/10.1155/2012/872875 - 45. Wang, C., Tao, W., Ping, M.U., Li, Z.C., and Yang, L. (2013) Quantitative Trait Loci for Mercury Tolerance in Rice Seedlings. Rice Science, 20, 238-242.
https://doi.org/10.1016/S1672-6308(13)60124-9 - 46. Rascio, N. and Navari-Izzo, F. (2011) Heavy Metal Hyperaccumulating Plants: How and Why Do They Do It? And What Makes Them So Interesting? Plant Science, 180, 169-181.
https://doi.org/10.1016/j.plantsci.2010.08.016 - 47. Jogawat, A., Yadav, B. and Narayan, O.P. (2021) Metal Transporters in Organelles and Their Roles in Heavy Metal Transportation and Sequestration Mechanisms in Plants. Physiologia Plantarum, 173, 259-275.
https://doi.org/10.1111/ppl.13370 - 48. Van De Mortel, J.E., Almar Villanueva, L., Schat, H., Kwekkeboom, J., Coughlan, S., Moerland, P.D., et al. (2006) Large Expression Differences in Genes for Iron and Zinc Homeostasis, Stress Response, and Lignin Biosynthesis Distinguish Roots of Arabidopsis thaliana and the Related Metal Hyperaccumulator Thlaspi caerulescens. Plant Physiology, 142, 1127-1147.
https://doi.org/10.1104/pp.106.082073 - 49. Weber, M., Trampczynska, A. and Clemens, S. (2006) Comparative Transcriptome Analysis of Toxic Metal Responses in Arabidopsis thaliana and the Cd2+-Hypertolerant Facultative Metallophyte Arabidopsis halleri. Plant, Cell & Environment, 29, 950-963.
https://doi.org/10.1111/j.1365-3040.2005.01479.x - 50. Courbot, M., Willems, G., Motte, P., Arvidsson, S., Roosens, N., Saumitou-Laprade, P. and Verbruggen, N. (2007) A Major Quantitative Trait Locus for Cadmium Tolerance in Arabidopsis halleri Colocalizes with HMA4, a Gene Encoding a Heavy Metal ATPase. Plant Physiology, 144, 1052-1065.
https://doi.org/10.1104/pp.106.095133 - 51. Gao, J., Sun, L., Yang, X. and Liu, J.-X. (2013) Transcriptomic Analysis of Cadmium Stress Response in the Heavy Metal Hyperaccumulator Sedum alfredii Hance. PLOS ONE, 8, e64643.
https://doi.org/10.1371/journal.pone.0064643 - 52. Zhang, H., Zhao, S., Li, D., Xu, X. and Li, C. (2017) Genome-Wide Analysis of the ZRT, IRT-Like Protein (ZIP) Family and Their Responses to Metal Stress in Populus trichocarpa. Plant Molecular Biology Reporter, 35, 534-549.
- 53. Talke, I.N., Hanikenne, M. and Krämer, U. (2006) Zinc-Dependent Global Transcriptional Control, Transcriptional Deregulation, and Higher Gene Copy Number for Genes in Metal Homeostasis of the Hyperaccumulator Arabidopsis halleri. Plant Physiology, 142, 148-167.
https://doi.org/10.1104/pp.105.076232 - 54. Corso, M., Schvartzman, M.S., Guzzo, F., Souard, F., Malkowski, E., Hanikenne, M. and Verbruggen, N. (2018) Contrasting Cadmium Resistance Strategies in Two Metallicolous Populations of Arabidopsis halleri. New Phytologist, 218, 283-297.
https://doi.org/10.1111/nph.14948 - 55. Merlot, S., Garcia de la Torre, V.S. and Hanikenne, M. (2021) Physiology and Molecular Biology of Trace Element Hyperaccumulation. In: van der Ent, A., Baker, A.J., Echevarria, G., Simonnot, MO. and Morel, J.L., Eds., Agromining: Farming for Metals. Mineral Resource Reviews, Springer, Cham, 155-181.
https://doi.org/10.1007/978-3-030-58904-2_8 - 56. Zhai, Z., Gayomba, S.R., Jung, H.I., Vimalakumari, N.K., Piñeros, M., Craft, E., et al. (2014) OPT3 Is a Phloem-Spe- cific Iron Transporter That Is Essential for Systemic Iron Signaling and Redistribution of Iron and Cadmium in Arabidopsis. The Plant Cell, 26, 2249-2264.
https://doi.org/10.1105/tpc.114.123737 - 57. Wang, J., Liang, S., Xiang, W., et al. (2019) A Repeat Region from the Brassica juncea HMA4 Gene BjHMA4R Is Specifically Involved in Cd2+ Binding in the Cytosol under Low Heavy Metal Concentrations. BMC Plant Biology, 19, Article No. 89.
https://doi.org/10.1186/s12870-019-1674-5 - 58. Mills, R.F., Krijger, G.C., Baccarini, P.J., Hall, J.L. and Williams, L.E. (2003) Functional Expression of AtHMA4, a P1B-Type ATPase of the Zn/Co/Cd/Pb Subclass. The Plant Journal, 35, 164-176.
https://doi.org/10.1046/j.1365-313X.2003.01790.x - 59. Yokosho, K., Yamaji, N. and Ma, J.F. (2014) Global Transcriptome Analysis of Al-Induced Genes in an Al-Accumu- lating Species, Common Buckwheat (Fagopyrum esculentum Moench). Plant and Cell Physiology, 55, 2077-2091.
https://doi.org/10.1093/pcp/pcu135 - 60. Ariani, A., Di Baccio, D., Romeo, S., Lombardi, L., Andreucci, A., Lux, A., Horner, D.S. and Sebastiani, L. (2015) RNA Sequencing of Populus x canadensis Roots Identifies Key Molecular Mechanisms Underlying Physiological Adaption to Excess Zinc. PLOS ONE, 10, e0117571.
https://doi.org/10.1371/journal.pone.0117571 - 61. Chang, J.-D., Huang, S., Yamaji, N., et al. (2020) OsNRAMP1 Transporter Contributes to Cadmium and Manganese Uptake in Rice. Plant, Cell & Environment, 43, 2476-2491.
https://doi.org/10.1111/pce.13843 - 62. Xu, X., Zhang, S., Cheng, Z., et al. (2020) Transcriptome Analysis Revealed Cadmium Accumulation Mechanisms in Hyperaccumulator Siegesbeckia orientalis L. Environmental Science and Pollution Research, 27, 18853-18865.
https://doi.org/10.1007/s11356-020-08387-y - 63. Wei, W., Chai, T., Zhang, Y., Han, L., Xu, J. and Guan, Z. (2009) The Thlaspi caerulescens NRAMP Homologue TcNRAMP3 Is Capable of Divalent Cation Transport. Molecular Biotechnology, 41, 15-21.
https://doi.org/10.1007/s12033-008-9088-x - 64. Liu, H., Zhao, H., Wu, L., Liu, A., Zhao, F.-J. and Xu, W. (2017) Heavy Metal ATPase 3 (HMA3) Confers Cadmium Hypertolerance on the Cadmium/Zinc Hyperaccumulator Sedum plumbizincicola. New Phytologist, 215, 687-698.
https://doi.org/10.1111/nph.14622 - 65. Zhao, H., Wang, L., Zhao, F.-J., Wu, L., Liu, A. and Xu, W. (2019) SpHMA1 Is a Chloroplast Cadmium Exporter Protecting Photochemical Reactions in the Cd Hyperaccumulator Sedum plumbizincicola. Plant, Cell & Environment, 42, 1112-1124.
https://doi.org/10.1111/pce.13456 - 66. Wang, L., Zheng, B., Yuan, Y., Xu, Q. and Chen, P. (2020) Transcriptome Profiling of Fagopyrum tataricum Leaves in Response to Lead Stress. BMC Plant Biology, 20, Article No. 54.
https://doi.org/10.1186/s12870-020-2265-1 - 67. Wu, D., Yamaji, N., Yamane, M., Kashino-Fujii, M., Sato, K. and Ma, J.F. (2016) The HvNramp5 Transporter Mediates Uptake of Cadmium and Manganese, but Not Iron. Plant Physiology, 172, 1899-1910.
https://doi.org/10.1104/pp.16.01189 - 68. Feng, S., Tan, J., Zhang, Y., Liang, S., Xiang, S., Wang, H. and Chai, T. (2017) Isolation and Characterization of a Novel Cadmium-Regulated Yellow Stripe-Like Transporter (SnYSL3) in Solanum nigrum. Plant Cell Reports, 36, 281-296.
https://doi.org/10.1007/s00299-016-2079-7 - 69. Peng, F., Wang, C., Zhu, J., Zeng, J., Kang, H., Fan, X., et al. (2018) Expression of TpNRAMP5, a Metal Transporter from Polish Wheat (Triticum polonicum L.), Enhances the Accumulation of Cd, Co and Mn in Transgenic Arabidopsis Plants. Planta, 247, 1395-1406.
https://doi.org/10.1007/s00425-018-2872-3 - 70. Zhang, X., Li, X., Tang, L., Peng, Y., Qian, M., Guo, Y., et al. (2020) The Root Iron Transporter 1 Governs Cadmium uptake in Vicia sativa Roots. Journal of Hazardous Materials, 398, Article ID: 122873.
https://doi.org/10.1016/j.jhazmat.2020.122873
